Abstract
Theoretical and experimental analyses of deep brain stimulation (DBS) in the subthalamic nucleus (STN) show both excitatory and inhibitory effects on the neural elements surrounding the electrode. Given these observations, the mechanism underlying the therapeutic effect of STN DBS on parkinsonian motor signs remains under debate. One hypothesis suggests abnormal levels of bursting activity in the pallidum play a key role in the development of parkinsonian motor signs and that STN DBS may exert its beneficial effect by modifying this type of activity. We quantified the changes in bursting activity of globus pallidus internus (GPi) and externus (GPe) neurons before and during ineffective (subtherapeutic) and effective (therapeutic) STN DBS in two monkeys rendered parkinsonian by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Compared to pre-stimulation control values, the population mean firing rate increased during therapeutic stimulation significantly in both GPe (from 41.7Hz±2.8 to 71.4Hz±7.8) and GPi (from 58.8Hz±4.2 to 71.5Hz±6.2). The burst rate, however, increased significantly in GPe (from 80.1 bursts/min ±10.0 to 103.1 bursts/min ±11.1) and decreased significantly in GPi (from 104.2 bursts/min ±8.3 to 75.8 bursts/min ±10.8). Although both animals showed improvement in parkinsonian motor signs, changes in rate and bursting activity in GPi were significant only in one animal. These data suggest that while changes in rate and bursting activity may contribute to the improvement in PD motor signs during STN DBS, one cannot explain the therapeutic effects of stimulation in all cases solely on changes in these parameters. Other physiological changes that contribute to its therapeutic effect must also occur.
Keywords: Subthalamic Nucleus, Macaque, Basal Ganglia, Parkinson’s Disease, MPTP, Network
Introduction
Parkinsonian motor signs (akinesia, bradykinesia, rigidity and tremor) have been linked to abnormal neuronal activity in the basal ganglia (DeLong, 1990; Bergman et al., 1994; Wichmann et al., 1994; Brown et al., 2001). Changes in neuronal activity characteristics such as rate and pattern have been shown when comparing normal and parkinsonian animals. In general, the parkinsonian state is characterized by an increased rate in the subthalamic nucleus (STN) and globus pallidus internus (GPi), and a decreased rate in the globus pallidus externus (GPe) and motor thalamus (Elder and Vitek, 2001). Neurons in all three nuclei also exhibit more bursts, or abruptly occurring sequences of closely spaced action potentials and synchronized oscillatory activity in the beta range (Bergman et al., 1994; Nini et al, 1995; Montgomery and Baker, 2000; Soares et al., 2004; Brown et al 2001).
Lesioning or deep brain stimulation (DBS) of the subthalamic region have both been used to successfully treat parkinsonian symptoms (Bergman et al., 1990; Limousin et al.,1998; Benabid et al., 2001a,b; Walter and Vitek, 2004; Alvarez et al., 2005). However, lesioning and DBS generate different effects on neuronal activity of the pallidum. Lesions of the STN decrease pallidal firing rates and remove altered patterns of neuronal activity (Wichmann et al., 1994; Trost et al., 2006), while STN DBS increases pallidal firing rates and tends to organize the timing of pallidal firing relative to the stimulus pulses (Hashimoto et al., 2003). In turn, emphasis has been placed not only on changes in firing rates, but also on changes in the firing pattern to explain the pathological neuronal activity correlate of parkinsonism (Bergman et al., 1994; Nini et al, 1995; Montgomery and Baker, 2000; Soares et al., 2004). Hashimoto et al. (2003) reported that during DBS of the subthalamic region pallidal spikes occurred with a bimodal probability distribution following the stimulus pulses. That manuscript, however, did not address the effect of DBS on the bursting characteristics of pallidal neurons. We therefore extend the original analysis to investigate the higher order bursting characteristics recorded in pallidal neurons during therapeutic high frequency subthalamic DBS.
We examined the differences in firing properties of neurons from GPe and GPi before stimulation and during subtherapeutic and therapeutic high frequency STN DBS in two parkinsonian non-human primates. When comparing pallidal activity without stimulation to pallidal activity during therapeutic stimulation we found reduced bursting in GPi and enhanced bursting in GPe. The transition from subtherapeutic to therapeutic stimulation showed a significant overall shift toward larger decreases in GPi burstiness, increases in GPe burstiness and increases in pallidal firing rates.
Materials and Methods
All surgical procedures and behavioral protocols were approved by the Institutional Animal Care and Use Committee and complied with United States Public Health Service policy on the humane care and use of laboratory animals.
