Abstract
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid that mediates a wide array of biologic effects through its interaction with a family of five G protein-coupled receptors. Cytokines and growth factors interact with this signaling pathway in a variety of ways, including both activation and regulation of the expression of the enzymes that regulate synthesis and degradation of S1P. Not only do many growth factors and cytokines stimulate S1P production, leading to transactivation of S1P receptors, ligation of S1P receptors by S1P can also transactivate growth factor tyrosine kinase receptors and stimulate growth factor and cytokine signaling cascades.XXX This review discusses the mechanisms involved in cross-talk between S1P, cytokines, and growth factors and the impact of that cross-talk on cell signaling and cell biology.
Keywords: transforming growth factor beta, sphingosine kinase, G protein-coupled receptor, growth factor receptor
The biological effects of sphingosine-1-phosphate (S1P) overlap with those of a wide array of cytokines and growth factors. There is increasing evidence that this overlap in functions relates to both the ability of growth factors and cytokines to transactivate S1P signaling cascades as well as the ability of S1P to transactivate growth factor and cytokine signaling cascades. This mutual pathway cross-talk affects cell growth, differentiation, and movement and is integral to a variety of normal processes as well as disease processes, including inflammation and cancer. This review will focus on the ways in which S1P and growth factor or cytokine signaling pathways interact.
S1P
S1P is a potent bioactive lipid that regulates processes that are essential to cellular homeostasis, including cell viability, differentiation, and motility (1–3). Most of its best-characterized actions are mediated through a family of five G protein-coupled receptors, (GPCRs) S1P1–5 (4, 5). The S1P receptors couple to a variety of G proteins, allowing them to mediate many different biologic responses (6, 7).
One mechanism for cross-talk between cytokine or growth factor pathways and S1P signaling involves regulation of S1P metabolism. Sphingolipid metabolism was the focus of a recent excellent review (3) and will not be discussed in detail here. Cellular levels of S1P are regulated by the activities of the enzymes that are responsible for its synthesis and degradation. In mammalian cells, two sphingosine kinases, SphK1 and SphK2, catalyze the phosphorylation of sphingosine to generate S1P (8). Although both isozymes phosphorylate sphingosine, they differ in their enzymatic properties, cellular location, and biological roles (9). SphK1 is generally regarded as providing pro-survival signals, whereas SphK2, at least when overexpressed, induces apoptosis in several cell types (10). S1P is degraded to sphingosine by specific S1P phosphatases (11) or cleaved to palmitaldehyde and phosphoethanolamine by S1P lyase (12). Thus, it is clear that S1P levels can potentially be altered by stimuli that affect activity or expression of any of these enzymes. However, the majority of cytokines and growth factors that coopt regulation of S1P metabolism to augment signaling do so by regulating either expression or activation of SphKs, especially SphK1.
SPHK ACTIVATION
Generally, SphKs are found mostly, but not exclusively in the cytosol. However, initial cell fractionation studies demonstrated that active SphK was both associated with endoplasmic reticulum of smooth muscle cells and membrane bound (13). Numerous cytokines and growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and tumor necrosis factor alpha (TNF-α), induce rapid activation and translocation of SphK1 from the cytosol to the plasma membrane (Fig. 1) (14). In the case of PDGF, which also induces cell motility, SphK1 is specifically translocated to the leading edge of the plasma membrane lamella of migrating cells (15), suggesting that translocation may reflect associated alterations in cell behavior (16). The mechanism of SphK1 translocation is not understood. However, it appears to involve association with other proteins, e.g., calmodulin (17), suggesting that trafficking of associated proteins may drive subcellular localization of SphK1. Interestingly, in macrophages, the localization and activity of SphK1 are coordinately regulated with actin dynamics during macrophage activation (18).
Fig. 1.
Inside-out signaling by sphingosine-1-phosphate (S1P). Activation of various growth factor tyrosine kinase receptors (RTK) by growth factors induces activation and translocation of sphingosine kinase 1 (SphK1) to the plasma membrane, where localized formation of S1P leads to activation of its receptors and their downstream signaling. GF, growth factor; P, phosphate; S1PR, S1P receptor; Sph, sphingosine.
Agonists, such as phorbol ester and TNF-α, that increase SphK1 activity and induce its translocation were also shown to increase SphK1 phosphorylation, specifically on a single serine, Ser-225 (19). Phosphorylation of Ser-225 was sufficient to induce a 14-fold increase in enzymatic activity (19) and enhanced its membrane affinity and plasma membrane selectivity, presumably enhancing its interaction with membrane acidic phospholipids (20). Both TNF-α- and PMA-induced phosphorylation of SphK1 are ERK1/2 dependent (19). Although PMA is a more potent activator of ERK1/2 than TNF-α, the two agonists are equally efficient at inducing SphK1 phosphorylation. Prior to demonstrating that TNF-α activated SphK1 in an ERK1/2-dependent manner, Pitson et al. (21) demonstrated that TNF-α activation of ERK1/2, like VEGF activation of ERK1/2 (22), is SphK1 dependent. These observations suggested that activation of SphK1 may serve to amplify or prolong agonist-mediated ERK1/2 activation. SphK2, like SphK1, is activated by ERK-induced phosphorylation (23). So far this has only been demonstrated for EGF- and PMA-induced activation of SphK2, but it is possible that other growth factors might also utilize the ERK pathway to activate SphK2. Although phosphorylation of a single residue is sufficient for ERK-mediated activation of SphK1, phosphorylation of two residues, Ser-351 and Thr-578, is required for activation of SphK2 (23). Recently, it was shown that phosphorylation of SphK2 by protein kinase D, which is also stimulated by PMA, leads to its nuclear export for subsequent cellular signaling (24). Intriguingly, although SphK2 has a nuclear localization signal that is required for its inhibition of DNA synthesis, and in many cell types is mainly localized to the nucleus (25), in mast cells, it is cytosolic and translocated to the plasma membrane after FcepsilonRI cross-linking (26).
