Abstract
Transient receptor potential (TRP) genes encode subunits that form cation-selective ion channels in a variety of organisms and cell types. TRP channels serve diverse functions ranging from thermal, tactile, taste, and osmolar sensing to fluid flow sensing. TRPC1 and TRPC6 belong to the TRPC subfamily, members of which are thought to contribute to several cellular events such as regulated migration of neuronal dendrites, contractile responses of smooth muscle cells and maintenance of the structural integrity of kidney podocytes. Pathogenic roles have been suggested for TRPC1 in asthma and chronic obstructive pulmonary disease, and TRPC6 dysfunction was recently linked to proteinuric kidney disease. To explore the potential roles for TRPC channels in zebrafish organ function, we cloned zebrafish trpC1 and trpC6 cDNAs, and investigated their expression during zebrafish development. We detected trpC1 expression in the head, in cells surrounding the outflow tract of the heart, and in the ganglion cells as well as the inner nuclear layer of the eye. trpC6 expression was detected in the head, pectoral fins, aortic endothelial cells, and gastrointestinal smooth muscle cells. Our results point to roles of TRPC channels in several tissues during zebrafish development, and suggest that the zebrafish may be a suitable model system to study the pathophysiology of TRPC1 and TRPC6 in specific cell types.
Keywords: Transient receptor potential, ion channel, smooth muscle, in situ hybridization
1. Results and Discussion
Transient receptor potential (TRP) genes are widely expressed in a number of organs and cell types throughout the species (Ramsey et al., 2006). Since the discovery of the first TRP channel in Drosophila (Montell et al., 1985), encoded by the gene trp, 27 structurally related TRP proteins in humans and more than 60 orthologs in other species including flies, worms, and mice have been identified. Together they form the TRP superfamily, which is subdivided into the TRPC, TRPV, TRPM, TRPP, TRPN, and TRPML subfamilies (Montell, 2005).
All TRP proteins have six predicted transmembrane segments, intracellular N- and C-termini, and share highly conserved motifs within and downstream of the putative channel pore domain. TRP channels have been shown to mediate receptor-operated calcium entry and are also candidate channels for store-operated calcium entry. They contribute to signaling pathways involved in sensing and responding to environmental stimuli which include mechanical/physical stimuli (such as temperature, light, and pressure), or chemical stimuli (including phorbol esters, hormone ligands, and metabolites of arachadonic acid) (Clapham, 2003).
In zebrafish, five TRP channel genes have thus far been described. trpM7 was identified as the gene defective in the mutant touchtone/nutria (Elizondo et al., 2005) which exhibits altered skeletal structure, a diminished response to touch, and kidney stones. trpM7 is expressed in pronephric and mesonephric kidney tubules, corpuscles of Stannius, and the liver (Liu et al., 2007). trpA1 and trpN (also known as NOMPC) have been shown to contribute to ear and lateral line hair cell function (Sidi et al., 2003; Corey et al., 2004). trpC2, which is a pseudogene in humans, is expressed in the adult olfactory epithelium superficial layer (Sato et al., 2005). Recently, the osmosensory channel trpV4 has been detected in multiple developing organs in zebrafish (Mangos et al., 2007).
Within the TRP superfamily, the TRPC subfamily shares the highest homology with the original TRP channel discovered in the fly. TRPCs have been implicated in a wide range of diseases, including asthma, chronic obstructive pulmonary disorder, and defective immune responses involving both B cells and T cells (Nilius et al., 2007). Furthermore, it was recently shown that TRPC6 plays a role in genetic and acquired proteinuric kidney diseases (Winn et al., 2005, Reiser et al., 2005, Moller et al., 2007). In mice and humans, TRPC6 protein is expressed in kidney podocytes in close proximity to the filtration slits of the glomerular filter. Recent studies support the notion that TRPC6-mediated calcium signaling contributes to the maintenance of the glomerular slit diaphragm and the regulation of glomerular permselectivity (Huber et al., 2006). TRPC6 knockout mice display defective vasomotor control and a sensitized myogenic response, suggesting an important role in smooth muscle contractility (Dietrich et al. 2005, Weissmann et al., 2006). Based on these observations, which implicate TRPC channels in the function of a number of cell types, in particular smooth muscle cells and kidney podocytes, we analyzed the developmental expression pattern of trpC1 and trpC6 in zebrafish.