Surgical procedures
Two rhesus monkeys (Macaca mulatta; R7160 and R370) were used in this study. Hashimoto et al. (2003) used these same monkeys in a previous report of neural activity during subthalamic DBS. A hemiparkinsonian syndrome was induced by unilateral intracarotid injections of the neurotoxin 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP, 0.4 - 0.6 mg/kg over the course of ∼10 minutes) during aseptic surgical procedures under isoflurane anesthesia until a stable parkinsonian state characterized by contralateral rigidity and bradykinesia was achieved. A craniotomy was trephined and recording chamber implanted over the craniotomy in a subsequent aseptic procedure under isoflurane anesthesia with the head held in a primate stereotaxic instrument. Stainless steel screws were secured to the skull, and the implant system consisting of screws, recording chamber and head stabilization receptacle was bonded together with dental acrylic. During a 2-week postoperative recovery period, the monkeys were given unlimited food and water, analgesics, and extra fruit. Prophylactic antibiotics were given preoperatively and continued postoperatively for 10 days.
Electrical stimulation and single neuron recording
The locations of the subthalamic nucleus (STN) and nearby structures were identified by mapping the activity of single neurons with a microelectrode during a series of electrode penetrations. During recording sessions the monkeys were seated in a primate chair with the head restrained. Glass-insulated platinum-iridium microelectrodes (impedance 0.5 - 1.0 MΩ at 1 KHz) were advanced by a microdrive (Narishige Scientific Instruments) attached to the recording chamber. Signals from the microelectrode were amplified and filtered using standard techniques. The filtered analog signals were sampled with a resolution of 20μs and stored on a computer hard disk for later offline analysis.
An electrophysiological map of the area around the STN was compiled by classifying neural activity using the same techniques commonly used during human functional neurosurgery (Vitek et al., 1998). A scaled-down version of a DBS lead used in humans (Model 3387, Medtronic Inc.) was then implanted into the subthalamic region as described in Elder et al. (2005). The DBS lead had four metal contacts each with a diameter of 0.76 mm, a height of 0.50 mm, and a separation between contacts of 0.50 mm. The lead was connected to a programmable pulse generator (Itrel II, Medtronic Inc.) implanted subcutaneously between the scapulae. The maximum therapeutic benefit with bipolar stimulation (to minimize stimulation artifacts) was determined by systematically varying contacts, pulse duration, frequency, and voltage as described in Hashimoto et al. (2003). Stimulation using the voltage that ultimately produced the best therapeutic benefit was considered therapeutic stimulation, whereas the maximum reduced voltage where no therapeutic benefit was detected was considered ineffective stimulation. After the DBS lead was implanted, spontaneous activity of single neurons was recorded in the pallidum before, during and after high frequency (136 Hz) stimulation while the monkey sat quietly in the primate chair. All neuronal data was analyzed offline. Stimulation artifacts were removed from the digitized neuronal recordings as described in Hashimoto et al. (2002), and action potentials were discriminated. The sequence of action potential and stimulation times was stored for further analysis. Data from both animals were pooled for combined analysis, except where noted.
Data analysis
Bursting, while not rigidly defined in the literature, is generally characterized as short intervals of time when action potential firing frequency is highly elevated compared to the baseline rate. We identified bursts using a modified version of the rate independent Poisson surprise method (Legendy and Salcman, 1985). Putative bursts were first identified as sequences of interspike intervals (ISI) that were less than the mean ISI of the series. These putative bursts were further refined by calculating the surprise index (SI) of every combination of contiguous spikes and taking the combination with the highest SI (lowest probability of occurrence). Spikes removed from putative bursts during this procedure were reassigned to the adjacent non-burst period. Bursts found in this manner were rejected if they did not consist of a minimum of 3 spikes or have an SI value of at least 3 (Wichmann and Soares, 2006), with the rejected spikes being reassigned to the adjacent non-burst period.
The discharge properties within burst and non-burst periods were analyzed separately (means values are reported +/- SEM). The mean firing rate and ISI coefficient of variation (CV) was calculated for each burst and non-burst period, as well as the time intervals between the onset of burst and non-burst periods. The mean values of these firing parameters during the control and stimulation period were compared using t-tests (α = 0.05). Differences between the proportion of neurons exhibiting changes in these parameters during therapeutic and ineffective stimulation were analyzed using a chi-square test (α = 0.05). The magnitude of parameter changes from control to stimulation conditions was calculated. The mean and distribution of magnitudes for the subtherapeutic and therapeutic stimulation conditions were compared (t-test and Kolmogorov-Smirnov respectively, α = .05). Magnitude distributions were binned, normalized and smoothed with a 5-point moving average.