SPHK ACTIVATION AT THE CROSSROADS OF CROSS-TALK
SphK1 and TNF-α
The fact that several cytokines and growth factors activate SphKs raises two questions, namely, what contribution do SphKs make to the biochemical and biologic consequences of the agonists that regulate their expression, and how do they mediate those effects? One cytokine for which activation of SphK1 plays a significant role is TNF-α, a major effector of innate immunity and inflammation. Activation of SphK1 plays at least two roles in the biochemical effects of TNF-α. One of those is augmentation of ERK1/2 activation (19). The second is the result of SphK1 binding to TRAF2, which is a mediator of TNF-α signaling and is required for TNF-α activation of nuclear factor kappa B (NF-κB) (27). NF-κB in turn provides a pro-survival or anti-apoptotic signal.
TNF-α is one of an array of cytokines produced by macrophages upon encounters with pathogens, and it has both local and systemic effects. The role of TNF-α in inflammation is due in part to its ability to induce the expression of other mediators of inflammation, including the chemokine interleukin-8 (IL-8) and prostaglandin E2 (PGE2). IL-8 is a chemoattractant for neutrophils, which are also a target of TNF-α (28). Recent studies have demonstrated that downregulating the expression of SphK1 blocked TNF-α induction of IL-8 in lung epithelial cells, supporting a role for their cross-talk in initiation of lung inflammation (29). In this regard, inhibition of SphK1 interferes with the ability of TNF-α and other priming agents to induce the hallmarks of neutrophil priming, changes in cell polarization and the augmentation of the fMetLeuPhe-induced superoxide anion generation (28). Together, these studies suggest that cross-talk between TNF-α and SphK1/S1P signaling plays a role in both initiation and maintenance of inflammatory lung diseases. These studies are complemented by several other studies demonstrating that SphK1 activation by cross-linking of FcepsilonRI with antigen and production of S1P is critical for the pathogenesis of asthma (6, 30–33). Inhibition of SphK1 also blocks TNF-α induction of COX-2 and PGE2 secretion by fibroblasts and macrophages (34, 35). There is yet another aspect to cross-talk between these pathways in inflammation: SphK1 is required for mycobacterium-induced production of TNF-α by macrophages (36).
Growth factors and SphK1 activation
Insulin-like growth factors (IGFs) stimulate biologic processes associated with glucose metabolism as well as cell growth. These effects are mediated by the interaction of IGFs with heterodimeric tyrosine kinase receptors that are able to activate Akt as well as the Ras-Raf- mitogen-activated protein kinase (MAPK) cascade. Recent studies have demonstrated that IGF-1 and IGF-2 cause a rapid activation of SphK1, as demonstrated by its translocation to the membrane as well as the expected increase in S1P levels (37). IGF-induced ERK1/2 stimulation was also dependent on both SphK1 and S1P receptor activation, indicating that cross-talk between IGFs and SphK/S1P signaling pathways involves the “inside-out” model previously described for PDGF (16). In this cross-talk model (Fig. 1), the agonist activates SphK1 to produce S1P that then leaves the cell and acts in either a paracrine or an autocrine manner to activate S1P receptors. As is the case with TNF-α signaling, in IGF signaling, SphK1 activation is likely to be both upstream and downstream of ERK1/2 activation because the receptor tyrosine kinase itself can activate ERK1/2, which appears to be required for the activation of SphKs. The subsequent S1P receptor-mediated activation of ERK1/2 probably plays a role in either amplifying or prolonging activation of this pathway. It should be noted that the activation of specific signaling cascades in this “inside-out” manner depends on the pattern of S1P receptor expression and potentially contributes to the cell type-specific effects of cytokines and growth factors that are involved. For example, whereas S1P1 receptors are involved in PDGF induction of cell motility (15, 16), the presence of S1P2 receptors inhibits PDGF-induced cell motility (38).
In an alternative model for pathway cross-talk mediated by the rapid activation of SphK1, the S1P that is generated as a consequence of SphK1 activation acts as an intracellular second messenger. This appears to be the case for VEGF, because VEGF-induced activation of ERK1/2 is SphK1 dependent, but not GPCR dependent (22). These two models of cross-talk are not mutually exclusive. For example, activation of SphK1 is involved in a wide array of effects of PDGF, including cell growth, cell survival, stress fiber formation and chemotaxis, activation of NADPH oxidase, and increased expression of delayed rectifier current (15, 39–41). However, whereas cell movement toward PDGF depends on S1P receptor activation, cell survival does not (39).
