Summary
The TLX1/HOX11 homeobox gene is frequently activated in T-cell acute lymphoblastic leukemia (T-ALL) by the t(10;14)(q24;q11) and t(7;10)(q35;q24) chromosomal translocations or by as yet unknown transcriptional mechanisms in the absence of 10q24 cytogenetic abnormalities. Almost all TLX1+ T-ALLs exhibit a CD4+CD8+ double-positive (DP) phenotype. To investigate the role of TLX1 as an initiating oncogene in T-ALL pathogenesis, we assessed the consequences of retroviral vector-directed TLX1 expression during the differentiation of murine and human thymocytes in fetal thymic organ cultures. Interestingly, enforced expression of TLX1 disrupted the differentiation of murine fetal liver precursors and human cord blood CD34+ stem/progenitor cells prior to the DP thymocyte stage. Although differentiation arrest was associated with an increased percentage of apoptotic thymocytes, it could only be partially bypassed by coexpression of transgenic BCL2. Mutation of the invariant asparagine residue at position 51 of the homeodomain – which is required for efficient DNA binding – released the block, consistent with the notion that TLX1 inhibits thymocyte differentiation and promotes T-cell oncogenesis by functioning as a transcription factor. The relevance of these findings is discussed in the context of activating NOTCH1 mutations and the other genetic lesions implicated in the multistep transformation process of TLX1+ T-ALL.
Keywords: TLX1/HOX11 oncogene, T-cell acute lymphoblastic leukemia, differentiation arrest, double, negative thymocytes, fetal thymic organ culture
Introduction
The TLX1/HOX11 oncogenic transcription factor is aberrantly expressed in 4–20% of pediatric and 14–30% of adult T-cell acute lymphoblastic leukemia (T-ALL) cases (Ferrando et al, 2002; Owens & Hawley, 2002; Kees et al, 2003). A common mechanism of TLX1 transcriptional activation in T-ALL involves the t(10;14)(q24;q11) and t(7;10)(q35;q24) chromosomal translocations, which juxtapose the intact TLX1 coding region downstream of T-cell receptor (TCR) δ or TCRβ regulatory sequences (Dube et al, 1991; Hatano et al, 1991; Kennedy et al, 1991; Lu et al, 1991). During normal T cell development, recombination of the TCR variable, diversity and joining gene segments occurs at the transition from the CD4−CD8− double-negative (DN) stage to the CD4+CD8+ double-positive (DP) stage (Borowski et al, 2002; Ceredig & Rolink, 2002; Varas et al, 2003). Notably, TLX1+ T-ALL samples are virtually all arrested at the CD4+CD8+ DP early cortical T-cell stage (Ferrando et al, 2002; Asnafi et al, 2004; Soulier et al, 2005).
The TCR translocation-generated 10q24 chromosomal aberrations that occur during thymocyte differentiation are presumed to be initiating genetic lesions in TLX1+ T-ALL. Indeed, the T-cell tumorigenic activity of TLX1 has been confirmed experimentally in studies of transgenic mice in which the LCK proximal promoter was used to express TLX1 in immature thymocytes (Hatano et al, 1992), and in murine bone marrow transplant recipients that received hematopoietic stem cells expressing a retrovirally-delivered TLX1 transgene (Hawley et al, 1997). However, a long latency of tumor development in both model systems indicated the requirement for additional neoplastic mutations, consistent with epidemiological data implicating a multistep process of pathogenesis of T-ALL (Pui et al, 2004). In this regard, the INK4A-ARF tumor suppressor locus is inactivated by homozygous deletion in most TLX1+ cases of T-ALL (Cayuela et al, 1996; Krug et al, 2002; Ferrando et al, 2002). Moreover, it is now appreciated that more than 50% of human T-ALL cases, including TLX1+ samples, harbor activating mutations in the NOTCH1 gene (Weng et al, 2004). In addition, recent work has identified two variant ABL1 fusion genes encoding constitutively activated tyrosine kinases in at least seven TLX1+ T-ALL cases (Graux et al, 2004; De Keersmaecker et al, 2005; Soulier et al, 2005). These findings, considered together with a growing body of evidence that TLX1 expression is frequently dysregulated in the absence of cytogenetic abnormalities at 10q24 (Salvati et al, 1995; Ferrando et al, 2002; Kees et al, 2003; Berger et al, 2003; Ferrando et al, 2004a; Ferrando et al, 2004b; Gottardo et al, 2005), raise the question as to the frequency of TLX1 involvement in T-ALL as a secondary as opposed to a primary oncogenic event.
While in vitro transforming function (operationally defined as the ability to induce increased replicative capacity) of TLX1 has been demonstrated in murine hematopoietic progenitor cell immortalization experiments, the cell lines established from bone marrow, yolk sac and embryonic stem cell-derived embryoid bodies have been solely of nonlymphoid origin (Hawley et al, 1994a; Hawley et al, 1997; Keller et al, 1998; Yu et al, 2002; Owens et al, 2003). Investigations of the role of TLX1 in T-ALL pathobiology would thus benefit from the availability of appropriate in vitro experimental systems. Here, we evaluated the consequences of ectopically expressing TLX1 in murine T-cell precursors using fetal thymic organ culture (FTOC) methodology in which fetal thymi fully support differentiation through the CD4−CD8−DN and CD4+CD8+ DP stages into mature CD4+ and CD8+ T cells (Jenkinson & Owen, 1990). Hematopoietic stem/progenitor cells in murine fetal liver (FL) were transduced with wild-type and mutant TLX1 retroviral vectors (Owens et al, 2003) followed by in vitro repopulation of irradiation-depleted fetal thymic lobes (Owens et al, 2004). We hypothesized that inappropriate expression of TLX1 during FTOC-supported thymopoiesis should result predominantly in T-cell developmental arrest at the CD4+CD8+ DP stage. Unexpectedly, TLX1 impeded the differentiation of murine FL-derived precursors at the CD4−CD8− DN stage of thymocyte differentiation, with subsequent apoptotic cell death that could not be completely overcome by a BCL2 transgene. The effect of retroviral-mediated TLX1 expression was then examined using human cord blood CD34+ hematopoietic stem/progenitor cells as the starting cell population and hybrid human/murine FTOCs (Plum et al, 1994; Plum et al, 2000), where a similar block of human T cell development prior to the CD4+CD8+ DP stage was also observed. These observations are discussed in the context of TLX1 mechanism in the etiology of T-ALL.
Materials and methods
Mice
BALB/c mice were obtained from National Cancer Institute Animal Production Area (Frederick, MD). Eμ-bcl-2-36 mice carrying the human BCL2 transgene (Strasser et al, 1991) were obtained from the Jackson Laboratory (Bar Harbor, ME). All procedures involving mice followed Institutional Animal Care and Use Committee guidelines.
Cell lines
The Phoenix-Eco packaging cell line (ATCC No. SD 3444; American Type Culture Collection, Manassas, VA) and 293T cells (obtained from M. Eiden, National Institute of Mental Health, National Institutes of Health, Bethesda, MD) were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen Corp., Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Cambrex Bio Science Walkersville, Inc., Walkersville, MD) and 10 mM HEPES (Invitrogen Corp.). Primary murine hematopoietic progenitor lines derived from FL precursors were cultured in Iscove’s Modified Dulbecco’s medium (IMDM; Invitrogen Corp.) supplemented with 10% FBS, 10% X630-rIL3 conditioned medium (a source of IL-3), 1% antibiotic/antimycotic (Invitrogen Corp.), 10 mM HEPES and 50 μM β-mercaptoethanol (Owens et al, 2003). All cells were maintained at 37ºC in a humidified incubator containing 5% CO2.
Construction of TLX1 retroviral vectors
The MSCV-GW retroviral vector was generated from the murine stem cell virus (MSCV) backbone (Hawley et al, 1994b) by insertion of the enhanced green fluorescent protein (GFP) gene and Woodchuck hepatitis virus posttranscriptional regulatory element from the SIN-CMV-GFP-W vector (Ramezani et al, 2000) downstream of an internal phosphoglycerate kinase (PGK) promoter. Wild-type TLX1 and several previously described TLX1 mutants (Owens et al, 2003) – TLX1 T47I, TLX1 N51A and TLX1 M5 – were subcloned into the EcoRI/HpaI sites of MSCV-GW upstream of the PGK promoter, to generate the MSCV-GW-TLX1, MSCV-GW-TLX1 T47I, MSCV-GW-TLX1 N51A, and MSCV-GW-TLX1 M5 retroviral vectors, respectively.
Retroviral transduction of FL cells
For production of ecotropic retroviral vector supernatants, Phoenix-Eco packaging cells (4 × 106 cells/dish) cultured in 100 mm dishes were transiently transfected with MSCV-GW or MSCV-GW-TLX1 vectors (15 μg/dish) using calcium phosphate precipitation. The cells were washed the following day and vector supernatants were collected 24 and 48 h later (Owens et al, 2003). FL cells were obtained on embryonic day 14 (E14) and prestimulated for 2 days in IMDM supplemented with 10% FBS, 10% IL-3-conditioned medium and 10% kit ligand (KL)-conditioned medium from Chinese hamster ovary cells transfected with a KL expression vector (Hawley et al, 1996). Cells were resuspended in vector supernatants and spinoculated on 2 consecutive days in the presence of IL-3- and KL- conditioned medium and 6 μg/ml polybrene at 2,000g for 1 h (Owens et al, 2004). The percentage of transduced cells, determined by expression of GFP, ranged from 15–90%. In some experiments, transduced cells were enriched by sorting on a FACSVantage SE/Diva instrument (BD Biosciences, San Jose, CA). Viable cells were gated by a combination of forward and orthogonal light scatter, and data was acquired and analyzed using FACSDiva software (BD Biosciences).
FTOC
Timed pregnancies were set up for collection of embryonic day 16 (E16) fetal thymi. Sorted or unsorted FL cells were centrifuged and resuspended in a volume of media equivalent to 35 μl per thymic lobe, and 35 μl aliquots of cells were placed in wells of a Terasaki dish. One irradiated thymic lobe (25 Gy) was placed in each well of cells, dishes were inverted to form hanging drop preparations and placed in a humidified container for 24–72 h. Lobes were then placed in organ culture and incubated for 5–18 days, with weekly media changes as described (Owens et al, 2004).