1.1 Cloning and bioinformatic analysis of trpC1 and trpC6 in zebrafish
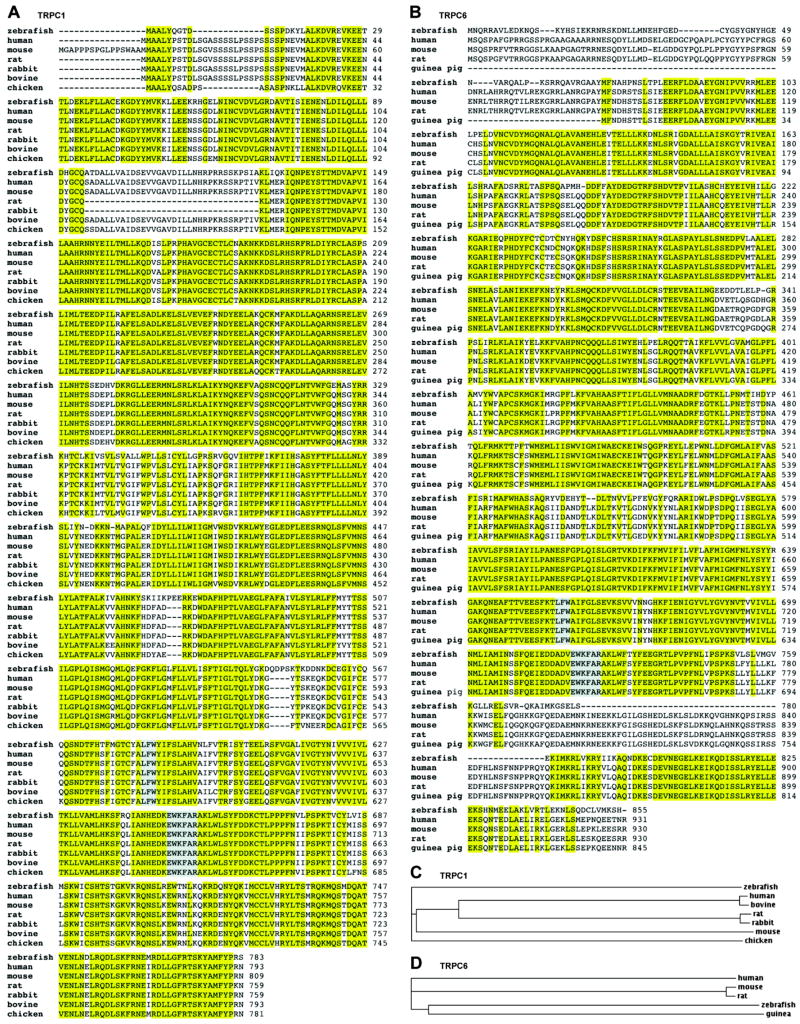
A tBLASTn search of the Ensembl zebrafish cDNA database, using the protein sequences for human TRPC1 and TRPC6 as queries, revealed trpC1 and trpC6 orthologs on chromosomes 24 and 21, respectively. Based on hypothetical sequence information available in the National Center for Biotechnology Information (NCBI) CoreNucleotide database (Accession Number XM_694363), we designed 5′ and 3′ primers to amplify full-length zebrafish trpC1 cDNA by RT-PCR. The zebrafish trpC1 gene is composed of 13 exons and encodes a predicted protein of 783 amino acids. The trpC1 amino acid sequence is highly conserved throughout the species (Fig. 1 A,C) and zebrafish trpC1 shares 81% sequence identity with human TRPC1. For zebrafish trpC6, provisional sequence information is available in the NCBI CoreNucleotide database (Accession Number NM_001030282); the zebrafish trpC6 gene is composed of 11 exons and encodes a 855 amino acid protein sharing sequence similarity with orthologs described in human, mouse, rat, and guinea pig (Fig. 1 B,D). The sequence identity of zebrafish trpC6 to human TRPC6 is 71%.