Results
The goal of this study was to investigate the relationship between therapeutic DBS and the characteristics of bursting in pallidal neurons. Recordings were made from pallidal neurons of two MPTP-treated monkeys prior to and during high frequency stimulation of the subthalamic region. Spontaneous movement increased and muscle tone was reduced for stimulation at 136 Hz at therapeutic voltages (1.8V/210μsec for R7160 and 3.0V/90 μsec for R370), but not at slightly lower voltages (1.4V/210μsec for R7160 and 2.0V/90 μsec for R370; for details see Hashimoto et al. (2003)). Not every cell in this study was recorded at both therapeutic and subtherapeutic voltages. Accordingly, for GPe a total of 38 cells were analyzed, 16 cells using both therapeutic and subtherapeutic voltages, 4 cells using therapeutic voltage only, and 18 cells using subtherapeutic voltage only. For GPi a total of 66 cells were analyzed, 21 cells using both therapeutic and subtherapeutic voltages, 15 cells using therapeutic voltage only, and 30 cells using subtherapeutic voltage only. The therapeutic and subtherapeutic voltages were determined for each animal individually.
Effects of subthalamic DBS on pallidal firing patterns
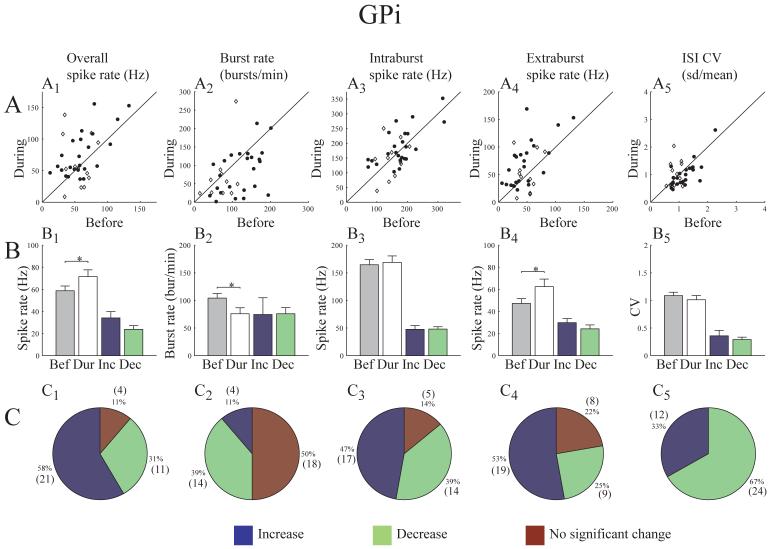
Although high frequency electrical stimulation in the STN generated a range of responses for individual neurons in the pallidum compared to pre-stimulation control values, the population mean firing rate increased significantly in both GPe (from 41.7 Hz ±2.8 to 71.4 Hz ±7.8) and GPi (from 58.8 Hz ±4.2 to 71.5 Hz ±6.2), while the burst rate increased significantly in GPe (from 80.1 bursts/min ±10.0 to 103.1 bursts/min ±11.1) and decreased significantly in GPi (from 104.2 bursts/min ±8.3 to 75.8 bursts/min ±10.8) (Figs. 1 and 2). For each neuron a comparison was made between the overall spike train during the pre-stimulation and on-stimulation epochs. Each cell was classified as having either a significant increase or decrease or no significant change in each characteristic. The mean change was calculated separately for cells where the value increased and for those where the value decreased. Figures 1B and 2B show the pre-stimulation and on-stimulation population mean rates, as well as the mean change in spike firing rate and incidence of bursting for each group. The majority of cells responded to effective stimulation by increasing spike firing rate (70% GPe, 58% GPi) (see Table 1). Significant changes in bursting also occurred in a substantial proportion of pallidal neurons with 39% of GPi cells showing a decrease and 30% of GPe cells showing an increase in the incidence of burst activity in the spike train (see Table 1).
Figure 1.
Changes in GPe spike train characteristics during effective stimulation. A) For each cell, feature values before stimulation are plotted on the x-axis and during stimulation on the y-axis (R7160 - open diamond, R370 closed circle). B) Population mean feature values before and during stimulation (* indicates significance; t-test, α=.05). The last two bars represent the mean change in feature value for subpopulations of cells with a significant increase or decrease respectively. C) Proportion of cells responding with a significant increase or decrease or no significant change (number of cells given in parentheses).
Figure 2.
Changes in GPi spike train characteristics during effective stimulation. A) For each cell, feature values before stimulation are plotted on the x-axis and during stimulation on the y-axis (R7160 - open diamond, R370 closed circle). B) Population mean feature values before and during stimulation (* indicates significance; t-test, α=.05). The last two bars represent the mean change in feature value for subpopulations of cells with a significant increase or decrease respectively. C) Proportion of cells responding with a significant increase or decrease or no significant change (number of cells given in parentheses).
Table 1.