A tripartite mechanism of signaling involving estradiol, SphK1, and EGF receptor has recently been described (42). Like other growth factors, estrogen is capable of inducing a rapid and transient activation of SphK1, which in turn activates the ERK1/2 pathway and promotes growth of breast cancer cells (43). The S1P that is generated by estradiol activation of SphK1 acts through S1P3 to activate the EFG receptor in a metalloprotease-dependent fashion (42). Thus, these findings reveal a key role for SphK1 in the coupling of the signals between three membrane-spanning events induced by estradiol, S1P, and EGF, called “criss-cross” transactivation (42).
IL-12 and SphK2
Another mechanism of cytokine-S1P cross-talk that plays a role in innate immunity and inflammation is the unique interaction between the IL-12 and S1P signaling pathways. IL-12 is a pro-inflammatory cytokine that acts as an interface between the innate and adaptive immune systems (44). One way in which it shifts the balance of the immune response is by inducing production of IFN-γ and promoting the development of the subset of helper T cells, TH1, that promote innate immune responses. IL-12 is the ligand for a heterodimeric receptor formed by IL-12β1 and IL-12β2 that has a unique interaction with SphK2 (45). Moreover, overexpression of SphK2 augmented IL-12 induction of STAT4-dependent transcription, whereas introduction of a dominant-negative SphK2 blocked IL-12-induced production of IFN-γ (45). It is not yet clear how this interaction affects IL-12 signaling or whether it leads to activation of SphK2.
Transcriptional regulation of SphK1
In addition to rapidly activating SphKs, cytokines and growth factors can regulate their expression. In fibroblasts, transforming growth factor beta (TGFβ) increased the enzymatic activity of SphK1, but not SphK2, indirectly by increasing expression of SphK1 protein over a prolonged period of time (46). In parallel, TGFβ decreased expression of S1P phosphatase, suggesting that it increases the level of S1P over time. The prolonged activation of SphK1 plays a role in TGFβ induction of TIMP-1 mRNA. SphK1 expression in myofibroblasts was also increased by TGFβ (47). Interestingly, TGFβ also induced a rapid increase in intracellular S1P in these cells (47), suggesting that TGFβ regulation of SphK1 may also occur both transcriptionally and posttranslationally.
Similarly, nerve growth factor (NGF) regulates SphK1 both posttranslationally and transcriptionally (48, 49). NGF rapidly induces activation of SphK1, leading to generation of S1P, which acts extracellularly via S1P1 to promote neurite extension (48). Understanding regulation of SphK1 expression by NGF is complicated by the existence of multiple transcriptional start sites and differential use of exons in the 5′ untranslated region (50). However, using PC12 cells as a model, the promoter element that confers NGF regulation to SphK1 has been identified, and it was demonstrated that the transcription factor Sp1 is important for regulation of SphK1 expression by NGF (49).
RECEPTOR-RECEPTOR INTERACTIONS
There is also some evidence supporting a model in which cross-talk is mediated by stable S1P receptor-tyrosine kinase receptor complexes (51–53). Receptor complexes consisting of PDGFβ and a constitutively active S1P1 have been shown to form independently of stimulation with either PDGF or S1P in several cell types, including HEK 293, airway smooth muscle cells, and fibroblasts (51–53). When these cells are stimulated with PDGF, the complex of receptors is endocytosed in a c-Src/β-arrestin-dependent fashion. The endocytosed complex associates with and activates ERK1/2. Inhibition of S1P1 blocks both PDGF-induced activation of ERK1/2 and cell migration (53). In contrast to the models invoking activation of SphK1 and generation of S1P, which potentially provide a general mechanism for linking receptor tyrosine kinases to GPCR activation, to date this platform model has only been shown to be relevant to cross-talk between the PDGF and S1P signaling pathways.
S1P TRANSACTIVATION OF TYROSINE KINASE RECEPTORS
S1P interaction with growth factor signaling pathways is bidirectional. As with other GPCR ligands, S1P can also transactivate growth factor receptors. The first example of this type of cross-talk to be described is the interaction between VEGF and S1P. Two studies have demonstrated that at least some of the consequences of S1P stimulation of endothelial cells are due to its ability to activate the VEGF receptor, VEGF2, or Flk-1/KDR (54, 55). S1P activation of VEGF2 was shown to be both Gi protein and Src family kinase dependent and did not require synthesis of VEGF. These findings lead to a model in which S1P ligation of S1P2 causes Gi protein-dependent activation of phospholipase C and increased intracellular Ca+, which in turn leads to both phosphorylation of endothelial nitric oxide synthase and concomitant activation of VEGF2 and Src family kinases. In addition, transactivation of VEGF2 and Src kinase activation result in increased membrane ruffling via activation of CrkII (55). VEGF can activate SphK1, which is important for its ability to induce both DNA synthesis and ERK1/2 activation in endothelial cells (22). Furthermore, VEGF can increase expression of S1P receptors (56), enhancing the potential for S1P to activate VEGF receptors. Therefore, S1P-VEGF signaling is bi-directional.