Fig. 1.
Sequence analysis of zebrafish trpC1 and trpC6. (A,B) Alignments of the zebrafish trpC1 and trpC6 sequences to known homologs in other species using the ClustalW algorithm. Conserved amino acids are highlighted in yellow. The highly conserved channel pore domain (LFW) and TRP box (EWKFAR) are highlighted in gray. (C,D) Phylogenetic trees representative of evolutionary relationships between the zebrafish trpC1 and trpC6 ortholog and cloned full-length TRPC1 and TRPC6 channels of other species. Branch length is proportional to evolutionary distance.
1.2 Expression of trpC1 in embryonic and early laval development
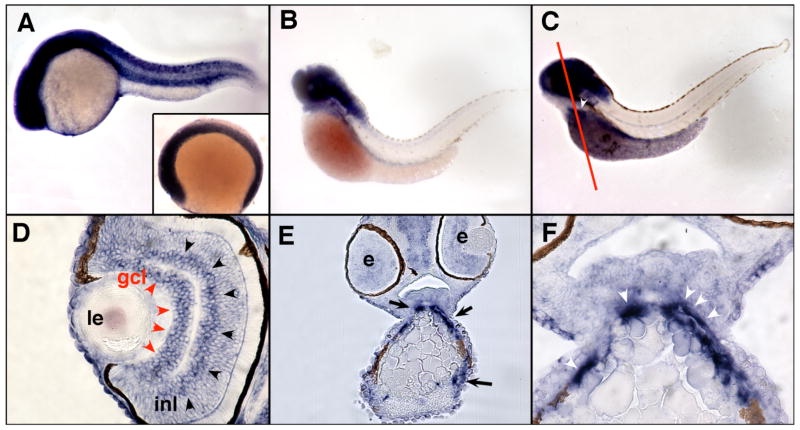
The expression of zebrafish trpC1 was studied by whole mount in situ hybridization and histological analysis of zebrafish embryos. trpC1 expression was ubiquitous up to 24 hours post-fertilization (hpf) (Fig. 2 A). At 56 hpf, expression was restricted to the head with no detectable expression in the trunk (Fig. 2 B), and strong head expression of trpC1 persisted until 72 hpf (Fig. 2 C). At 72 hpf, expression of trpC1 was also detected in cells surrounding the outflow tract of the heart (Fig. 2 C,E,F). trpC1 expression associated with the outflow tract of the heart is consistent with the published work on TRPC1 expression in the mammalian heart (Dietrich et al., 2007). trpC1 expression was also detected in the ganglion cell layer and the inner nuclear layer of the eye in 72 hpf embryos (Fig. 2 D). These structures contain neuronal cells appearing early on the second day post-fertilization (Hu and Easter, 1999). This is consistent with the general role of TRPC channels in sensory physiology (Montell, 1997), and the previously reported immunolocalization of TRPC1 in the chicken retina (Crousillac et al., 2003).
Fig. 2.
Expression of zebrafish trpC1 by whole mount in situ hybridization and histological analysis. Expression of trpC1 mRNA is ubiquitous in 6 somite embyos (A; inset) and stages up to and including 24 hpf (A). At 56 hpf, expression is restricted to the head with no detectable expression in the trunk (B). Strong head expression of trpC1 persists until 72 hpf (C), in addition to expression in the outflow tract of the heart (white arrowhead; white line denotes plane of section in E and F). Histological examination of 72 hpf embryos reveals specific expression of trpC1 in the ganglion cell layer of the eye (gcl, red arroheads) and in the inner nuclear layer (inl, black arrowheads). Anterior sections of 72 hpf embryos (line in C) confirms expression of trpC1 in the outflow track (E, black arrows). A magnified view (F) shows a high level of expression in the cells associated with the outflow tract (white arrowheads). Le=lens, e=eye, gcl=ganglion cell layer, inl=inner nuclear layer.