Comparison of population rate changes in response to effective and ineffective stimulation
| GPe | GPi | ||
|---|---|---|---|
| % Increased / % Decreased / % No change | % Increased / % Decreased / % No change | ||
| Spike firing rate | Ineffective Effective |
24 / 44 / 32 70 / 5 / 25 * |
45 / 45 / 10 58 / 31 / 11 |
| Bursting rate | Ineffective Effective |
12 / 12 / 76 30 / 5 / 65 |
20 / 16 / 65 11 / 39 / 50 * |
indicates significance, chi-square test, α=.05
The spike train is a combination of both high frequency burst episodes and lower frequency activity between bursts (extraburst activity). The spike firing rates during burst and extraburst episodes were analyzed separately. The resulting rates for the pre-stimulation and on-stimulation epochs were then compared. Figure 1 shows that GPe cells increased rate significantly both during bursts (70%, mean change 51.7 Hz ±13.2) and between bursts (60%, mean change 30.9 Hz ±10.0). The extraburst firing rate was also significantly increased in GPi cells (from 47.3 Hz ±4.3 to 62.4 Hz ±6.9; Fig. 2). There was no significant change in the intraburst firing rate for GPi cells.
The coefficient of variation (CV) is one measure of the regularity of a spike train. The CV for pre-stimulation and on-stimulation epochs were calculated and compared. Fifty percent of GPe cells and sixty seven percent of GPi cells had a lower CV during therapeutic stimulation. Since the CV is a single measure for a given episode, a classification of no change was not meaningful. However, the population mean was found to increase only slightly in GPe (from 1.3 ±.08 to 1.5 ±.12) and decrease slightly in GPi (from 1.1 ±.08 to 1.0 ±.08). While neither of these differences was found to be statistically significant, the potential relevance of these changes on a physiological level and the effect of decreasing the variability of the spike train in such a large population of neurons remain to be determined.
Stimulation location and pallidal bursting
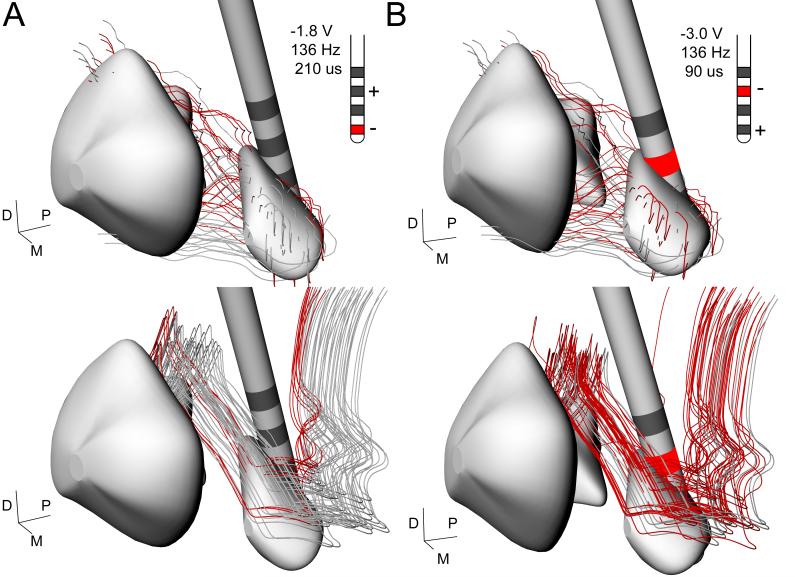
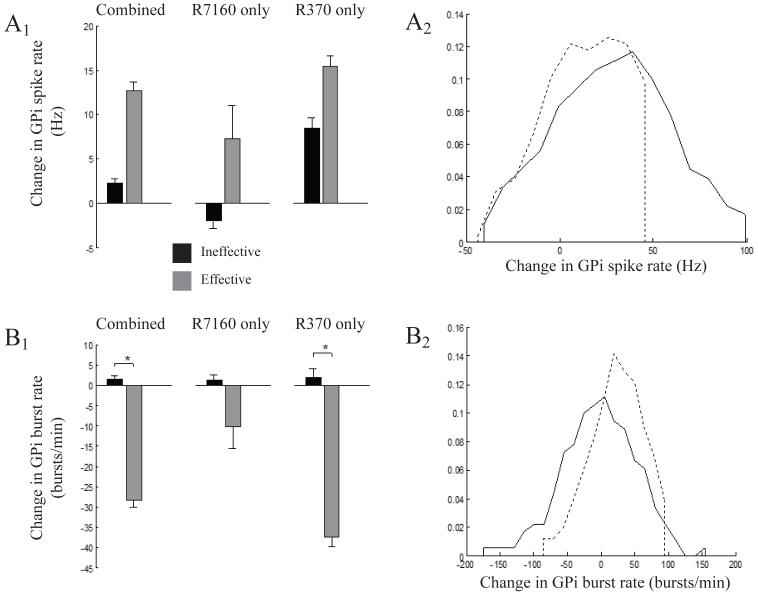
Miocinovic et al (2006) constructed 3D brain atlases of monkeys R7160 and R370, defining the position of the DBS electrode relative to the basal ganglia anatomy. The anatomical models were then coupled to electrical models of the voltage distribution generated in the tissue medium by the stimulation. These electric field models were coupled to biophysical models of STN projection neurons and GPi fibers of passage in the lenticular fasiculus. The model predicted direct axonal activation of both STN and GPi neurons during DBS (Miocinovic et al., 2006). Figure 3 shows a reconstruction of the neurons that were predicted to be activated for each animal given the specific location of the contact used for effective stimulation. The level of STN activation was similar in both monkeys, but activation of pallido-thalamic fibers differed substantially. Direct stimulation of the lenticular fasicuclus (LF) was strong in R370 and weak in R7160 (Fig. 3). The net effects of therapeutic stimulation on GPi spike firing and bursting rates also differed between animals (Fig. 4). In both animals the mean discharge rate of spike firing increased and the burst rate decreased; however, these changes were significant only for R370. Thus, although both animals demonstrated significant improvement in parkinsonian motor signs during DBS, the increase in mean discharge rate and reduction in bursting activity in GPi was only significant for the animal with strong LF activation.