Transduction of human CD34+ cord blood cells and hybrid human/murine FTOC
Human CD34+ hematopoietic stem/progenitor cells were isolated from anonymous cord blood units, which were obtained after informed consent in conformity with an Institutional Review Board protocol, by Ficoll-Paque gradient separation (Ramezani et al, 2000). Vesicular stomatitis virus G protein (VSV-G)-pseudotyped retroviral vector supernatants were generated by cotransfection of 293T cells with control or TLX1 vectors (15 μg), pEQPAM3-E gag-pol packaging plasmid (10 μg) and a VSV-G envelope plasmid (6.7 μg) as described (Owens et al, 2004). CD34+ cells were isolated by labeling with magnetic-bead conjugated anti-human CD34 antibody and separating using a MACS column (Miltenyi Biotec Inc., Auburn, CA). CD34+ cells were prestimulated in X-Vivo 15 medium (Cambrex Bio Science Walkersville, Inc.) supplemented with 10% BIT (bovine serum albumin, insulin and human transferrin) serum substitute (StemCell Technologies, Vancouver, British Columbia, Canada), recombinant human IL-3, IL-6, TPO (each at 20 ng/ml), KL and Flt3-L (each at 100 ng/ml; all growth factors from PeproTech Inc., Rocky Hill, NJ) and plated onto dishes coated with full-length human fibronectin (BD Biosciences) for 3 days and then spinoculated in the presence of VSV-G pseudotyped vector supernatants containing either the MSCV-GW or the MSCV-GW-TLX1 vector at 2,000g for 1 h on 3 consecutive days. GFP+ cells were enriched by sorting for GFP expression on a FACSVantage SE/Diva (Owens et al, 2004). Sorted GFP+ human CD34+ hematopoietic stem/progenitor cells transduced with either the control or TLX1 vectors were incubated in hanging drops with irradiated (25 Gy) E16 BALB/c thymic lobes (1–2 × 104 cells per lobe) for 48–72 h. Thymi were then set up in organ culture for 25 days and media was changed weekly until analysis as described (Owens et al, 2004). Cells were harvested from thymi and immunophenotyped with human-specific antibodies.
Identification of apoptotic cells
For determination of apoptotic cells in FTOC samples (Schmid et al, 1994), control and TLX1 retroviral vector-transduced thymocytes were isolated from day 5 FTOCs and resuspended in 200 μl PBS plus 2% FBS. The vital dye 7-AAD (5 μl; BD Biosciences Pharmingen, San Jose, CA) was added to each cell sample and incubated on ice for 10 min. Cells were immediately analyzed for GFP and 7-AAD expression on a BD LSR instrument using CellQuest software (BD Biosciences).
Isolation and transduction of DN thymocytes
To enrich for DN thymocytes, cells were isolated from the thymi of 4–6 week old mice and labeled with magnetic bead-conjugated anti-CD4 and anti-CD8 antibodies (Miltenyi Biotec) at 4°C for 15 min. Flow-through cells were collected and analyzed for enrichment of DN cells by flow cytometry. DN cells to be transduced with retroviral vectors were resuspended in concentrated (10x) vector supernatants in the presence of IL-7 and IL-2 (10 ng/ml each) and 6 μg/ml polybrene and spinoculated for 1 h at 2,000g. This was repeated the following day. After two days of transduction, cells were sorted for GFP expression and used in hanging drop preparations as described (Owens et al, 2004). Thymocytes were analyzed on day 15 of FTOC.
Immunophenotyping
For analysis of murine T cell differentiation, thymi were mechanically disrupted using a tissue processor and cells from each lobe were aliquotted into two wells of a 96-well plate. Fc receptors were blocked in 2.4G2 hybridoma supernatant (BD Biosciences Pharmingen), washed and stained for surface expression of T cell differentiation antigens in PBS plus 2% FBS. Cells were immunophenotyped with the following antibodies: CD25-PE, CD44-APC, CD4-biotin, CD4-APC, CD8-biotin, CD8-TC (all from Caltag Laboratories, Burlingame, CA); Red670-avidin (Invitrogen Corp.); and CD44-APC, CD11b (Mac-1)-PE, B220-PerCP and CD49b-APC (all from BD Biosciences Pharmingen). For analysis of human thymocyte development, cells were stained with the following fluorochrome-conjugated antibodies directed against human surface markers: CD45-APC and CD3-APC (BD Biosciences Pharmingen), CD1a-PE, CD2-TC, CD4-PE and CD8-TC (Caltag Laboratories). All flow cytometry data were acquired on a BD LSR instrument and four-color analysis was performed using FlowJo software (Tree Star, Inc., San Carlos, CA).
Western blotting
Western blotting was carried out as described (Owens et al, 2003; Riz & Hawley, 2005). In brief, cells were lysed on ice in RIPA buffer (50 mM Tris-HCl pH 7.4, 1% NP40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM NaF, 1 mM NaV3O4, 1 mM PMSF, 10 mg/ml leupeptin, 10 μg/ml aprotinin) for 30 min. Insoluble materials were removed by centrifugation at 18,300g at 4°C for 10 min. To detect TLX1 proteins in stably transduced Jurkat cells, 20 μg whole cell lysates were separated by 12% SDS-PAGE, transferred to a PVDF-Plus membrane (Osmonics, Inc., Minnetonka, MN) and probed with an affinity-purified anti-TLX1 rabbit polyclonal antibody (HOX11 C18; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed using the enhanced chemiluminescence technique and analyzed on a Storm 860 instrument equipped with ImageQuant software (Amersham Biosciences Corp., Piscataway, NJ).
Statistical analysis
Data were analyzed with the Student’s t test using Microsoft Excel software and presented as mean ± the standard error of the mean (SEM).
Results
TLX1 blocks murine thymocyte development at the DN1/DN2 stage of differentiation
Because lack of in vitro transformation of lymphoid precursors in our earlier studies may have been due to inherent limitations of the culture conditions, the effect of TLX1 expression on thymocyte differentiation was examined in the FTOC model system (Jenkinson & Owen, 1990). For these experiments, we constructed a series of TLX1 retroviral vectors in the MSCV-GW backbone (Fig 1A) that also contained the GFP gene as a fluorescence-activated cell sorter-selectable reporter (Cheng et al, 1997; Hawley et al, 2001). The vector design was such that TLX1 coding regions are transcribed from the retroviral 5’ long terminal repeat while the GFP gene is transcribed from an internal PGK promoter. This double-promoter format was chosen over a bicistronic configuration in an attempt to avoid concomitant selection of cells with excessively high levels of TLX1 expression as a consequence of sorting for linked GFP expression (Leung et al, 1999). Western blot analysis of Jurkat cells stably transduced with MSCV-GW-TLX1 demonstrated that TLX1 protein levels were similar to those obtained with the MSCVhyg-TLX1 retroviral vector containing the hygromycin resistance gene as selectable marker (Fig 1B), which were previously shown to be in the range observed in the TLX1+ K3P and ALL-SIL T-ALL cell lines (Riz & Hawley, 2005). Murine E14 FL precursors were transduced with MSCV-GW-TLX1 and control MSCV-GW vector and used to reconstitute E16 fetal thymic organs (Owens et al, 2004) as outlined in Fig 1C. As a positive control for TLX1 transforming function, transduced FL precursors were maintained in parallel in liquid cultures containing IL-3-supplemented medium, conditions that were previously conducive to the immortalization of myeloid precursors in murine bone marrow (Hawley et al, 1994a; Hawley et al, 1997; Owens et al, 2003). After 15–18 days in FTOCs, thymocytes were analyzed for expression of GFP and markers of T cell differentiation. Control FTOCs displayed a similar proportion of GFP+ cells (33 ± 25%, N = 9 from 2 independent experiments; Fig 1D, lower left plot) compared with FL cells maintained in liquid culture (40% GFP+ cells; Fig 1D, upper left plot). In contrast, GFP expression was almost completely absent from TLX1 FTOCs (0.7 ± 0.3%, N = 9, P < 0.0007; Fig 1D, lower right plot); by comparison, expression of the TLX1 vector was selectively maintained in the IL-3-containing FL liquid cultures (97% GFP+ cells; Fig 1D, upper right plot), ultimately giving rise to IL-3-dependent myeloid cell lines with a surface immunophenotype similar to that described for TLX1-immortalized bone marrow progenitor lines (Hawley et al, 1994a; Hawley et al, 1997; Owens et al, 2003). Analysis of thymocyte differentiation based on surface expression of CD3, CD4 and CD8 indicated that FL precursors expressing the MSCV-GW vector developed normally while very few cells in the TLX1-expressing FTOCs progressed to mature T-cell stages (data not shown; see below).
Fig 1.
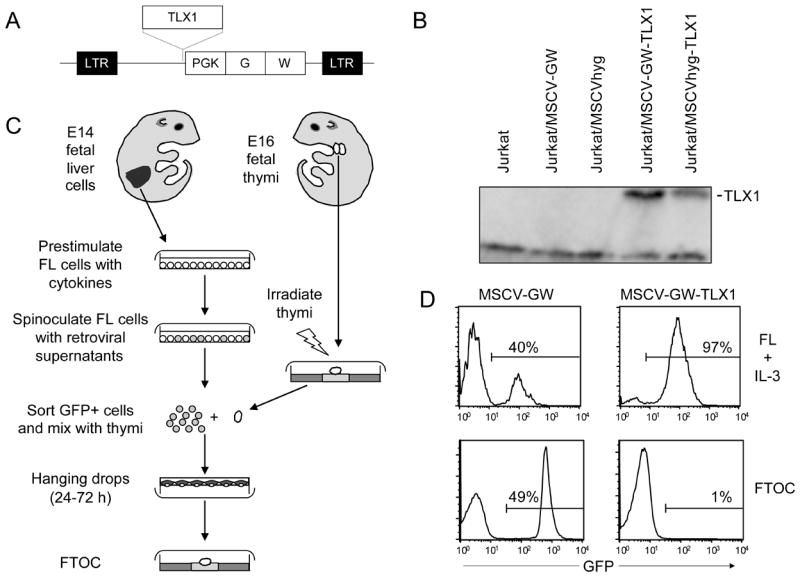
TLX1 impedes thymocyte differentiation. (A) TLX1 coding regions were subcloned upstream of the phosphoglycerate kinase promoter in the MSCV-GW retroviral vector. Abbreviations: LTR, long terminal repeat; PGK, phosphoglycerate kinase; G, GFP gene; W, woodchuck hepatitis virus posttranscriptional regulatory element. (B) Western blot analysis demonstrating TLX1 protein levels in Jurkat cells stably transduced with the MSCV-GW-TLX1 and MSCVhyg-TLX1 retroviral vectors. (C) Protocol used to evaluate effect of TLX1 expression on primary thymocyte differentiation. (D) Upper plots GFP expression in FL precursors transduced with MSCV-GW or MSCV-GW-TLX1 vectors on day 18 of liquid culture in the presence of IL-3. Lower plots GFP expression in FTOCs on day 18 of culture. Data are representative of nine thymic lobes from two independent experiments.