1.3 Expression of trpC6 in embryonic and early laval development
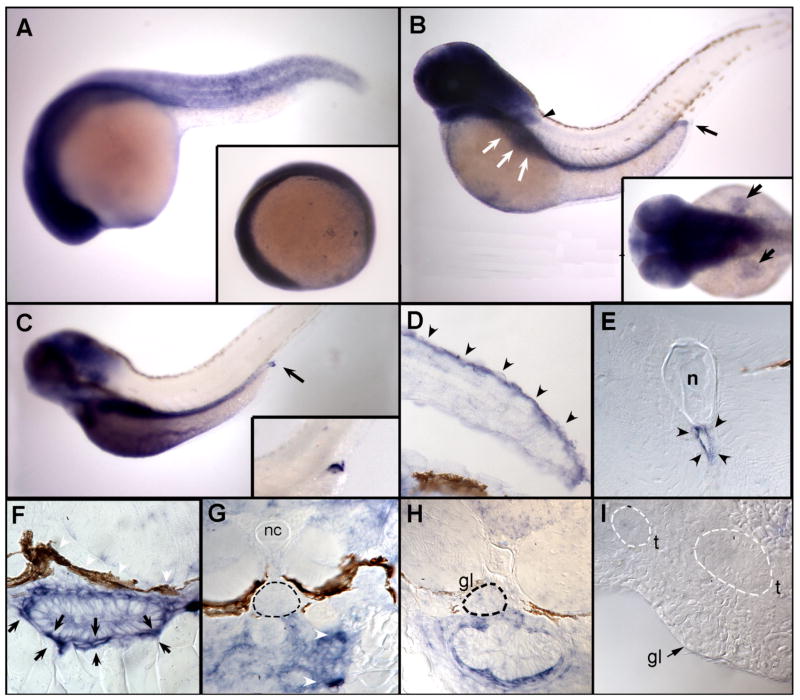
In mammals TRPC6 channels are expressed in cells responding to changes in hydrostatic pressure such as vascular smooth muscle cells. It is abundant in the pulmonary system and in vascular tissues, and contributes to membrane polarization and subsequent vasoconstriction induced by elevated intravascular pressure, which represents the important myogenic constriction response in arteries also known as the Bayliss effect (Welsh et al., 2002). In zebrafish embryos, trpC6 mRNA was ubiquitously expressed up to 24 hpf (Fig. 3 A). At 48 hpf, expression became restricted to the head, pectoral fins, and the posterior extension of the gut (Fig. 3 B). In the gut, trpC6 expression persisted to 72 hpf, where expression in the most posterior region of the gut remained high while more proximal regions of the gut showed diminished expression (Fig. 3 C). Histological examination revealed that trpC6 was highly expressed in cells that surround and encapsulate the gut at 72 hpf (Fig. 3 F). The first detectable smooth muscle cell markers are detected in the vicinity of the gut at approximately 48 hpf (Georgijevic et al., 2007) in cells similar to trpC6-expressing cells. From this we conclude that trpC6 is expressed in gastrointestinal smooth muscle cells that contribute to the stability, contractility and elasticity of the zebrafish gut (Holmberg et al., 2004). trpC6 expression was also detected in cells lining the aorta (Fig. 3 E). Recent reports indicate important roles of trpC6 in the mammalian cardiopulmonary vasculature (reviewed in Dietrich et al., 2007), which would be consistent with trpC6 expression in the zebrafish aorta. In histological sections, trpC6 expression in the pectoral fins appeared to be strongest on the dorsal surface (Fig. 3 D). The pectoral fin is composed of two simple muscles, the abductor and adductor (Thorsen and Hale, 2005), as well as large dorsal and ventral nerve branches (Thorsen and Hale, 2007). Our sections indicate that trpC6 is primarily restricted to the dorsal dermal layer of the fin and excluded from muscle and nerve. Despite the published role for TRPC6 in the pathopyhsiology of glomerular kidney disease in the human and in rodents, trpC6 expression was not detected in the glomeruli of 3 days post-fertilization (dpf) larvae (Fig. 3 G). Even though all glomerular cell types are present at this stage of development and the zebrafish pronephros is required to function as an osmoregulatory kidney, it has been shown that podocyte foot processes are not fully mature yet, and slit-diaphragms between foot processes are rarely observed (Kramer-Zucker et al., 2005). In contrast, at 4 dpf, the filtration apparatus appears mature with podocyte foot processes present as fine, evenly spaced cell processes separated by slit-diaphragm cell–cell junctions (Kramer-Zucker et al., 2005). This is why we studied trpC6 expression in glomeruli also in 5 dpf larvae (Fig. 3 H) and in adult zebrafish kidney (Fig. 3 I). At neither of these later stages were we able to observe a specific labeling pattern of trpC6 in podocytes.