CROSS-TALK BETWEEN TGFβ AND S1P SIGNALING
More-recent studies have demonstrated that S1P can transactivate the TGFβ pathway. TGFβ is produced by many cell types and induces a wide range of biologic effects that depend on both cell type and the state of differentiation of the cell (as reviewed in Ref. 57). It initiates signal transduction by stimulating the formation of a multimeric complex consisting of two pairs of heterodimers (57). Each heterodimer contains two serine-threonine kinases, a type 1 TGFβ receptor (TβRI) and a type II (TβRII) receptor. The canonical Smad pathway is activated by ligand binding to TβRII, which phosphorylates TβRI. In the case of TGFβ, an ALK5 TβRI phosphorylates one of the two receptor-activated Smads, Smad2 or Smad3. Other members of the TGFβ superfamily use different combinations of receptors and phosphorylate different Smads. The receptor-activated Smad binds to Smad4 and enters the nucleus. The subsequent gene response is controlled by the interaction of Smads with both transcriptional activators and repressors. In addition to the canonical Smad pathway, TGFβ also activates non-Smad signaling cascades, including MAPK and PI-3K, but the mechanism for activation of those pathways is not well established (58).
TGFβ was originally identified by its ability to induce anchorage-independent growth of fibroblasts (59). It was subsequently shown to induce a reversible G1 arrest in several cell types, including epithelial cells and lymphocytes (57). The observation that S1P can also have dichotomous, cell type-dependent effects on cell growth prompted Kleuser and colleagues (60) to explore the interaction between TGFβ and S1P signaling. They observed that S1P and TGFβ induced phosphorylation of Smad2 and Smad3 in cultures of primary human keratinocytes with similar kinetics. Using a combination of molecular and pharmacological approaches, they demonstrated that S1P induction of keratinocyte migration as well as Smad3 phosphorylation required both S1P and TGFβ receptors (60). Furthermore, S1P activation of Smad3 was shown to be GPCR dependent. The notion that Smad3 activation was required for S1P induction of keratinocytes was further confirmed by the observation that neither S1P nor TGFβ could induce chemotaxis in keratinocytes from Smad3−/− mice. These findings suggested a model for S1P-TGFβ cross-talk that depends on activation of both S1P receptors and TGFβ receptors. Additional support for this model was derived from studies in myofibroblasts (61). FTY720 is an immunomodulator that is a substrate for SphK2. In its activated, or phosphorylated, form, it is a ligand for S1P receptors. FTY720-P was shown to cause myofibroblast differentiation in an S1P3, G protein-coupled receptor-dependent fashion (61). The induction of myofibroblast differentiation also required Smad3 activation, but did not require secretion of TGFβ.
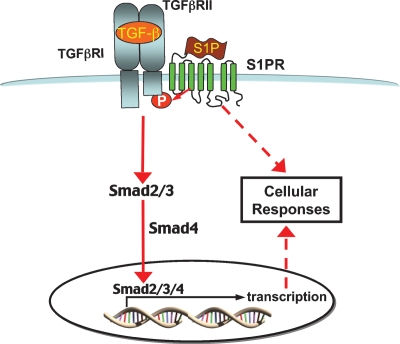
Cross-talk between S1P and TGFβ signaling pathways has also been observed in renal mesangial cells (62, 63). In these cells, S1P activates ERK1/2 and phosphorylates Smad1 and Smad2, albeit with different kinetics. Unlike S1P activation of ERK1/2, S1P activation of Smads was shown to be Gi protein-coupled receptor independent. Additional experiments demonstrated that TβRII was required for S1P activation of Smads and that S1P appears to induce TGFβ receptor oligomerization. The latter results led to a model in which S1P induces the formation of an S1P receptor-TβRII receptor complex that forms a heteromer with TβRI, resulting in phosphorylation of Smads 1, 2, and 3 (62) (Fig. 2). This model was also reinforced through studies with the immunomodulator FTY720 (63). Like S1P, both FTY720 and FTY720-P are able to activate MAPK and stress kinase pathways in renal mesangial cells, in addition to inducing phosphorylation of Smads 1, 2, and 3 (63). Furthermore, FTY720 activation of Smads is TβRII dependent, but Gi protein-coupled receptor independent. The discrepancy between the models of S1P-TGFβ cross-talk in renal mesangial cells and keratinocytes or myofibroblasts could potentially be explained by differences in TGFβ signaling in these cells. Generally, TGFβ signaling involves TβRII recruitment of an ALK5-type TβRI receptor and phosphorylation of Smad2 and Smad3. However, in some cell types, e.g., endothelial cells, TβRII can also recruit an ALK1-type TβRI receptor to phosphorylate Smad1 and Smad5 (64).
Fig. 2.
S1P induces the formation of an S1P receptor-TβRII receptor complex that forms a heteromer with TβRI resulting in phosphorylation of Smads 1, 2, and 3. P, phosphate; TGFβR, TGFβ receptor.
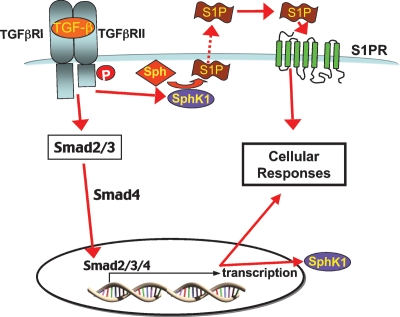
Regardless of the precise mechanism for S1P activation of Smad signaling, it is becoming increasingly clear that cross-talk between these two complex pathways has a significant role in a variety of biologic processes. TGFβ is important for progression of pulmonary fibrosis. TGFβ stimulates SphK1, leading to an increase in S1P that in turn binds to its receptors, leading to upregulation of α-smooth muscle actin expression, which is involved in the lung fibrogenic process (47) (Fig. 3). In renal mesangial cells, Smad3 is required for induction of connective tissue growth factor and collagen expression (62). Combined with the observations that TGFβ regulates SphK1 expression to increase TIMP-1 expression in fibroblasts (46) and regulates differentiation of myofibroblasts (61), those studies suggest a role for bi-directional TGFβ-S1P cross-talk in lung pathology and tissue remodeling. Smad3 is also involved in cross-talk between S1P and IL1β, acting to block IL1β-induced expression of iNOS, cPLA2, and MMP-9 (62), suggesting that S1P-TGFβ pathway cross-talk plays a role in inflammation and innate immunity. This notion is further supported by the observation that S1P induces dendritic cell migration by activation of Smad3 (65).