Figure 3.
Impact of stimulation parameters on basal ganglia network components. STN efferents (top) and GPi projection axons (bottom) activated in the model by effective stimulation parameters for R7160 (A) and R370 (B) are shown in red. Inset shows stimulation parameters, contacts and polarity used in vivo.
Figure 4.

Comparison of stimulation effects on GPi for individual subjects. Population mean spike firing rate (A1,B1) and burst rate (A2,B2) before and during effective stimulation (* indicates significance; t-test, α=.05) for cells from R7160 (A) and R370 (B).
Effective versus Ineffective Stimulation
Stimulation with a voltage below the therapeutic level (ineffective stimulation) did not produce any significant changes in the firing pattern of pallidal neurons. The population mean spike firing rate in GPe during no stimulation compared to ineffective stimulation was 42.0 Hz ±3.0 and 38.8 Hz ±4.1, respectively and for GPi it was 59.4 Hz ±2.8 versus 61.7 Hz ±4.0, respectively. Similarly, there was no significant change in the population mean burst rate during no stimulation versus ineffective stimulation conditions in GPe (from 72.7 bursts/min ±7.0 to 72.2 bursts/min ±7.0) or GPi (from 101.4 bursts/min ±7.8 to 102.8 bursts/min ±8.3). However, when comparing effective to ineffective stimulation parameters, significant changes were observed. Table 1 shows a comparison for ineffective versus effective stimulation of the proportion of GPe and GPi cells that had significant increases or decreases, or no significant response. During ineffective stimulation most GPi cells (65%) did not have a significant change in burst rate. During effective stimulation, however, there was a reduction in burst rate in a significantly greater proportion of GPi cells (39% effective versus 16% ineffective, table 1). Two additional measures of burstiness, percentage of spikes in bursts and percentage of time in bursts, also decreased in a significantly greater proportion of GPi cells during effective stimulation (Table 2). In addition to the fraction of cells affected, the size of the rate changes due to stimulation measured in individual cells also differed between effective and ineffective stimulation. The mean and distribution of rate changes in the GPi population were calculated (Fig. 5). Effective stimulation resulted in smaller decreases in spike firing rate when decreases were observed and larger increases. Similarly, burst rate increases were smaller and decreases in burst rate were larger when they occurred. The distribution of observed rate changes in individual cells during effective stimulation was significantly different than ineffective stimulation for GPe and GPi, for both spike firing rate and burst rate.
Table 2.
Comparison of population burst structure changes in response to effective and ineffective stimulation
| GPe | GPi | ||
|---|---|---|---|
| % Increased / % Decreased | % Increased / % Decreased | ||
| % spikes in bursts | Ineffective Effective |
62 / 38 60 / 40 |
57 / 43 28 / 72 * |
| % time in bursts | Ineffective Effective |
50 / 50 65 / 35 |
63 / 37 33 / 67 * |
indicates significance, chi-square test, α=.05
Figure 5.
Magnitude of changes in GPi firing patterns during effective and ineffective stimulation. Comparison of mean change in spike firing rate (A1) and bursting rate (B1) (* indicates significance; t-test, α=.05). The distribution of rate shifts was significantly different for all categories (Kolmogorov-Smirnov, α=.05); shown is Combined for spike firing rate (A2) and bursting rate (B2) during ineffective (dashed line) and effective (solid line) stimulation.