Fig. 3.
Expression of zebrafish trpC6 by whole mount in situ hybridization and histological analysis. Expression of trpC6 mRNA is ubiquitous at 6 somites (A, inset) and in all stages tested up to and including 24 hpf (A). (B) At 48 hpf, expression becomes restricted to the head, pectoral fins (black arrowhead), the area of the gut (white arrows) extending to the posterior end (black arrow). Dorsal view of expression in fins at 48 hpf (B, inset). This pattern of trpC6 expression persists to 72 hpf (C), where expression in the most posterior region of the gut (black arrow and inset) remains high while more proximal regions of the gut show diminished expression. Histological examination reveals that trpC6 expression in the pectoral fins is restricted to the dorsal surface (D, black arrowheads). Sectioning of the trunk of 72 hpf embryos shows that trpC6 mRNA is expressed in cells lining the dorsal aorta (E, black arrowheads). A closer examination of the gut reveals that trpC6 is highly expressed in cells that surround and encapsulate the gut (F, black arrows). (G) Sections through the glomerulus of a 3 dpf larva (G, dashed black circle) demonstrate that trpC6 RNA is not detectably expressed podocytes, whereas cells encapsulating the anterior gut are positive for trpC6 (G, white arrowheads). Later stage, 5 dpf (H) and adult glomeruli (I) do not display specific labeling for trpC6. nc=notochord, gl=glomerulus, t=tubules.
1.4 Conclusion
In the zebrafish, trpC1 and trpC6 are expressed in cell types that respond to physiological mechanical signals including neurons, smooth muscle cells, and endothelial cells. Notably, despite the published role for TRPC6 in the pathopyhsiology of glomerular kidney disease, trpC6 expression was not detected in pronephric podocytes. This could be due to low abundance of trpC6 channels in these cells or expression only under pathophysiological conditions. Equally possible is the notion that trpC6 does not play the same role in glomerular filtration in the human and fish. Albeit zebrafish and higher vertebrate share a high degree of similarity of organ cell types and tissue substructures, obviously fish organ shape and size is different from the human and other mammalian model systems, with fish e.g. lacking collecting and complex nephron systems. Further studies of trpC6 expression in genetic and inducible zebrafish models of glomerular injury will be informative about the role it plays in physiological regulation.
2. Experimental Procedures
2.1 Zebrafish embryos
Wild-type TL or TÜAB zebrafish lines were maintained and raised as previously described (Westerfield, 1995). Embryos were reared at 28.5 °C in E3 solution with 0.003% PTU (1-Phenyl-2-thiourea, Sigma) added to retard pigment formation. Embryonic staging was performed as previously described (Westerfield, 1995). All animal studies were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital.