Fig. 3.
TβRII activates TβRI, resulting in the phosphorylation of Smad2 and Smad3. The phosphorylated Smad alone or together with Smad4 enters the nucleus. This leads to regulation of gene expression, including upregulation of SphK1. In addition, TβRs also stimulate SphK1, leading to formation of S1P that in turn acts in an autocrine manner to activate S1P receptors. P, phosphate.
CONCLUSIONS
Co-opting of the S1P signaling pathway appears to be a common theme for growth factors and cytokines. This occurs primarily through activation of SphKs and generation of S1P, which acts both intracellularly and extracellularly. S1P mediates a wide array of cell type-dependent biologic effects as a consequence of its activation of a family of specific cell surface receptors, each of which activates distinct signaling cascades and which are expressed in cell-specific patterns. Consequently, cross-talk between cytokines, growth factors, and S1P pathways is likely to explain many cell type-specific responses. S1P also coopts other signaling pathways to mediate its biologic effects. Of particular interest in this regard is its interaction with the TGFβ pathway, which is equally notorious for its ability to mediate a wide array of effects in a highly cell type-dependent fashion. Future efforts to dissect the molecular interactions between these pathways have the potential to significantly impact our understanding of the pathogenesis of several diseases processes, including inflammation and cancer.
Abbreviations
EGF, epidermal growth factor
GPCR, G protein-coupled receptor
IGF, insulin-like growth factor
IL, interleukin
MAPK, mitogen-activated protein kinase
NF-κB, nuclear factor kappa B
NGF, nerve growth factor
PDGF, platelet-derived growth factor
PGE2, prostaglandin E2
S1P, sphingosine-1-phosphate
SphK, sphingosine kinase
TGFβ, transforming growth factor beta
TβR, TGFβ receptor
TNF-α, tumor necrosis factor alpha
VEGF, vascular endothelial growth factor
Published, JLR Papers in Press, April 2, 2008.
Footnotes
This work was supported by National Institutes of Health Grants R01AI-50094 (S.S.), R01CA-61774 (S.S.) and R37GM-043880 (S.S.) and in part by Philip Morris, USA, Inc. and Philip Morris International (D.A.L.).
This paper is dedicated to Professor Herbert Carter, whose pioneering work laid the foundation for the field of sphingolipid chemistry.
References
- 1.Alvarez S. E., S. Milstien, and S. Spiegel. 2007. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol. Metab. 18 300–307. [DOI] [PubMed] [Google Scholar]
- 2.Schwab S. R., and J. G. Cyster. 2007. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8 1295–1301. [DOI] [PubMed] [Google Scholar]
- 3.Hannun Y. A., and L. M. Obeid. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9 139–150. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel S., and S. Milstien. 2003. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat. Rev. Mol. Cell Biol. 4 397–407. [DOI] [PubMed] [Google Scholar]
- 5.Saba J. D., and T. Hla. 2004. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ. Res. 94 724–734. [DOI] [PubMed] [Google Scholar]
- 6.Rosen H., and E. J. Goetzl. 2005. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat. Rev. Immunol. 5 560–570. [DOI] [PubMed] [Google Scholar]
- 7.Chun J., and H. Rosen. 2006. Lysophospholipid receptors as potential drug targets in tissue transplantation and autoimmune diseases. Curr. Pharm. Des. 12 161–171. [DOI] [PubMed] [Google Scholar]
- 8.Spiegel S., and S. Milstien. 2007. Functions of the multifaceted family of sphingosine kinases and some close relatives. J. Biol. Chem. 282 2125–2129. [DOI] [PubMed] [Google Scholar]
- 9.Hait N. C., C. A. Oskeritzian, S. W. Paugh, S. Milstien, and S. Spiegel. 2006. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim. Biophys. Acta. 1758 2016–2026. [DOI] [PubMed] [Google Scholar]
- 10.Liu H., R. E. Toman, S. Goparaju, M. Maceyka, V. E. Nava, H. Sankala, S. G. Payne, M. Bektas, I. Ishii, J. Chun, et al. 2003. Sphingosine kinase type 2 is a putative BH3-Only protein that induces apoptosis. J. Biol. Chem. 278 40330–40336. [DOI] [PubMed] [Google Scholar]
- 11.Le Stunff H., C. Peterson, H. Liu, S. Milstien, and S. Spiegel. 2002. Sphingosine-1-phosphate and lipid phosphohydrolases. Biochim. Biophys. Acta. 1582 8–17. [DOI] [PubMed] [Google Scholar]
- 12.Saba J. D. 2004. Lysophospholipids in development: miles apart and edging in. J. Cell. Biochem. 92 967–992. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh T. K., J. Bian, and D. L. Gill. 1994. Sphingosine 1-phosphate generated in the endoplasmic reticulum membrane activates release of stored calcium. J. Biol. Chem. 269 22628–22635. [PubMed] [Google Scholar]