We next examined whether the TLX1 retroviral vector-transduced FL precursors gained access to the thymus by analyzing for the presence of GFP expression in fetal thymi at 0, 24 and 48 h after the hanging drop step. During this period, the percentage of GFP-expressing cells was comparable to input levels (data not shown). This analysis was then extended over an 8 day observation period, which revealed that the proportion of GFP+ cells progressively decreased in the TLX1 FTOCs from day 2 onward; concomitantly, expression of the TLX1 vector was selected for in the IL-3-supplemented FL liquid cultures (Fig 2A).
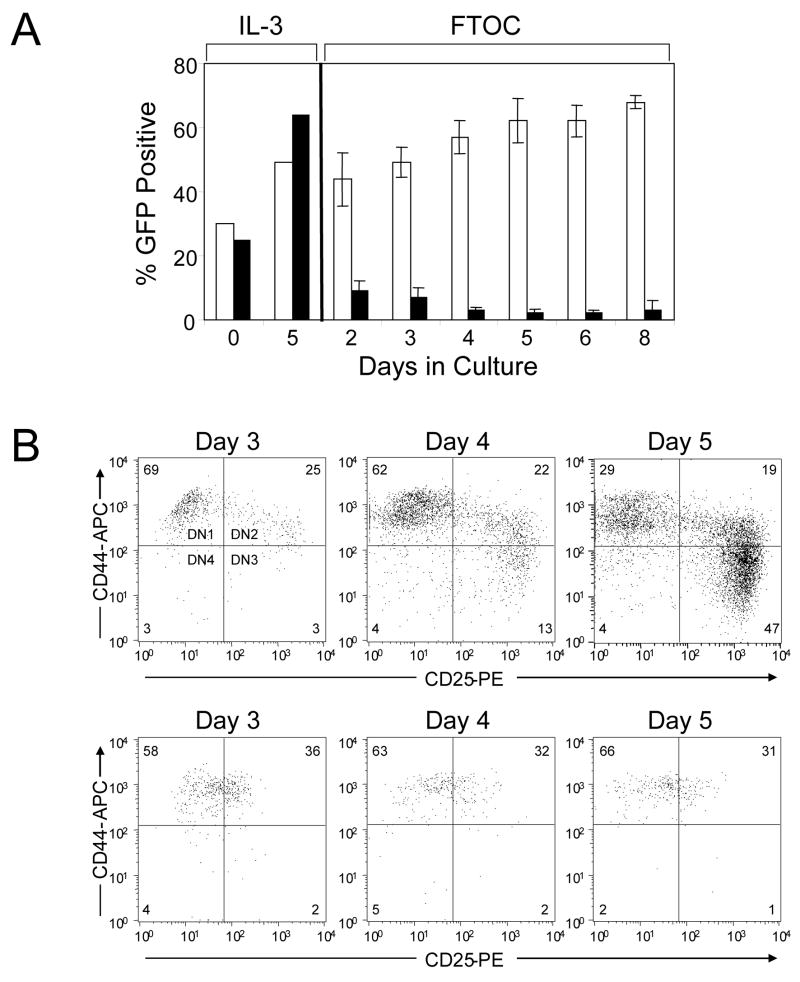
Fig 2.
TLX1 blocks thymocyte differentiation at the DN stage. (A) Time course analysis of TLX1-dependent thymocyte arrest. FL precursors transduced with either MSCV-GW (white bars) or TLX1 (black bars) were used in FTOCs. Aliquots of transduced FL precursors were maintained in parallel in liquid cultures containing IL-3. Thymocytes from organ cultures were analyzed at indicated time points for expression of GFP. Data represent the mean ± SEM for 3–4 lobes analyzed per time point. (B) Analysis of DN differentiation of FL precursors transduced with MSCV-GW (upper plots) or TLX1 (lower plots) retroviral vectors. Thymocytes from FTOCs were processed on days 3, 4 and 5 of culture and immunophenotyped for analysis of CD44/CD25 populations by gating on GFP+CD4−CD8− cells. Bivariate histograms are representative of 6–8 thymic lobes from two independent experiments.
In mice, the DN T-cell compartment, which comprises less than 1% of thymocytes, can be subdivided into four sequential populations based on surface expression of the hyaluronan receptor (CD44) and the IL-2 receptor α chain (CD25) – DN1 (CD44+CD25−), DN2 (CD44+CD25+), DN3 (CD44−CD25+) and DN4 (CD44−CD25−) – during which the TCRδ, TCRγ and TCRβ loci undergo rearrangement, followed by TCRa rearrangement concomitant with progression to the CD4+CD8+ DP stage, and subsequent maturation into CD4+CD3+ or CD8+CD3+ single-positive (SP) T cells (Godfrey et al, 1993). To determine the DN stage at which TLX1 arrested thymocyte development, GFP+ FL precursors were sorted to >95% purity and DN thymocyte differentiation was analyzed based on CD44 and CD25 expression in FTOCs. MSCV-GW-transduced cells underwent normal DN differentiation, progressing through the DN1, DN2 and DN3 stages of development over a 5-day period (Fig 2B, upper plots), whereas analysis of TLX1 retroviral vector-transduced FL precursors revealed a block in thymocyte differentiation at the transition from the DN1 to the DN2 stage (Fig 2B, lower plots). In addition to the block in differentiation, the overall cell recovery was reduced in the TLX1 thymic lobes, indicating that TLX1-expressing thymocyte progenitors were at a survival or proliferative disadvantage. Even at day 3 of culture, the percentage of GFP+ cells was decreased in the TLX1 FTOCs (57 vs 41%, P < 0.01 for MSCV-GW and TLX1, respectively) (Fig 3A). Over the next 48 h, while the proportion of GFP+ cells was maintained or increased in control FTOCs, the percentage of GFP-expressing cells in TLX1 FTOCs remained at significantly reduced levels (Fig 3A). The data indicated that TLX1 retroviral vector-transduced FL precursors were so severely compromised in T-cell developmental potential that a small fraction of untransduced cells were capable of out-competing them to reconstitute the thymic lobes.
Fig 3.
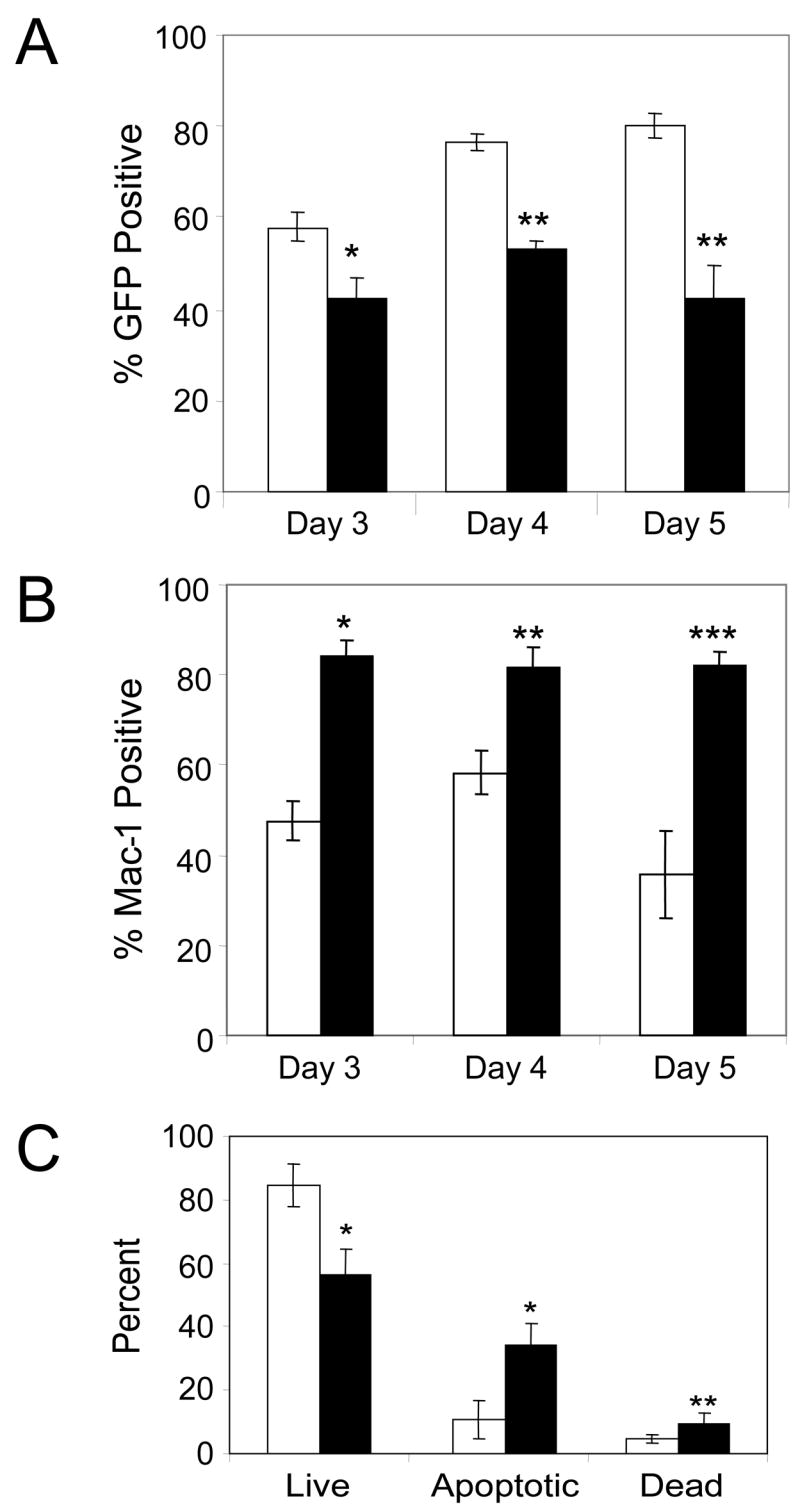
Decreased numbers of thymocytes in TLX1 FTOCs. (A) GFP expression in thymocytes from days 3, 4 and 5 of FTOCs. Data represent the mean ± SEM of 8–10 fetal thymi per time point from two independent experiments for MSCV-GW (white bars) and TLX1 (black bars); * P < 0.003, ** P < 0.0001. (B) MSCV-GW- (white bars) or TLX1- (black bars) expressing thymocytes were analyzed at FTOC days 3, 4 and 5 for surface expression of the FL stem cell/myeloid marker Mac-1. Data are based on a GFP+ gate and represent the mean ± SEM of 6 lobes; * P < 0.0002, ** P < 0.011, ***P < 0.0001. (C) Viability of thymocytes in day 5 FTOCs. MSCV-GW- (white bars) and TLX1 retroviral vector- (black bars) transduced thymocytes were stained with 7-AAD. Cells were gated on GFP fluorescence and the proportions of 7-AAD negative (live), dim (early apoptotic) and bright (late apoptotic or necrotic, dead) cells were determined. Data represent the mean ± SEM of at least four lobes per vector; * P < 0.0016, ** P < 0.036.