2.2 Cloning
The protein sequences for human TRPC1 (NCBI accession: NP_003295) and TRPC6 (NP_004621) were used as queries for a tBLASTn search of the Ensembl zebrafish cDNA database (www.ensembl.org/Danio_rerio), and sequences coding for the putative zebrafish orthologs were identified for trpC1 on chromosome 24 and for trpC6 on chromosome 21. We isolated total RNA from 2-day old zebrafish embryos using Trizol reagent (Invitrogen) and performed reverse transcription with olido dT-Primers. Based on hypothetical sequence information for trpC1 available in the NCBI CoreNucleotide database (Accession Number XM_694363), we designed 5′ (5′-ATGGCTGCTCTATATCAGGGC-3′) and 3′ (5′-TTAGCTTCTGGGGTAGAACATG-3′) primers to amplify the actual full-length zebrafish trpC1 ortholog. trpC1 was then subcloned into the pCRII-TOPO vector (Invitrogen) and four different clones containing the trpC1 open reading frame were sequenced using T7 forward and SP6 reverse primers. For zebrafish trpC6, provisional sequence information recently became available (Accession Number NM_001030282). Based on this information, we designed 5′ (5′-ATTGGCCAGTCCGGCTTACC-3′) and 3′ (5′-CCTTGGGACCAGATCTCCTT-3′) primers to amplify a sequence region spanning 711 base pairs specific for zebrafish trpC6 (bases 796-1506). The amplified trpC6 cDNA fragment was then subcloned into the pCRII-TOPO vector (Invitrogen) for antisense riboprobe generation. Sequencing of the fragment revealed identity to the provisional sequence in 708 of 711 base pairs. There were also three base pair mismatches (C1018 vs. T1018; C1144 vs. T1144; A1162 vs. C1162), all of which represented synonymous single nucleotide polymorphisms. Multiple sequence alignments were performed using the ClustalW algorithm, Version 1.83 (Thompson et al., 1994). Phylogenetic trees were generated in the Phylip type.
2.3 In situ hybridization and histology
Whole-mount in situ hybridization was performed as previously described (Thisse and Thisse, 1999). For trpC1 and trpC6 antisense probes, the templates (pCRII-TOPO-trpC1 and pCRII-TOPO-trpC6) were linearized with NotI (New England Biolabs) and antisense riboprobes were transcribed using SP6 RNA polymerase (Ambion). Embryos were hybridized with digoxigenin-labeled riboprobes at 65 °C. Anti-DIG-AP (1:5,000) and the NBT/BCIP substrate (Roche Diagnostics) were used to detect the probe. After the color reaction was stopped, embryos were washed with methanol and equilibrated in clearing solution (1/3 benzoyl-alcohol and 2/3 benzoyl-benzoate) and photographed using a Leica MZ12 dissecting microscope (Leica). Histological analysis on embryos after in situ hybridization analysis was carried out after stained embryos were fixed in 4% paraformaldehyde then dehydrated through a series of methanol/PBST washes of 25%/75%, 50%/50%, 75%/25%, and finally 100% methanol for 10 min each followed by embedding in JB-4 (Polysciences). A Riechert–Jung Supercut 2065 (Leica) microtome was used to generate 10 μm sections. A Nikon E800 microscope equipped with a Spot Image digital camera was used for photography (Nikon). In situ hybridizations were carried out 3 different times using between 12 and 24 embryos each time with consistent results. Sense probes did not produce a detectable background signal when applied to otherwise equally treated embryos [Mangos et al., 2007].
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 DK73495-01 to J.R. and DK54711 to I.A.D. C.C.M. was funded by a pre-doctoral scholarship from the Deutscher Akademischer Austausch Dienst (DAAD). S.M. was funded by post-doctoral fellowship 126a2f from the Polycystic Kidney Disease Foundation. We thank Mélanie Becker for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Crousillac S, LeRouge M, Rankin M, Gleason E. Immunolocalization of TRPC channel subunits 1 and 4 in the chicken retina. Vis Neurosci. 2003;20:453–463. doi: 10.1017/s0952523803204107. [DOI] [PubMed] [Google Scholar]
- Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amaltano A, Cheung EL, DerXer BH, Duggan A, Geleoc GS, Gray PA, HoVman MP, Rehm HL, Tamasauskas D, Zhang DS. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Mederos y Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol. 2005;25:6980–6989. doi: 10.1128/MCB.25.16.6980-6989.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Kalwa H, Fuchs B, Grimminger F, Weissmann N, Gudermann T. In vivo TRPC functions in the cardiopulmonary vasculature. Cell Calcium. 2007;42:233–244. doi: 10.1016/j.ceca.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Elizondo MR, Arduini BL, Paulsen J, MacDonald EL, Sabel JL, Henion PD, Cornell RA, Parichy DM. Defective skeletogenesis with kidney stone formation in dwarf zebraWsh mutant for trpm7. Curr Biol. 2005;15:667–671. doi: 10.1016/j.cub.2005.02.050. [DOI] [PubMed] [Google Scholar]
- Georgijevic S, Subramanian Y, Rollins EL, Starovic-Subota O, Tang AC, Childs SJ. Spatiotemporal expression of smooth muscle markers in developing zebrafish gut. Dev Dyn. 2007;236:1623–1632. doi: 10.1002/dvdy.21165. [DOI] [PubMed] [Google Scholar]
- Holmberg A, Schwerte T, Pelster B, Holmgren S. Ontogeny of the gut motility control system in zebrafish Danio rerio embryos and larvae. J Exp Biol. 2004;207:4085–4094. doi: 10.1242/jeb.01260. [DOI] [PubMed] [Google Scholar]
- Hu M, Easter SS. Retinal neurogenesis: the formation of the initial central patch of postmitotic cells. Dev Biol. 1999;207:309–321. doi: 10.1006/dbio.1998.9031. [DOI] [PubMed] [Google Scholar]
- Huber TB, Schermer B, Muller RU, Hohne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstadt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol. 2005;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pathak N, Kramer-Zucker A, Drummond IA. Notch signaling controls the differentiation of transporting epithelia and multiciliated cells in the zebrafish pronephros. Development. 2007;134:1111–1122. doi: 10.1242/dev.02806. [DOI] [PubMed] [Google Scholar]
- Mangos S, Liu Y, Drummond IA. Dynamic expression of the osmosensory channel TRPV4 in multiple developing organs in zebrafish. Gene Expr Patterns. 2007;7:480–484. doi: 10.1016/j.modgep.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C, Jones K, Hafen E, Rubin G. Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science. 1985;230:1040–1043. doi: 10.1126/science.3933112. [DOI] [PubMed] [Google Scholar]
- Montell C. New light on TRP and TRPL. Mol Pharmacol. 1997;52:755–763. doi: 10.1124/mol.52.5.755. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE 2005. 2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Moller CC, Wei C, Altintas MM, Li J, Greka A, Ohse T, Pippin JW, Rastaldi MP, Wawersik S, Schiavi S, Henger A, Kretzler M, Shankland SJ, Reiser J. Induction of TRPC6 channel in acquired forms of proteinuric kidney disease. J Am Soc Nephrol. 2007;18:29–36. doi: 10.1681/ASN.2006091010. [DOI] [PubMed] [Google Scholar]
- Nilius B. TRP channels in disease. Biochim Biophys Acta. 2007;1772:805–812. doi: 10.1016/j.bbadis.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Ramsey S, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR. TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Miyasaka N, Yoshihara Y. Mutually exclusive glomerular innervation by two distinct types of olfactory sensory neurons revealed in transgenic zebrafish. J Neurosci. 2005;25:4889–4897. doi: 10.1523/JNEUROSCI.0679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidi S, Friedrich RW, Nicolson T. NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science. 2003;301:96–99. doi: 10.1126/science.1084370. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development. 1999;126:229–240. doi: 10.1242/dev.126.2.229. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen DH, Hale ME. Development of zebrafish (Danio rerio) pectoral fin musculature. J Morphol. 2005;266:241–255. doi: 10.1002/jmor.10374. [DOI] [PubMed] [Google Scholar]
- Thorsen DH, Hale ME. Neural development of the zebrafish (Danio rerio) pectoral fin. J Comp Neurol. 2007;504:168–184. doi: 10.1002/cne.21425. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh D, Morielli A, Nelson M, Brayden J. Transient receptor potential channels regulate myogenic tone of resistance arteries. Circ Res. 2002;90:248–250. doi: 10.1161/hh0302.105662. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. University of Oregon Press: Eugene, Oregon; 1995. [Google Scholar]
- Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB. A Mutation in the TRPC6 Cation Channel Causes Familial Focal Segmental Glomerulosclerosis. Science. 2005;308:1801–1804. doi: 10.1126/science.1106215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.