Discussion
The overall effect of STN DBS on the GPi population was a significant increase in spike firing rate and a decrease in burst rate. In GPe, the population mean spike firing rate and burst rate both increased significantly. Therapeutic stimulation differed significantly from subtherapeutic stimulation in several respects. Most notably, a greater proportion of GPe cells increased their average rate of spike firing and a greater proportion of GPi cells decreased their average rate of burst firing. In addition, during therapeutic stimulation a significantly greater proportion of GPi neurons also had a decrease in the percentage of spikes found in bursts and a decrease in the percentage of time spent in bursts. Although both animals showed improvement in parkinsonian motor signs, changes in rate and bursting activity in GPi were significant only in one animal. Thus, the relative role of these changes in producing the observed therapeutic effect is not well defined. We would predict that changes in GPi activity would play a relatively greater role when compared to GPe in producing the therapeutic effect given its position in the basal ganglia thalamocortical network. However, the reduction in burst activity observed in GPi is likely only one of several critical physiological factors that are modified during DBS that contribute to the alleviation of PD motor signs. In addition to changes in mean discharge rate and bursting activity there are a number of other physiological parameters that are altered in the parkinsonian state that may also contribute to the development of parkinsonian motor signs and are affected by stimulation. These include changes in the power and frequency of oscillatory activity, synchronization and receptive field characteristics of neurons. One animal exhibited therapeutic improvement with stimulation that resulted in changes in mean discharge rates and bursting. Those changes trend in the same direction as the other animal, but did not reach significance. Based on this observation, our data provide compelling evidence to suggest that changes in other physiological parameters may be equally important in mediating the beneficial effects of DBS.
Different neuronal elements may be differentially affected by stimulation. Figure 3 illustrates two major neural components that can be stimulated by DBS electrodes in the subthalamic region. Projection neurons surrounding the DBS electrode may be directly excited or indirectly inhibited by the applied electric field (McIntyre et al., 2004b; Miocinovic et al., 2006). Fibers of passage coursing through the region of the electrode will also be activated by DBS (McIntyre et al., 2004c; Miocinovic et al., 2006). Action potentials generated by DBS will propagate in both an orthodromic and antidromic direction. Multiple pathways are likely to be involved in modulation of pallidal activity by subthalamic DBS. Direct activation of STN output would increase the excitatory synaptic drive on both GPe and GPi. Direct activation of the lenticular fasiculus would generate antidromic invasion of GPi as well as orthodromic activation to the pallidal receiving area of the motor thalamus. Stimulation induced trans-synaptic inhibition of STN neurons (via direct activation of GPe afferent inputs into the STN) would reduce ambient excitatory drive on the pallidum. At the same time, GPe afferents to the STN would be antidromically activated. For a given pallidal neuron, the net effect of subthalamic DBS depends on the particular spatial relationship of its afferents and efferents relative to the DBS electric field. Since axonal trajectories are highly irregular, it is not surprising that DBS of the subthalamic region does not have uniform effects on the individual cells of the pallidum. Therapeutic stimulation may therefore depend on a combination of electrode position and stimulation parameters such that a sufficient proportion of pallidal neurons are shifted away from pathological firing patterns.
During effective stimulation, the overall GPi population is shifted to a pattern involving higher spike firing rates, but less bursting. Interestingly, these shifts may be directly regulated by activation of the lenticular fasicuclus (Miocinovic et al., 2006). Results from monkey R370 support this concept where strong electrophysiological changes in GPi and strong theoretical activation of the lenticular fasicuclus were seen. On the other hand, results from monkey R7160 indicate that dynamic changes leading to therapeutic effects are possible through an alternative mechanism as discussed above.
A popular hypothesis on the therapeutic mechanism of DBS is uniform regularization that results in a reduction of the pathological content of the spike train (Montgomery and Baker, 2000; Vitek, 2002; Rubin and Terman, 2004; Grill et al., 2004; McIntyre et al., 2004a). If we use the argument that the coefficient of variation (CV) measures periodicity, then on average, the periodicity of pallidal neurons did not significantly change during stimulation. A periodic signal has a CV of 0 and a Poisson process has a CV of 1. The population mean CV for GPi was near 1 before and during therapeutic stimulation. Still, the proportion of cells that demonstrated an increase in periodicity, as indicated by a decrease in the CV, was significantly increased to 67% during effective versus ineffective stimulation. The CV is not the only measure of regularity. Neuronal responses of GPi neurons tend to occur at specific times relative to the stimulation pulse, but may not occur with each stimulation pulse. Since pallidal spike timing is driven by the stimulus timing, the summation of post-synaptic currents in the thalamus may be made more regular even though GPi activity does not appear significantly more periodic.