Cells from FTOCs were also immunophenotyped for non-T lineage surface antigens, including B220 (B cell marker), CD49b (NK/NKT cell marker) and Mac-1 (myeloid cell marker that is found at high levels on FL-derived hematopoietic stem cells (Morrison et al, 1995)). Expression of B220 and CD49b in fetal thymi was low or absent following transduction with both MSCV-GW and TLX1 vectors, and did not differ significantly between the two groups (data not shown). However, a higher percentage of cells expressed Mac-1 in the TLX1 cultures compared with controls, even on day 3 of FTOC. On day 5 of FTOC, a time point at which 70% of cells in the control cultures were in DN2, 3 or 4, and ~36% of the cells expressed Mac-1 (Fig 3B, compare with Fig 2B), ~82% of the cells in the TLX1 cultures were Mac-1+. As shown in the bivariate histograms of Fig 2B, the majority of cells in the TLX1 cultures remained in the DN1 and DN2 quadrants. These data indicated that inappropriate TLX1 expression in murine FL precursors effectively blocks their differentiation during the earliest stages of T cell development.
Since proliferative capacity of the cells appeared to be inhibited by ectopic TLX1 expression, we suspected that TLX1-expressing thymocyte progenitors may be dying by apoptosis. MSCV-GW- and TLX1 retroviral vector-transduced FL precursors were sorted and analyzed in day 5 FTOCs. Thymocytes were stained with the vital dye 7-AAD to identify early apoptotic and late apoptotic/necrotic dead cell populations (Schmid et al, 1994). Analysis of GFP+/7-AAD+ cells indicated that cells in the TLX1 FTOCs exhibited increased numbers of early apoptotic and dead cells relative to control cultures (Fig 3C).
TLX1 blocks human thymocyte development prior to the DP stage of differentiation
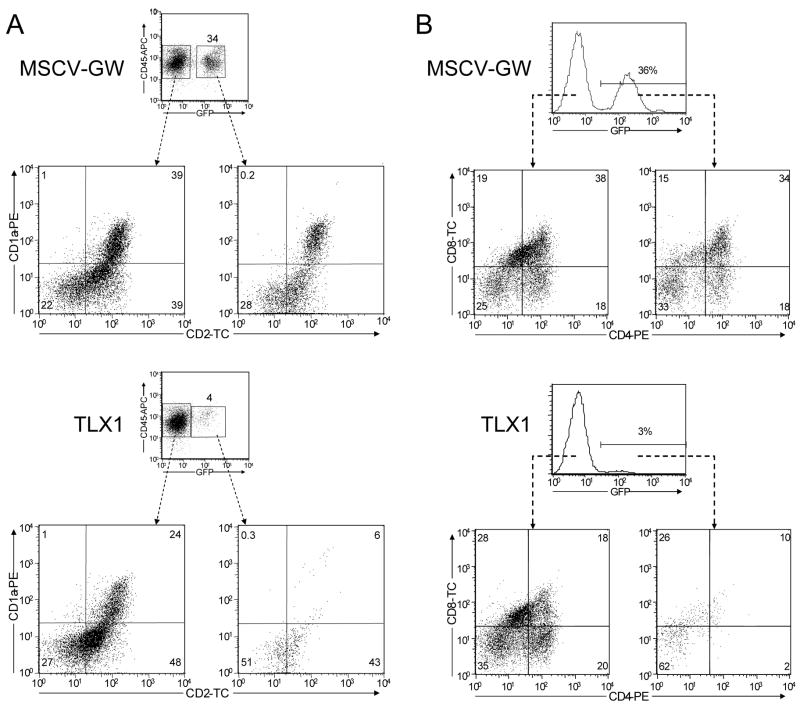
In view of the unexpected murine results, we decided to investigate the impact of dysregulated TLX1 expression on primary human thymocyte differentiation using a hybrid human/murine FTOC protocol (Plum et al, 1994; Plum et al, 2000). Cord blood-derived CD34+ hematopoietic stem/progenitor cells were transduced with either MSCV-GW or TLX1 VSV-G-pseudotyped retroviral vectors, enriched for GFP expression by cell sorting, and incubated with irradiated murine E16 fetal thymic lobes (Owens et al, 2004). After 25 days of culture, the percentage of cells expressing GFP was significantly higher in control MSCV-GW FTOCs (30 ± 9%) compared with TLX1 FTOCs (3 ± 1%, N = 5, P < 0.0002). Expression of several markers of T cell differentiation was analyzed including CD1a, which is expressed on cortical thymocytes, and CD2, a molecule that is up-regulated on the cell surface following pre-TCR signaling and which is present on ~80–90% of human peripheral blood T cells (Plum et al, 2000). In this respect, it is noteworthy that TLX1+ T-ALL lymphoblasts express high levels of CD1a but have low levels of CD2 (Ferrando et al, 2002). GFP+ and GFP− populations in control cultures expressed significant levels of CD1a (25 ± 9% vs 30 ± 8%, respectively; Fig 4A, upper plots) and high levels of CD2 were detected in both the GFP+ and GFP− cell populations (72 ± 4% vs 73 ± 7%, respectively; Fig 4A, upper plots). Notably, CD1a+GFP+ cell numbers were greatly reduced in the TLX1 cultures compared with either the GFP− cells from the same fetal thymi (7 ± 3% vs 24 ± 10%, P < 0.0047; Fig 4A, lower plots) or with the thymocytes in the control cultures, and fewer GFP+ cells present in the TLX1 cultures expressed CD2 (49 ± 5% vs 72 ± 2%, P < 0.0001; Fig 4A, lower plots). In addition, whereas the majority of cells in the control cultures expressed CD4 and/or CD8 (Fig 4B, upper plots), CD4−CD8−GFP+ cells comprised the major population in the TLX1 cultures (Fig 4B, lower plots). Taken together, these findings indicated that ectopic TLX1 expression disrupts differentiation of human thymocyte progenitors prior to the CD4+CD8+ DP early cortical thymocyte stage, extending and validating the observations in the syngeneic murine FTOC model.
Fig 4.
TLX1 blocks human thymocyte differentiation prior to the DP stage. CD34+ human cord blood stem/progenitor cells were transduced with MSCV-GW and TLX1 retroviral vectors and used in hybrid human/murine FTOCs. Thymocytes were harvested and analyzed after 25 days for expression of (A) CD1a and CD2 on GFP−CD45+ and GFP+CD45+ cells, and (B) CD4 and CD8 expression. Bivariate histograms are representative of thymocytes from five thymic lobes.