- 14.Wattenberg B. W., S. M. Pitson, and D. M. Raben. 2006. The sphingosine and diacylglycerol kinase superfamily of signaling kinases: localization as a key to signaling function. J. Lipid Res. 47 1128–1139. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeldt H. M., J. P. Hobson, M. Maceyka, A. Olivera, V. E. Nava, S. Milstien, and S. Spiegel. 2001. EDG-1 links the PDGF receptor to Src and focal adhesion kinase activation leading to lamellipodia formation and cell migration. FASEB J. 15 2649–2659. [DOI] [PubMed] [Google Scholar]
- 16.Hobson J. P., H. M. Rosenfeldt, L. S. Barak, A. Olivera, S. Poulton, M. G. Caron, S. Milstien, and S. Spiegel. 2001. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 291 1800–1803. [DOI] [PubMed] [Google Scholar]
- 17.Young K. W., J. M. Willets, M. J. Parkinson, P. Bartlett, S. Spiegel, S. R. Nahorski, and R. A. Challiss. 2003. Ca2+/calmodulin-dependent translocation of sphingosine kinase: role in plasma membrane relocation but not activation. Cell Calcium. 33 119–128. [DOI] [PubMed] [Google Scholar]
- 18.Kusner D. J., C. R. Thompson, N. A. Melrose, S. M. Pitson, L. M. Obeid, and S. S. Iyer. 2007. The localization and activity of sphingosine kinase 1 are coordinately-regulated with actin cytoskeletal dynamics in macrophages. J. Biol. Chem. 282 23147–23162. [DOI] [PubMed] [Google Scholar]
- 19.Pitson S. M., P. A. Moretti, J. R. Zebol, H. E. Lynn, P. Xia, M. A. Vadas, and B. W. Wattenberg. 2003. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 22 5491–5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahelin R. V., J. H. Hwang, J. H. Kim, Z. Y. Park, K. R. Johnson, L. M. Obeid, and W. Cho. 2005. The mechanism of membrane targeting of human sphingosine kinase 1. J. Biol. Chem. 280 43030–43038. [DOI] [PubMed] [Google Scholar]
- 21.Pitson S. M., P. A. Moretti, J. R. Zebol, P. Xia, J. R. Gamble, M. A. Vadas, R. J. D'Andrea, and B. W. Wattenberg. 2000. Expression of a catalytically inactive sphingosine kinase mutant blocks agonist-induced sphingosine kinase activation: a dominant negative sphingosine kinase. J. Biol. Chem. 275 33945–33950. [DOI] [PubMed] [Google Scholar]
- 22.Shu X., W. Wu, R. D. Mosteller, and D. Broek. 2002. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol. Cell. Biol. 22 7758–7768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hait N. C., A. Bellamy, S. Milstien, T. Kordula, and S. Spiegel. 2007. Sphingosine kinase type 2 activation by ERK-mediated phosphorylation. J. Biol. Chem. 282 12058–12065. [DOI] [PubMed] [Google Scholar]
- 24.Ding G., H. Sonoda, H. Yu, T. Kajimoto, S. K. Goparaju, S. Jahangeer, T. Okada, and S. Nakamura. 2007. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J. Biol. Chem. 282 27493–27502. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi N., T. Okada, S. Hayashi, T. Fujita, S. Jahangeer, and S. I. Nakamura. 2003. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem. 278 46832–46839. [DOI] [PubMed] [Google Scholar]
- 26.Olivera A., N. Urtz, K. Mizugishi, Y. Yamashita, A. M. Gilfillan, Y. Furumoto, H. Gu, R. L. Proia, T. Baumruker, and J. Rivera. 2006. IgE-dependent activation of sphingosine kinases 1 and 2 and secretion of sphingosine 1-phosphate requires Fyn kinase and contributes to mast cell responses. J. Biol. Chem. 281 2515–2525. [DOI] [PubMed] [Google Scholar]
- 27.Xia P., L. Wang, P. A. Moretti, N. Albanese, F. Chai, S. M. Pitson, R. J. D'Andrea, J. R. Gamble, and M. A. Vadas. 2002. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J. Biol. Chem. 277 7996–8003. [DOI] [PubMed] [Google Scholar]
- 28.MacKinnon A. C., A. Buckley, E. R. Chilvers, A. G. Rossi, C. Haslett, and T. Sethi. 2002. Sphingosine kinase: a point of convergence in the action of diverse neutrophil priming agents. J. Immunol. 169 6394–6400. [DOI] [PubMed] [Google Scholar]
- 29.Chandru H., and V. Boggaram. 2007. The role of sphingosine 1-phosphate in the TNF-alpha induction of IL-8 gene expression in lung epithelial cells. Gene. 391 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolly P. S., M. Bektas, A. Olivera, C. Gonzalez-Espinosa, R. L. Proia, J. Rivera, S. Milstien, and S. Spiegel. 2004. Transactivation of sphingosine-1-phosphate receptors by Fc{epsilon}RI triggering is required for normal mast cell degranulation and chemotaxis. J. Exp. Med. 199 959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitra P., C. A. Oskeritzian, S. G. Payne, M. A. Beaven, S. Milstien, and S. Spiegel. 2006. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc. Natl. Acad. Sci. USA. 103 16394–16399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oskeritzian C. A., S. Milstien, and S. Spiegel. 2007. Sphingosine-1-phosphate in allergic responses, asthma and anaphylaxis. Pharmacol. Ther. 115 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olivera A., K. Mizugishi, A. Tikhonova, L. Ciaccia, S. Odom, R. L. Proia, and J. Rivera. 2007. The sphingosine kinase-sphingosine-1-phosphate axis is a determinant of mast cell function and anaphylaxis. Immunity. 26 287–297. [DOI] [PubMed] [Google Scholar]