During therapeutic DBS, a marked reduction in the incidence of bursts and a reduced number of spikes within each burst were observed. These pattern changes may have important physiological implications for motor symptoms (Kaneoke and Vitek, 1996). A significant decrease in burst frequency alone might be expected to result in a reduced CV. Large differences between spike firing rates during bursts and between bursts can account for a large overall CV (Gabbiani and Koch, 1998; Wilbur and Rinzel, 1983). However, the variation in pallidal spike patterns in our data arises from irregular background firing between bursts more than from a pronounced bimodal interspike interval (ISI) distribution due to bursting. Therefore, a decrease in regular burst firing is not necessarily accompanied by a substantial reduction in CV. In addition, any decrease in CV due to fewer bursts may be offset by an increase from non-periodic spike series occurring in place of the bursts. A reduction in burst frequency may instead have a post-synaptic impact that is distinct from dynamic changes due to an output that is dominated by the timing of the stimulus pulse train and therefore more regular.
The overriding goal of this analysis was to investigate whether a change in the rate and/or bursting of pallidal neural activity could be consistently and significantly related to therapeutic benefit. The quantitative analysis techniques used in this study did find statistically significant changes in rate and bursting. In both animals, the greater GPi spike rate shifts observed during effective stimulation compared to ineffective stimulation were accompanied by large decreases in burst rate as well as the percentage of spikes in bursts and percentage of time in bursts. Shi et al. (2006) measured changes in rate and bursting during behaviorally effective DBS in rat. Mean firing rates in GP (rodent GPe) and SNr (output nucleus of BG along with GPi or EP in rat) did not change significantly, though excitatory and inhibitory responses in individual cells were reported. Bursting, measured during periods following the cessation of effective stimulation, decreased in GP and did not change in SNr. It is unclear whether these differences are attributable to differences in animal model or experimental methods.
Shi et al (2006) did not find mean rate changes in SNr and GP, even though behavioral improvements were observed. Our analysis shows changes in GPe and GPi that accompanied therapeutic stimulation. It is possible that microstimulation of the rat inhibits STN soma, either directly or indirectly, but fails to activate a substantial proportion of STN efferents. Since stimulation has the desired behavioral effect, this same stimulation presumably also activates other pallidofugal pathways in the rat. Thalamic recordings in rat during similar experiments could confirm this therapeutic mechanism. A second important factor that could explain these results is the stimulation protocols used. Hashimoto et al (2003) used ∼30 second periods of stimulation that were classified as either therapeutic or subtherapeutic. Shi et al (2006) used 20 second periods of stimulation that were further broken down in to 3 seconds on/2 seconds off within each period. It is unclear what lingering effects on the dynamics of the network this stimulation induces.
Lesion data cast doubt on the therapeutic effect of spike rate increases; but based on these data it would appear that changes in the incidence of bursting and burst characteristics may be one physiological factor that plays an important role in eliciting the therapeutic benefits observed with DBS. However, given that DBS is similarly effective with a wide range of stimulation parameter settings and electrode locations within the brain, as well as the variety of physiological changes that may be affected during stimulation, there are potentially multiple combinations of pallidal firing pattern changes that could be induced by DBS to generate a therapeutic outcome. This question can only be addressed with further investigation on a larger number of animals.
The increase in rate and decrease in bursting observed in this study may have direct dynamic consequences on thalamic targets and/or induce changes in network properties that rely on the temporal and spatial precision of spike timing (Montgomery, 2005). Our observed reduction in the burstiness of GPi spike trains supports each of these mechanisms. It remains uncertain what properties of pallidothalamic activity are pathological in Parkinson’s disease, though interference in thalamocortical circuits are likely (Magnin et al., 2000; Smith and Sherman, 2002; Rubin and Terman, 2004; Vitek and Giroux 2000). Preliminary analysis of thalamic recordings from these same animals suggests that therapeutic STN DBS reduced bursting and oscillatory activity in the pallidal receiving area (Xu et al., 2006). In turn, a more comprehensive understanding of the network interactions induced by DBS is of great importance for the next steps toward defining the therapeutic mechanisms of this powerful medical technology.