TLX1-dependent block of T cell development requires distinct functional domains
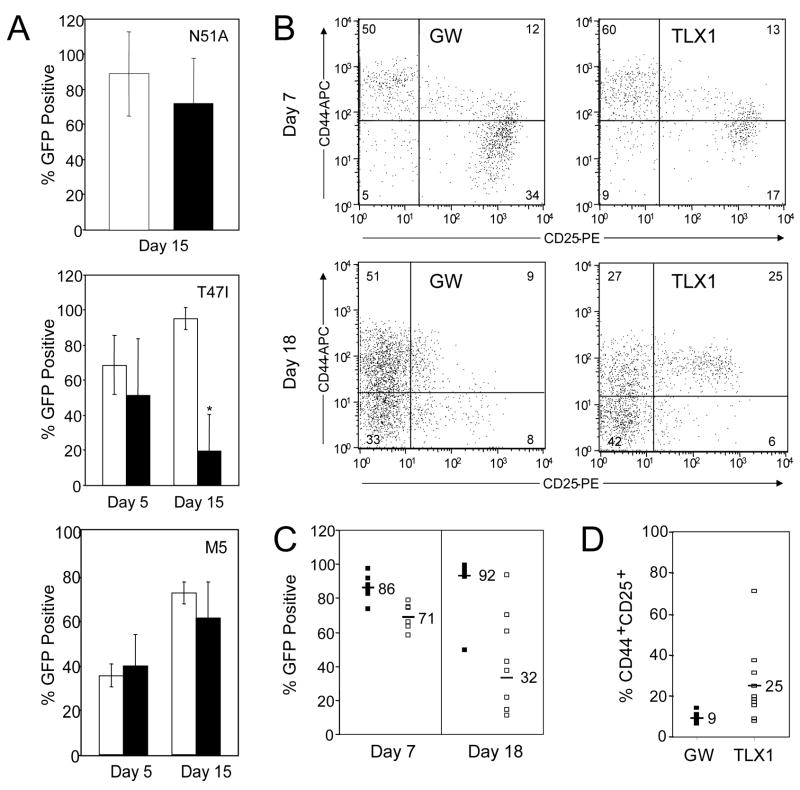
In a previous study (Owens et al, 2003), we generated a series of mutant TLX1 proteins containing modifications within structural domains required for in vitro DNA binding specificity and activity, and association with PBX homeodomain-containing cofactor proteins. Two TLX1 homeodomain point mutants, TLX1 T47I and TLX1 N51A, were chosen for analysis in the FTOC system. The TLX1 T47I mutation alters the in vitro DNA binding specificity of the protein from TAAC/T to TAAT only (Dear et al, 1993), while the TLX1 N51A mutation disrupts DNA binding without altering the overall structure of the homeodomain (Ades & Sauer, 1995). The TLX1 T47I mutant immortalized bone marrow precursors, but the myeloid cell lines established displayed a slightly more differentiated phenotype (as indicated by increased Mac-1 expression), whereas the TLX1 N51A mutant lacked myeloid precursor immortalizing function. The mutants were tested for their ability to block thymocyte differentiation at the DN stage (day 5) and the DP and SP stages (day 15). In contrast to wild-type TLX1, both TLX1 homeodomain point mutants allowed progression to the DN3 stage of thymocyte differentiation at day 5 (data not shown). No significant difference between the MSCV-GW control cultures and the TLX1 N51A cultures was observed in the CD3/CD4/CD8 subsets on day 15 of FTOC (Fig 5A, upper graph; and data not shown), indicating that the invariant asparagine residue at position 51 of the TLX1 homeodomain was critical for the DN1/DN2 differentiation block. By comparison, the percentage of GFP+ thymocytes at day 15 expressing the TLX1 T47I mutant was consistently lower than observed for control cultures [GFP% = 91.2% (N = 11) vs 21.2% (N = 12) for MSCV-GW and TLX1 T47I, respectively, P < 0.0001; Fig 5A, middle graph], suggesting that the mutant TLX1 protein inhibited thymocyte proliferation or survival to some extent. While no clear difference in expression of CD3, CD4 or CD8 on DP and SP subsets was observed in the TLX1 T47I cultures compared with controls, there was an increase in the percentage of CD25-expressing cells in the CD4−CD8− subset (44.6% vs 25.3% for TLX1 T47I vs MSCV-GW, respectively, P < 0.026). The TLX1 T47I homeodomain mutant therefore appeared to partially block thymocyte differentiation a later stage of T cell development than the wild-type TLX1 protein.
Fig 5.
Mechanistic aspects of TLX1 inhibition of thymocyte differentiation. (A) Disruption of residues implicated in DNA binding and PBX cofactor interactions abrogates TLX1-mediated differentiation block. Fetal thymocytes transduced with TLX1 N51A, TLX1 T47I and TLX1 M5 (black bars) were analyzed from day 5 and/or day 15 FTOCs for GFP expression versus MSCV-GW (white bars). Graphs present the mean ± SEM of data from 7–14 lobes per retroviral vector and 2–3 independent experiments; *P < 0.0001. (B–D) Transgenic BCL2 partially overcomes TLX1-inhibited thymic repopulation. (B) Phenotype of BCL2-expressing DN thymocytes at day 7 of FTOCs transduced with MSCV-GW or TLX1 retroviral vectors. Representative bivariate histograms of CD44 versus CD25 gated on GFP+/CD4-CD8- cells. (C) GFP expression in day 7 (MSCV-GW: N = 6; TLX1: N = 6) and day 18 (MSCV-GW: N = 12; TLX1: N = 8) BCL2-expressing thymocytes. MSCV-GW (black bars) and TLX1 (white bars). Data shown were combined from two independent experiments on day 18 and the means for the data subsets are indicated. Day 18 percentage of TLX1+GFP+ cells is significantly different; P < 0.0001. (D) Percentage of CD44+CD25+ cells is increased in TLX1+ BCL2-expressing thymocytes compared with MSCV-GW cells analyzed at day 18. MSCV-GW: N = 12; TLX1: N = 10. Means of the subsets are indicated; P < 0.0076.
The role of PBX interactions in TLX1-dependent thymocyte differentiation arrest was examined next. We showed previously that disruption of the PBX interaction motif (PIM) was incompatible with establishment of TLX1-immortalized myeloid progenitor lines (Owens et al, 2003). Furthermore, since high levels of PBX2 are detected in TLX1+ T-ALL cells, allowing for potential interaction and cooperative binding at TLX1-PBX2 DNA target sequences (Allen et al, 2000), PBX2 cofactor interactions may be important for TLX1-mediated leukemogenesis. The PIM mutant TLX1 M5 in which the entire PIM was mutated (FPWME NGSSR) was evaluated. Differentiation of TLX1 M5-transduced thymocytes to the DN3 stage by day 5 of FTOC was observed and percent GFP expression was not different from control cultures (data from 2 independent experiments; Fig 5A, lower graph). In day 15 FTOCs, there was also no significant difference in the percentage of GFP+ cells in the TLX1 M5 cultures compared with controls (83.1% vs 90.1%, P < 0.358; Fig 5A, lower graph). Moreover, differentiation to DP and SP cells was observed in all TLX1 M5 cultures examined (data not shown).
Transgenic BCL2 partially rescues TLX1-induced DN1/DN2 block
During thymocyte differentiation, BCL2 is expressed in a biphasic pattern (Gratiot-Deans et al, 1993; Veis et al, 1993; Moore et al, 1994). High levels of BCL2 are initially detected during the DN1-DN3 thymocyte stages but its expression is down-regulated in DN4 and CD4+CD8+CD3lo DP cortical thymocytes that are actively undergoing positive and negative selection. BCL2 expression is up-regulated again in mature CD4+CD3+ and CD8+CD3+ cells. In DN thymocytes, BCL2 expression is downstream of IL-7 receptor (IL-7R) signaling and its expression pattern can be correlated with IL-7 responsiveness (Kim et al, 1998). Since the survival of DN1-DN3 thymocytes is dependent on BCL2 expression, we examined whether the developmental block in differentiation at the DN1/DN2 transition could be overcome by a BCL2 transgene. BCL2 transgenic FL precursors were transduced with MSCV-GW and TLX1 retroviral vectors and sorted for GFP expression to greater than 95% purity. FTOCs were set up and analyzed for GFP positivity and T cell differentiation antigens. In both MSCV-GW and TLX1 FTOCs, cell recovery was greater on day 7 and day 18 than in previous experiments with BALB/c FL precursors (data not shown), indicating that the BCL2 transgene was conferring a survival advantage to the transduced cells. However, on day 7 of culture, GFP percentages were reduced in TLX1 FTOCs relative to input levels and control FTOCs (86.2% vs 71.3% for MSCV-GW and TLX1 respectively, P < 0.011; Fig 5C). Although the number of thymi evaluated was small (6 for each group), the trend was observed in all of the lobes analyzed. Two out of six of the TLX1-expressing FTOCs exhibited DN differentiation into DN2 and DN3, although progression into DN3 appeared to be delayed relative to MSCV-GW FTOCs (Fig 5B, upper plots).
Thymocytes were harvested and analyzed at day 18 from MSCV-GW and TLX1 FTOCs in 2 independent experiments. Despite the expression of transgenic BCL2, the percentage of GFP+ cells was markedly less in the TLX1 cultures relative to the controls (32.5% vs 92.2%, P < 0.0001; Fig 5C). Unlike earlier experiments with BALB/c FL precursors, however, sufficient numbers of GFP+ cells were recovered from the TLX1 cultures for analysis, indicating that BCL2 can prevent TLX1-associated thymocyte apoptosis to a certain degree. Based on expression of CD4, CD8 and CD3 at day 18 of FTOC, all thymocyte intermediate populations were represented in the TLX1 as well as the control cultures (data not shown), demonstrating that some differentiation did occur. Even so, a population of CD44+CD25+ DN2 cells persisted in the majority of lobes analyzed in day 18 TLX1 FTOCs (24.7% vs 8.8%, P < 0.0076; Fig 5B, lower plots and Fig 5D).