- 34.Pettus B. J., J. Bielawski, A. M. Porcelli, D. L. Reames, K. R. Johnson, J. Morrow, C. E. Chalfant, L. M. Obeid, and Y. A. Hannun. 2003. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 17 1411–1421. [DOI] [PubMed] [Google Scholar]
- 35.Hammad S. M., H. G. Crellin, B. X. Wu, J. Melton, V. Anelli, and L. M. Obeid. 2008. Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat. 85 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav M., L. Clark, and J. S. Schorey. 2006. Macrophage's proinflammatory response to a mycobacterial infection is dependent on sphingosine kinase-mediated activation of phosphatidylinositol phospholipase C, protein kinase C, ERK1/2, and phosphatidylinositol 3-kinase. J. Immunol. 176 5494–5503. [DOI] [PubMed] [Google Scholar]
- 37.El-Shewy H. M., K. R. Johnson, M. H. Lee, A. A. Jaffa, L. M. Obeid, and L. M. Luttrell. 2006. Insulin-like growth factors mediate heterotrimeric G protein-dependent ERK1/2 activation by transactivating sphingosine-1-phosphate receptors. J. Biol. Chem. 281 31399–31407. [DOI] [PubMed] [Google Scholar]
- 38.Goparaju S. K., P. S. Jolly, K. R. Watterson, M. Bektas, S. Alvarez, S. Sarkar, L. Mel, I. Ishii, J. Chun, S. Milstien, et al. 2005. The S1P2 receptor negatively regulates platelet-derived growth factor-induced motility and proliferation. Mol. Cell. Biol. 25 4237–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olivera A., H. M. Rosenfeldt, M. Bektas, F. Wang, I. Ishii, J. Chun, S. Milstien, and S. Spiegel. 2003. Sphingosine kinase type 1 induces G12/13-mediated stress fiber formation yet promotes growth and survival independent of G protein-coupled receptors. J. Biol. Chem. 278 46452–46460. [DOI] [PubMed] [Google Scholar]
- 40.Catarzi S., E. Giannoni, F. Favilli, E. Meacci, T. Iantomasi, and M. T. Vincenzini. 2007. Sphingosine 1-phosphate stimulation of NADPH oxidase activity: relationship with platelet-derived growth factor receptor and c-Src kinase. Biochim. Biophys. Acta. 1770 872–883. [DOI] [PubMed] [Google Scholar]
- 41.Soliven B., L. Ma, H. Bae, B. Attali, A. Sobko, and T. Iwase. 2003. PDGF upregulates delayed rectifier via Src family kinases and sphingosine kinase in oligodendroglial progenitors. Am. J. Physiol. Cell Physiol. 284 C85–C93. [DOI] [PubMed] [Google Scholar]
- 42.Sukocheva O., C. Wadham, A. Holmes, N. Albanese, E. Verrier, F. Feng, A. Bernal, C. K. Derian, A. Ullrich, M. A. Vadas, et al. 2006. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J. Cell Biol. 173 301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sukocheva O. A., L. Wang, N. Albanese, S. M. Pitson, M. A. Vadas, and P. Xia. 2003. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol. Endocrinol. 17 2002–2012. [DOI] [PubMed] [Google Scholar]
- 44.Trinchieri G., S. Pflanz, and R. A. Kastelein. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 19 641–644. [DOI] [PubMed] [Google Scholar]
- 45.Yoshimoto T., M. Furuhata, S. Kamiya, M. Hisada, H. Miyaji, Y. Magami, K. Yamamoto, H. Fujiwara, and J. Mizuguchi. 2003. Positive modulation of IL-12 signaling by sphingosine kinase 2 associating with the IL-12 receptor beta1 cytoplasmic region. J. Immunol. 171 1352–1359. [DOI] [PubMed] [Google Scholar]
- 46.Yamanaka M., D. Shegogue, H. Pei, S. Bu, A. Bielawska, J. Bielawski, B. Pettus, Y. A. Hannun, L. Obeid, and M. Trojanowska. 2004. Sphingosine kinase (SPHK1) is induced by TGF-beta and mediates TIMP-1 upregulation. J. Biol. Chem. 279 53994–54001. [DOI] [PubMed] [Google Scholar]
- 47.Kono Y., T. Nishiuma, Y. Nishimura, Y. Kotani, T. Okada, S. Nakamura, and M. Yokoyama. 2007. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am. J. Respir. Cell Mol. Biol. 37 395–404. [DOI] [PubMed] [Google Scholar]