Ectopic expression of TLX1 in committed DN thymocytes inhibits further differentiation
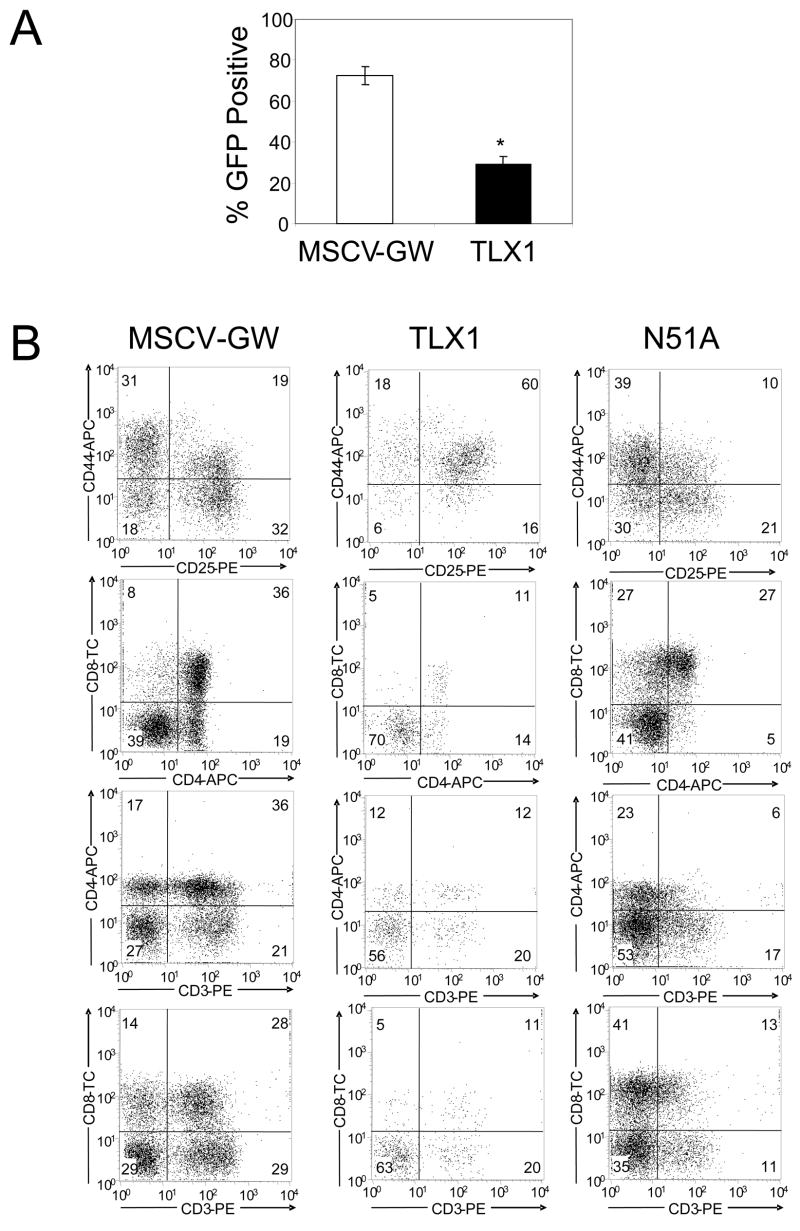
In an attempt to circumvent the TLX1-induced DN1/DN2 thymocyte block, DN thymocytes from 4–6 week old mice, which consist of greater than 80% DN3 and DN4 thymocytes, were transduced with the TLX1 vector. Two independent experiments were performed and GFP expression was analyzed after 15 days in FTOCs. The percentage of GFP+ cells in the TLX1 cultures (~29%) was significantly lower than in the MSCV-GW cultures (~73%; P < 0.00001; Fig 6A).
Fig 6.
TLX1 blocks further development of committed DN thymocytes. (A) DN thymocytes from BALB/c mice were transduced with MSCV-GW or TLX1 and used in FTOC experiments. Two independent experiments were performed and GFP expression was analyzed after 15–18 days. Data represent the mean ± SEM of GFP expression in MSCV-GW- or TLX1 retroviral vector-transduced thymocytes from 8–10 thymi. (B) TLX1 inhibits differentiation of BCL2 transgenic thymocytes. DN thymocytes from a 4 week old BCL2 transgenic mouse were transduced with MSCV-GW, TLX1 or TLX1 N51A for FTOCs. Thymocytes were analyzed after 17 days for expression of GFP and T cell differentiation antigens.
DN thymocytes were next isolated from BCL2 transgenic mice and transduced with the MSCV-GW, TLX1 and TLX1 N51A vectors. Cells were sorted for GFP+CD4−CD8− populations, introduced into irradiated FTOC, and analyzed at day 17. As seen in all previous experiments, lower percentages of expression of GFP were observed in the TLX1 FTOCs relative to control FTOCs (MSCV-GW GFP+ mean: 80%; TLX1+GFP+ mean: 11.5%). On the other hand, TLX1 N51A-expressing cells repopulated fetal thymi as well as control cells (TLX1 N51A GFP+ mean: 73%) and normal differentiation of DP and SP populations was observed. Consistent with the FL precursor experiments, enforced expression of TLX1 in BCL2 transgenic thymocytes caused a significant but partial block in differentiation, increasing the CD44+CD25+ DN2 subset and reducing the DP and SP subsets (Fig 6B).
Discussion
TLX1 expression in human hematologic malignancies is exclusive to T-ALL (Salvati et al, 1995; Kees et al, 2003), where it is almost universally associated with transformation of early cortical CD4+CD8+ thymocytes (Ferrando et al, 2002; Asnafi et al, 2004; Soulier et al, 2005). In this study, we sought to investigate the effects of inappropriate TLX1 expression in in vitro FTOC systems that support the full range of primary thymocyte differentiation (Jenkinson & Owen, 1990; Plum et al, 1994; Plum et al, 2000; Owens et al, 2004). Under these experimental conditions, TLX1 retroviral vector-transduced murine FL precursors had the ability to enter and seed the thymus but were consistently blocked at an early stage – the DN1/DN2 transition –during T-cell lineage commitment (Borowski et al, 2002; Ceredig & Rolink, 2002; Varas et al, 2003). A comparable block in differentiation was observed in human thymocytes derived from CD34+ cord blood stem/progenitor cells in hybrid human/murine FTOCs, which were similarly prevented from undergoing differentiation to the CD1a+CD2+ and CD4+CD8+ subsets, reinforcing the findings obtained in the homologous murine system.
Although we had previously shown that hematopoietic-specific expression of TLX1 impedes the differentiation programs of various myeloerythroid progenitors (Hawley et al, 1994a; Hawley et al, 1997; Keller et al, 1998; Yu et al, 2002; Owens et al, 2003), because TLX1 function like that of other homeodomain transcription factors may be influenced by cellular context and availability of cofactors (Allen et al, 2000; Owens & Hawley, 2002), it was important to validate the paradigm for progenitor cells belonging to the T-cell lineage. While it remains a formal possibility that differentiation arrest prior to the DP stage of thymocyte development induced by ectopic TLX1 expression in primary FL precursors is an experimental artifact, we note that we previously demonstrated that retroviral expression of TLX1 in murine bone marrow-derived hematopoietic stem cells transplanted into BALB/c recipients gave rise to T-ALL-like malignancies after long latency in two instances (Hawley et al, 1997). Interestingly, one of the leukemia/lymphomas had a DN1/DN2 phenotype, whereas the other tumor consisted of cells resembling CD4+CD8+ cortical thymocytes with acquired surface CD3 expression (Hawley et al, 1997). The latter finding indicates that the additional mutations required for overt malignancy circumvented the TLX1-mediated DN thymocyte differentiation block in this particular tumor. It is notable that signaling through the Notch1 pathway has been shown to be essential for the DN2 to DP thymocyte transition (Ciofani et al, 2004), and ectopic expression of an activated NOTCH1 gene in transplanted murine and human hematopoietic stem/progenitor cells induces extrathymic development of DP T cells in the murine bone marrow microenvironment (Allman et al, 2001; De Smedt et al, 2002). Of particular relevance to these observations is the mounting evidence that NOTCH1 gene activation is one of the most frequent genetic alterations in human T-ALL, including TLX1+ cases (Weng et al, 2004).
Expression of the DNA binding-defective TLX1 N51A mutant did not block thymocyte differentiation, indicating a requirement for an intact homeodomain. On the other hand, expression of the TLX1 T47I mutant showed that transduced thymocytes could bypass the DN1/DN2 differentiation arrest, but the thymocytes exhibited survival or proliferation defects. This result implies that thymocyte survival and proliferation are regulated by different genetic programs than differentiation, since only a subset of potential TLX1 target genes would be activated by the TLX1 T47I protein (Dear et al, 1993). These findings are consistent with previous results which show that survival, proliferation and differentiation of thymocytes are not strictly coupled, but rather are separately controlled by the activation of overlapping signaling pathways (Mombaerts et al, 1994; Gartner et al, 1999; Sohn et al, 2001; Michie et al, 2001). Mutation of the PIM in TLX1 M5 permitted full thymocyte differentiation, suggesting that cooperative DNA binding with PBX cofactor proteins is needed for TLX1-mediated arrest. A prior role for TLX1-PBX interactions was demonstrated in TLX1-induced focus formation of NIH3T3 fibroblasts (Allen et al, 2000). Collectively, these observations support the hypothesis that specific homeodomain-DNA-cofactor interactions are important for TLX1 disruption of thymocyte differentiation, paralleling our earlier findings in myeloid progenitor cell immortalization experiments (Owens et al, 2003). Nonetheless, these results are somewhat surprising in view of other recent work from our laboratory studying TLX1+ T-ALL cell lines (Riz & Hawley, 2005), which indicated that a significant component of TLX1 transforming function may occur via a homeodomain-independent mechanism involving inhibition of the protein serine/threonine phosphatases PP1 and PP2A (Kawabe et al, 1997). That study showed that TLX1 blockade of PP1/PP2A phosphatase activity indirectly results in the modulation of multiple transcriptional networks, including that downstream of the Wnt-β-catenin signaling cascade which is required for thymocyte progression to the DP T-cell stage (Verbeek et al, 1995), as well as those regulated by Rb-E2F and c-Myc. A major difference between the two study designs is the status of the INK4A-ARF locus, which is inactivated in most T-ALL cases (Cayuela et al, 1996; Krug et al, 2002; Ferrando et al, 2002). It is well appreciated that the Wnt-β-catenin, Rb-E2F, c-Myc and p14/p19ARF pathways communicate with the p53 tumor suppressor protein via feedback loops to affect different cell fate outcomes (Harris & Levine, 2005). Along similar lines, it is noteworthy that p16INK4a-deficient mice exhibit selective expansion of CD4+CD8+ DP thymocytes (Sharpless et al, 2001) whereas inappropriate p16INK4a expression, such as that resulting from oncogenic stress signals, leads to enhanced apoptosis and thymocyte arrest at the CD4−CD8− DN stage (Lagresle et al, 2002). Further examination of this issue in TLX1 FTOC experiments using primary T-cell precursors that are nullizygous for INK4A-ARF (Serrano et al, 1996) or by down-regulating p16INK4a-p14/p19ARF expression through RNA interference technology (Voorhoeve & Agami, 2003) may thus prove informative in this regard.