- 48.Toman R. E., S. G. Payne, K. Watterson, M. Maceyka, N. H. Lee, S. Milstien, J. W. Bigbee, and S. Spiegel. 2004. Differential transactivation of sphingosine-1-phosphate receptors modulates nerve growth factor-induced neurite extension. J. Cell Biol. 166 381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sobue S., K. Hagiwara, Y. Banno, K. Tamiya-Koizumi, M. Suzuki, A. Takagi, T. Kojima, H. Asano, Y. Nozawa, and T. Murate. 2005. Transcription factor specificity protein 1 (Sp1) is the main regulator of nerve growth factor-induced sphingosine kinase 1 gene expression of the rat pheochromocytoma cell line, PC12. J. Neurochem. 95 940–949. [DOI] [PubMed] [Google Scholar]
- 50.Imamura T., J. Ohgane, S. Ito, T. Ogawa, N. Hattori, S. Tanaka, and K. Shiota. 2001. CpG island of rat sphingosine kinase-1 gene: tissue-dependent DNA methylation status and multiple alternative first exons. Genomics. 76 117–125. [DOI] [PubMed] [Google Scholar]
- 51.Alderton F., S. Rakhit, K. K. Choi, T. Palmer, B. Sambi, S. Pyne, and N. J. Pyne. 2001. Tethering of the platelet-derived growth factor beta receptor to G-protein coupled receptors. A novel platform for integrative signaling by these receptor classes in mammalian cells. J. Biol. Chem. 276 28578–28585. [DOI] [PubMed] [Google Scholar]
- 52.Waters C., B. S. Sambi, K. C. Kong, D. Thompson, S. M. Pitson, S. Pyne, and N. J. Pyne. 2003. Sphingosine 1-phosphate and platelet-derived growth factor (PDGF) act via PDGF beta receptor-sphingosine 1-phosphate receptor complexes in airway smooth muscle cells. J. Biol. Chem. 278 6282–6290. [DOI] [PubMed] [Google Scholar]
- 53.Waters C. M., J. Long, I. Gorshkova, Y. Fujiwara, M. Connell, K. E. Belmonte, G. Tigyi, V. Natarajan, S. Pyne, and N. J. Pyne. 2006. Cell migration activated by platelet-derived growth factor receptor is blocked by an inverse agonist of the sphingosine 1-phosphate receptor-1. FASEB J. 20 509–511. [DOI] [PubMed] [Google Scholar]
- 54.Tanimoto T., Z. G. Jin, and B. C. Berk. 2002. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS). J. Biol. Chem. 277 42997–43001. [DOI] [PubMed] [Google Scholar]
- 55.Endo A., K. I. Nagashima, H. Kurose, S. Mochizuki, M. Matsuda, and N. Mochizuki. 2002. Sphingosine 1-phosphate induces membrane ruffling and increases motility of human umbilical vein endothelial cells via vascular endothelial growth factor receptor and CrkII. J. Biol. Chem. 277 23747–23754. [DOI] [PubMed] [Google Scholar]
- 56.Igarashi J., P. A. Erwin, A. P. Dantas, H. Chen, and T. Michel. 2003. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. USA. 100 10664–10669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Massague J., and R. R. Gomis. 2006. The logic of TGFbeta signaling. FEBS Lett. 580 2811–2820. [DOI] [PubMed] [Google Scholar]
- 58.Derynck R., and Y. E. Zhang. 2003. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 425 577–584. [DOI] [PubMed] [Google Scholar]
- 59.Roberts A. B., M. A. Anzano, L. C. Lamb, J. M. Smith, and M. B. Sporn. 1981. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc. Natl. Acad. Sci. USA. 78 5339–5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sauer B., R. Vogler, H. von Wenckstern, M. Fujii, M. B. Anzano, A. B. Glick, M. Schafer-Korting, A. B. Roberts, and B. Kleuser. 2004. Involvement of Smad signaling in sphingosine 1-phosphate-mediated biological responses of keratinocytes. J. Biol. Chem. 279 38471–38479. [DOI] [PubMed] [Google Scholar]
- 61.Keller C. D., P. Rivera Gil, M. Tolle, M. van der Giet, J. Chun, H. H. Radeke, M. Schafer-Korting, and B. Kleuser. 2007. Immunomodulator FTY720 induces myofibroblast differentiation via the lysophospholipid receptor S1P3 and Smad3 signaling. Am. J. Pathol. 170 281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xin C., S. Ren, B. Kleuser, S. Shabahang, W. Eberhardt, H. Radeke, M. Schafer-Korting, J. Pfeilschifter, and A. Huwiler. 2004. Sphingosine-1-phosphate cross-activates the Smad signaling cascade and mimics TGFbeta-induced cell responses. J. Biol. Chem. 279 35255–35262. [DOI] [PubMed] [Google Scholar]
- 63.Xin C., S. Ren, W. Eberhardt, J. Pfeilschifter, and A. Huwiler. 2006. The immunomodulator FTY720 and its phosphorylated derivative activate the Smad signalling cascade and upregulate connective tissue growth factor and collagen type IV expression in renal mesangial cells. Br. J. Pharmacol. 147 164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levy L., and C. S. Hill. 2006. Alterations in components of the TGF-beta superfamily signaling pathways in human cancer. Cytokine Growth Factor Rev. 17 41–58. [DOI] [PubMed] [Google Scholar]
- 65.Radeke H. H., H. von Wenckstern, K. Stoidtner, B. Sauer, S. Hammer, and B. Kleuser. 2005. Overlapping signaling pathways of sphingosine 1-phosphate and TGF-{beta} in the murine Langerhans cell line XS52. J. Immunol. 174 2778–2786. [DOI] [PubMed] [Google Scholar]