Disruption of the IL-7Ra results in profound thymic hypoplasia and decreased thymocyte differentiation prior to the DN2 stage (Peschon et al, 1994), whereas disruption of the IL-7Ra-associated common receptor γ chain (γc) in concert with the c-Kit receptor in γc−/− W/W mice results in a complete abrogation of thymocyte development (Rodewald et al, 1997). DN T-cell differentiation arrest by TLX1 was accompanied by increased apoptosis. Coexpression of TLX1 with transgenic BCL2 resulted in partial rescue of cellularity and differentiation through to DN3 in short-term FTOCs. The block in thymocyte differentiation that occurs in IL-7Ra-deficient mice can be partially bypassed by transgenic expression of BCL2 (Maraskovsky et al, 1997; Akashi et al, 1997), while the block in the γc−/−W/W double knockout mice could not be overcome using the Eμ-bcl2-25 strain employed in this study (Rodewald et al, 2001). TLX1-induced T-cell differentiation arrest thus appears analogous to the developmental impairment observed upon inhibition of IL-7R signaling. Therefore, activating mutations in this signaling pathway may be potential cooperating genetic changes in TLX1+ T-ALL (Barata et al, 2004). Recent observations of ABL1 tyrosine kinase activation in certain TLX1+ T-ALL samples by variant chromosomal translocations (Graux et al, 2004; De Keersmaecker et al, 2005; Soulier et al, 2005) are in accordance with this notion (Danial et al, 1995).
In conclusion, the results reported here in relevant experimental models of murine and human T cell development define a role of dysregulated TLX1 expression as contributing to the differentiation arrest exhibited by TLX1+ T-ALLs. However, they also raise questions as to the timing of TLX1 mutations with respect to the other genetic lesions frequently detected in TLX1+ leukemic samples. It will therefore be of much interest to extend this line of investigation to systematically assess the consequences of combinatorial INK4A-ARF inactivation and enforced expression of NOTCH1 and ABL1 on thymocyte differentiation processes and TLX1-driven oncogenesis. The importance of these cooperative genetic aberrations in the multistep transformation process of TLX1+ T-ALL is underscored by the ALL-SIL cell line (Hatano et al, 1991), which carries all of these alterations (Kitagawa et al, 2002; Graux et al, 2004; Weng et al, 2004).
Acknowledgments
We gratefully acknowledge Ali Ramezani for generous provision of reagents and helpful advice. This work was supported in part by National Institutes of Health grants R01HL65519, R01HL66305 and R24RR16209.
References
- Ades SE, Sauer RT. Specificity of minor-groove and major-groove interactions in a homeodomain-DNA complex. Biochemistry. 1995;34:14601–14608. doi: 10.1021/bi00044a040. [DOI] [PubMed] [Google Scholar]
- Akashi K, Kondo M, Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89:1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Allen TD, Zhu YX, Hawley TS, Hawley RG. TALE homeoproteins as HOX11-interacting partners in T-cell leukemia. Leukemia and Lymphoma. 2000;39:241–256. doi: 10.3109/10428190009065824. [DOI] [PubMed] [Google Scholar]
- Allman D, Karnell FG, Punt JA, Bakkour S, Xu L, Myung P, Koretzky GA, Pui JC, Aster JC, Pear WS. Separation of Notch1 promoted lineage commitment and expansion/transformation in developing T cells. Journal of Experimental Medicine. 2001;194:99–106. doi: 10.1084/jem.194.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asnafi V, Beldjord K, Libura M, Villarese P, Millien C, Ballerini P, Kuhlein E, Lafage-Pochitaloff M, Delabesse E, Bernard O, Macintyre E. Age-related phenotypic and oncogenic differences in T-acute lymphoblastic leukemias may reflect thymic atrophy. Blood. 2004;104:4173–4180. doi: 10.1182/blood-2003-11-3944. [DOI] [PubMed] [Google Scholar]
- Barata JT, Silva A, Brandao JG, Nadler LM, Cardoso AA, Boussiotis VA. Activation of PI3K is indispensable for interleukin 7-mediated viability, proliferation, glucose use, and growth of T cell acute lymphoblastic leukemia cells. Journal of Experimental Medicine. 2004;200:659–669. doi: 10.1084/jem.20040789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R, Dastugue N, Busson M, Van Den AJ, Perot C, Ballerini P, Hagemeijer A, Michaux L, Charrin C, Pages MP, Mugneret F, Andrieux J, Talmant P, Helias C, Mauvieux L, Lafage-Pochitaloff M, Mozziconacci MJ, Cornillet-Lefebvre P, Radford I, Asnafi V, Bilhou-Nabera C, Nguyen KF, Leonard C, Speleman F, Poppe B, Bastard C, Taviaux S, Quilichini B, Herens C, Gregoire MJ, Cave H, Bernard OA. t(5;14)/HOX11L2-positive T-cell acute lymphoblastic leukemia. A collaborative study of the Groupe Francais de Cytogenetique Hematologique (GFCH) Leukemia. 2003;17:1851–1857. doi: 10.1038/sj.leu.2403061. [DOI] [PubMed] [Google Scholar]
- Borowski C, Martin C, Gounari F, Haughn L, Aifantis I, Grassi F, von BH. On the brink of becoming a T cell. Current Opinion in Immunology. 2002;14:200–206. doi: 10.1016/s0952-7915(02)00322-9. [DOI] [PubMed] [Google Scholar]
- Cayuela JM, Madani A, Sanhes L, Stern MH, Sigaux F. Multiple tumor-suppressor gene 1 inactivation is the most frequent genetic alteration in T-cell acute lymphoblastic leukemia. Blood. 1996;87:2180–2186. [PubMed] [Google Scholar]
- Ceredig R, Rolink T. A positive look at double-negative thymocytes. Nature Reviews Immunology. 2002;2:888–897. doi: 10.1038/nri937. [DOI] [PubMed] [Google Scholar]
- Cheng L, Du C, Murray D, Tong X, Zhang YA, Chen BP, Hawley RG. A GFP reporter system to assess gene transfer and expression in viable human hematopoietic progenitors. Gene Therapy. 1997;4:1013–1022. doi: 10.1038/sj.gt.3300507. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, Maryanski JL, Zuniga-Pflucker JC. Obligatory role for cooperative signaling by pre-TCR and Notch during thymocyte differentiation. Journal of Immunology. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- Danial NN, Pernis A, Rothman PB. Jak-STAT signaling induced by the v-abl oncogene. Science. 1995;269:1875–1877. doi: 10.1126/science.7569929. [DOI] [PubMed] [Google Scholar]
- De Keersmaecker K, Graux C, Odero MD, Mentens N, Somers R, Maertens J, Wlodarska I, Vandenberghe P, Hagemeijer A, Marynen P, Cools J. Fusion of EML1 to ABL1 in T-cell acute lymphoblastic leukemia with cryptic t(9;14)(q34;q32) Blood. 2005;105:4849–4852. doi: 10.1182/blood-2004-12-4897. [DOI] [PubMed] [Google Scholar]
- De Smedt M, Reynvoet K, Kerre T, Taghon T, Verhasselt B, Vandekerckhove B, Leclercq G, Plum J. Active form of Notch imposes T cell fate in human progenitor cells. Journal of Immunology. 2002;169:3021–3029. doi: 10.4049/jimmunol.169.6.3021. [DOI] [PubMed] [Google Scholar]
- Dear TN, Sanchez-Garcia I, Rabbitts TH. The HOX11 gene encodes a DNA-binding nuclear transcription factor belonging to a distinct family of homeobox genes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:4431–4435. doi: 10.1073/pnas.90.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube ID, Kamel-Reid S, Yuan CC, Lu M, Wu X, Corpus G, Raimondi SC, Crist WM, Carroll AJ, Minowada J, Baker JB. A novel human homeobox gene lies at the chromosome 10 breakpoint in lymphoid neoplasias with chromosomal translocation t(10;14) Blood. 1991;78:2996–3003. [PubMed] [Google Scholar]
- Ferrando AA, Herblot S, Palomero T, Hansen M, Hoang T, Fox EA, Look AT. Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood. 2004a;103:1909–1911. doi: 10.1182/blood-2003-07-2577. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Neuberg DS, Dodge RK, Paietta E, Larson RA, Wiernik PH, Rowe JM, Caligiuri MA, Bloomfield CD, Look AT. Prognostic importance of TLX1 (HOX11) oncogene expression in adults with T-cell acute lymphoblastic leukaemia. Lancet. 2004b;363:535–536. doi: 10.1016/S0140-6736(04)15542-6. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Neuberg DS, Staunton J, Loh ML, Huard C, Raimondi SC, Behm FG, Pui CH, Downing JR, Gilliland DG, Lander ES, Golub TR, Look AT. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell. 2002;1:75–87. doi: 10.1016/s1535-6108(02)00018-1. [DOI] [PubMed] [Google Scholar]
- Gartner F, Alt FW, Monroe R, Chu M, Sleckman BP, Davidson L, Swat W. Immature thymocytes employ distinct signaling pathways for allelic exclusion versus differentiation and expansion. Immunity. 1999;10:537–546. doi: 10.1016/s1074-7613(00)80053-9. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. Journal of Immunology. 1993;150:4244–4252. [PubMed] [Google Scholar]
- Gottardo NG, Jacoby PA, Sather HN, Reaman GH, Baker DL, Kees UR. Significance of HOX11L2/TLX3 expression in children with T-cell acute lymphoblastic leukemia treated on Children's Cancer Group protocols. Leukemia. 2005;19:1705–1708. doi: 10.1038/sj.leu.2403834. [DOI] [PubMed] [Google Scholar]
- Gratiot-Deans J, Ding L, Turka LA, Nunez G. bcl-2 proto-oncogene expression during human T cell development. Evidence for biphasic regulation. Journal of Immunology. 1993;151:83–91. [PubMed] [Google Scholar]
- Graux C, Cools J, Melotte C, Quentmeier H, Ferrando A, Levine R, Vermeesch JR, Stul M, Dutta B, Boeckx N, Bosly A, Heimann P, Uyttebroeck A, Mentens N, Somers R, MacLeod RA, Drexler HG, Look AT, Gilliland DG, Michaux L, Vandenberghe P, Wlodarska I, Marynen P, Hagemeijer A. Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nature Genetics. 2004;36:1084–1089. doi: 10.1038/ng1425. [DOI] [PubMed] [Google Scholar]
- Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- Hatano M, Roberts CW, Kawabe T, Shutter J, Korsmeyer SJ. Cell cycle progression, cell death and T cell lymphoma in HOX11 transgenic mice. Blood. 1992;80:355a. [Google Scholar]
- Hatano M, Roberts CW, Minden M, Crist WM, Korsmeyer SJ. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science. 1991;253:79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- Hawley RG, Fong AZC, Lu M, Hawley TS. The HOX11 homeobox-containing gene of human leukemia immortalizes murine hematopoietic precursors. Oncogene. 1994a;9:1–12. [PubMed] [Google Scholar]
- Hawley RG, Fong AZC, Reis MD, Zhang N, Lu M, Hawley TS. Transforming function of the HOX11/TCL3 homeobox gene. Cancer Research. 1997;57:337–345. [PubMed] [Google Scholar]
- Hawley RG, Hawley TS, Fong AZC, Quinto C, Collins M, Leonard JP, Goldman SJ. Thrombopoietic potential and serial repopulating ability of murine hematopoietic stem cells constitutively expressing interleukin-11. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:10297–10302. doi: 10.1073/pnas.93.19.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley RG, Lieu FHL, Fong AZC, Hawley TS. Versatile retroviral vectors for potential use in gene therapy. Gene Therapy. 1994b;1:136–138. [PubMed] [Google Scholar]
- Hawley TS, Telford WG, Hawley RG. "Rainbow" reporters for multispectral marking and lineage analysis of hematopoietic stem cells. Stem Cells. 2001;19:118–124. doi: 10.1634/stemcells.19-2-118. [DOI] [PubMed] [Google Scholar]
- Jenkinson EJ, Owen JJ. T-cell differentiation in thymus organ cultures. Seminars in Immunology. 1990;2:51–58. [PubMed] [Google Scholar]
- Kawabe T, Muslin AJ, Korsmeyer SJ. HOX11 interacts with protein phosphatases PP2A and PP1 and disrupts a G2/M cell-cycle checkpoint. Nature. 1997;385:454–458. doi: 10.1038/385454a0. [DOI] [PubMed] [Google Scholar]
- Kees UR, Heerema NA, Kumar R, Watt PM, Baker DL, La MK, Uckun FM, Sather HN. Expression of HOX11 in childhood T-lineage acute lymphoblastic leukaemia can occur in the absence of cytogenetic aberration at 10q24: a study from the Children's Cancer Group (CCG) Leukemia. 2003;17:887–893. doi: 10.1038/sj.leu.2402892. [DOI] [PubMed] [Google Scholar]
- Keller G, Wall C, Fong AZC, Hawley TS, Hawley RG. Overexpression of HOX11 leads to the immortalization of embryonic precursors with both primitive and definitive hematopoietic potential. Blood. 1998;92:877–887. [PubMed] [Google Scholar]
- Kennedy MA, Gonzalez-Sarmiento R, Kees UR, Lampert F, Dear N, Boehm T, Rabbitts TH. HOX11, a homeobox-containing T-cell oncogene on human chromosome 10q24. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:8900–8904. doi: 10.1073/pnas.88.20.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lee CK, Sayers TJ, Muegge K, Durum SK. The trophic action of IL-7 on pro-T cells: inhibition of apoptosis of pro-T1, -T2, and -T3 cells correlates with Bcl-2 and Bax levels and is independent of Fas and p53 pathways. Journal of Immunology. 1998;160:5735–5741. [PubMed] [Google Scholar]
- Kitagawa Y, Inoue K, Sasaki S, Hayashi Y, Matsuo Y, Lieber MR, Mizoguchi H, Yokota J, Kohno T. Prevalent involvement of illegitimate V(D)J recombination in chromosome 9p21 deletions in lymphoid leukemia. Journal of Biological Chemistry. 2002;277:46289–46297. doi: 10.1074/jbc.M208353200. [DOI] [PubMed] [Google Scholar]
- Krug U, Ganser A, Koeffler HP. Tumor suppressor genes in normal and malignant hematopoiesis. Oncogene. 2002;21:3475–3495. doi: 10.1038/sj.onc.1205322. [DOI] [PubMed] [Google Scholar]
- Lagresle C, Gardie B, Eyquem S, Fasseu M, Vieville JC, Pla M, Sigaux F, Bories JC. Transgenic expression of the p16(INK4a) cyclin-dependent kinase inhibitor leads to enhanced apoptosis and differentiation arrest of CD4-CD8- immature thymocytes. Journal of Immunology. 2002;168:2325–2331. doi: 10.4049/jimmunol.168.5.2325. [DOI] [PubMed] [Google Scholar]
- Leung BL, Haughn L, Veillette A, Hawley RG, Rottapel R, Julius M. TcRαβindependent CD28 signaling and costimulation require non-CD4-associated Lck. Journal of Immunology. 1999;163:1334–1341. [PubMed] [Google Scholar]
- Lu M, Gong Z, Shen W, Ho AD. The tcl-3 proto-oncogene altered by chromosomal translocation in T-cell leukemia codes for a homeobox protein. The EMBO Journal. 1991;10:2905–2910. doi: 10.1002/j.1460-2075.1991.tb07840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- Michie AM, Soh JW, Hawley RG, Weinstein IB, Zuniga-Pflucker JC. Allelic exclusion and differentiation by protein kinase C-mediated signals in immature thymocytes. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:609–614. doi: 10.1073/pnas.021288598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Anderson SJ, Perlmutter RM, Mak TW, Tonegawa S. An activated lck transgene promotes thymocyte development in RAG-1 mutant mice. Immunity. 1994;1:261–267. doi: 10.1016/1074-7613(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Moore NC, Anderson G, Williams GT, Owen JJ, Jenkinson EJ. Developmental regulation of bcl-2 expression in the thymus. Immunology. 1994;81:115–119. [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens BM, Hawley RG. HOX and non-HOX homeobox genes in leukemic hematopoiesis. Stem Cells. 2002;20:364–379. doi: 10.1634/stemcells.20-5-364. [DOI] [PubMed] [Google Scholar]
- Owens BM, Hawley RG, Spain LM. Retroviral transduction in fetal thymic organ culture. Methods in Molecular Medicine. 2004;105:311–322. doi: 10.1385/1-59259-826-9:311. [DOI] [PubMed] [Google Scholar]
- Owens BM, Zhu YX, Suen TC, Wang PX, Greenblatt JF, Goss PE, Hawley RG. Specific homeodomain-DNA interactions are required for HOX11-mediated transformation. Blood. 2003;101:4966–4974. doi: 10.1182/blood-2002-09-2857. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. Journal of Experimental Medicine. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum J, De Smedt M, Defresne MP, Leclercq G, Vandekerckhove B. Human CD34+ fetal liver stem cells differentiate to T cells in a mouse thymic microenvironment. Blood. 1994;84:1587–1593. [PubMed] [Google Scholar]
- Plum J, De SM, Verhasselt B, Kerre T, Vanhecke D, Vandekerckhove B, Leclercq G. Human T lymphopoiesis. In vitro and in vivo study models. Annals of the New York Academy of Sciences. 2000;917:724–731. doi: 10.1111/j.1749-6632.2000.tb05436.x. [DOI] [PubMed] [Google Scholar]
- Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. New England Journal of Medicine. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- Ramezani A, Hawley TS, Hawley RG. Lentiviral vectors for enhanced gene expression in human hematopoietic cells. Molecular Therapy. 2000;2:458–469. doi: 10.1006/mthe.2000.0190. [DOI] [PubMed] [Google Scholar]
- Riz I, Hawley RG. G1/S transcriptional networks modulated by the HOX11/TLX1 oncogene of T-cells acute lymphoblastic leukemia. Oncogene. 2005;24:5561–5575. doi: 10.1038/sj.onc.1208727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald HR, Ogawa M, Haller C, Waskow C, DiSanto JP. Pro-thymocyte expansion by c-kit and the common cytokine receptor gamma chain is essential for repertoire formation. Immunity. 1997;6:265–272. doi: 10.1016/s1074-7613(00)80329-5. [DOI] [PubMed] [Google Scholar]
- Rodewald HR, Waskow C, Haller C. Essential requirement for c-kit and common gamma chain in thymocyte development cannot be overruled by enforced expression of Bcl-2. Journal of Experimental Medicine. 2001;193:1431–1437. doi: 10.1084/jem.193.12.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvati PD, Ranford PR, Ford J, Kees UR. HOX11 expression in pediatric acute lymphoblastic leukemia is associated with T-cell phenotype. Oncogene. 1995;11:1333–1338. [PubMed] [Google Scholar]
- Schmid I, Uittenbogaart CH, Keld B, Giorgi JV. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. Journal of Immunological Methods. 1994;170:145–157. doi: 10.1016/0022-1759(94)90390-5. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- Sohn SJ, Forbush KA, Pan XC, Perlmutter RM. Activated p56lck directs maturation of both CD4 and CD8 single-positive thymocytes. Journal of Immunology. 2001;166:2209–2217. doi: 10.4049/jimmunol.166.4.2209. [DOI] [PubMed] [Google Scholar]
- Soulier J, Clappier E, Cayuela JM, Regnault A, Garcia-Peydro M, Dombret H, Baruchel A, Toribio ML, Sigaux F. HOXA genes are included in genetic and biologic networks defining human acute T-cell leukemia (T-ALL) Blood. 2005;106:274–286. doi: 10.1182/blood-2004-10-3900. [DOI] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991;67:889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Varas A, Hager-Theodorides AL, Sacedon R, Vicente A, Zapata AG, Crompton T. The role of morphogens in T-cell development. Trends in Immunology. 2003;24:197–206. doi: 10.1016/s1471-4906(03)00033-4. [DOI] [PubMed] [Google Scholar]
- Veis DJ, Sentman CL, Bach EA, Korsmeyer SJ. Expression of the Bcl-2 protein in murine and human thymocytes and in peripheral T lymphocytes. Journal of Immunology. 1993;151:2546–2554. [PubMed] [Google Scholar]
- Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Voorhoeve PM, Agami R. The tumor-suppressive functions of the human INK4A locus. Cancer Cell. 2003;4:311–319. doi: 10.1016/s1535-6108(03)00223-x. [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JP, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Yu WM, Hawley TS, Hawley RG, Qu CK. Immortalization of yolk sac-derived precursor cells. Blood. 2002;100:3828–3831. doi: 10.1182/blood-2002-03-0937. [DOI] [PubMed] [Google Scholar]