Acknowledgments
This project was supported by the National Institutes of Health (R01 NS037019 and R01 NS047388).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez L, Macias R, Lopez G, Alvarez E, Pavon N, Rodriguez-Oroz MC, Juncos JL, Maragoto C, Guridi J, Litvan I, Tolosa ES, Koller W, Vitek J, DeLong MR, Obeso JA. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain. 2005;128:570–583. doi: 10.1093/brain/awh397. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Koudsie A, Benazzouz A, Piallat B, Krack P, Limousin-Dowsey P, Lebas JF, Pollak P. Deep brain stimulation for Parkinson’s disease. Adv. Neurol. 2001;86:405–412. [PubMed] [Google Scholar]
- Benabid AL, Koudsie A, Benazzouz A, Vercueil L, Fraix V, Chabardes S, Lebas JF, Pollak P. Deep brain stimulation of the corpus luysi (subthalamic nucleus) and other targets in Parkinson’s disease. Extension to new indications such as dystonia and epilepsy. J. Neurol. 2001;248(Suppl 3):III37–47. doi: 10.1007/pl00007825. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. ii. neuronal activity in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J. Neurosci. 2001;21:1033–1038. doi: 10.1523/JNEUROSCI.21-03-01033.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. TINS. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Elder CM, Hashimoto T, Zhang J, Vitek JL. Chronic implantation of deep brain stimulation leads in animal models of neurological disorders. J. Neurosci. Methods. 2005;142:11–16. doi: 10.1016/j.jneumeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Elder CM, Vitek JL. The motor thalamus: alteration of neuronal activity in the parkinsonian state. In: Kultas-Ilinsky K, Ilinsky IA, editors. Basal Ganglia and Thalamus in health and movement disorders. Kluwer/Plenum; 2001. pp. 257–266. [Google Scholar]
- Gabbiani F, Koch C. Principles of spike train analysis. In: Koch C, Segev I, editors. Methods in Neuronal Modeling. Bradford Books, MIT Press; 1998. pp. 313–360. [Google Scholar]
- Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–1140. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Okun MS, Patrick SK, Vitek JL. Stimulation of the subthalamic nucleus changes the firing pattern of pallidal neurons. J Neurosci. 2003;23:1916–1923. doi: 10.1523/JNEUROSCI.23-05-01916.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Elder CM, Vitek JL. A template subtraction method for stimulus artifact removal in high-frequency deep brain stimulation. J. Neurosci. Meth. 2002;113:181–186. doi: 10.1016/s0165-0270(01)00491-5. [DOI] [PubMed] [Google Scholar]
- Kaneoke Y, Vitek JL. Burst and oscillation as diparate neuronal properties. J. Neurosci. Meth. 1996;68:211–223. doi: 10.1016/0165-0270(96)00081-7. [DOI] [PubMed] [Google Scholar]
- Kita H, Nambu A, Kaneda K, Tachibana Y, Takada M. Role of ionotropic glutamatergic and GABAergic inputs on the firing activity of neurons in the external pallidum in awake monkeys. J. Neurophysiol. 2004;92:3069–3084. doi: 10.1152/jn.00346.2004. [DOI] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J. Neurosci. 2005;25:8611–8619. doi: 10.1523/JNEUROSCI.1719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legendy CR, Salcman M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J. Neurophysiol. 1985;53:926–939. doi: 10.1152/jn.1985.53.4.926. [DOI] [PubMed] [Google Scholar]
- Limousin P, Krack P, Pollak P, Benazzouz A, Ardouin C, Hoffmann D, Benabid AL. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. N. Engl. J. Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- Magnin M, Morel A, Jeanmonod D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000;96:549–564. doi: 10.1016/s0306-4522(99)00583-7. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J. Neurophysiol. 2004;91:1457–1469. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Mori S, Sherman DL, Thakor NV, Vitek JL. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin. Neurophysiol. 2004;115:589–595. doi: 10.1016/j.clinph.2003.10.033. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin. Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Miocinovic S, Parent M, Butson CR, Hahn PJ, Russo GS, Vitek JL, McIntyre CC. Computational analysis of subthalamic nucleus and lenticular fasciculus activation during therapeutic deep brain stimulation. J. Neurophysiol. 2006;96:1569–1580. doi: 10.1152/jn.00305.2006. [DOI] [PubMed] [Google Scholar]
- Montgomery EB, Baker KB. Mechanisms of deep brain stimulation and future technical developments. Neurol. Res. 2000;22:259–266. doi: 10.1080/01616412.2000.11740668. [DOI] [PubMed] [Google Scholar]
- Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the mptp model of parkinsonism. J. Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- Rubin JE, Terman D. High frequency stimulation of the subthalamic nucleus eliminates pathological thalamic rhythmicity in a computational model. J. Comput. Neurosci. 2004;16:211–235. doi: 10.1023/B:JCNS.0000025686.47117.67. [DOI] [PubMed] [Google Scholar]
- Shi L, Luo F, Woodward DJ, Chang JY. Basal ganglia neural responses during behaviorally effective deep brain stimulation of the subthalamic nucleus in rats performing a treadmill locomotion test. Synapse. 2006;59:445–457. doi: 10.1002/syn.20261. [DOI] [PubMed] [Google Scholar]
- Smith G, Sherman S. Detectability of excitatory versus in hibitory drive in an integrate-and-fire-or-burst thalamocortical relay neuron model. J. Neurosci. 2002;22:10242–10250. doi: 10.1523/JNEUROSCI.22-23-10242.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J. Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trost M, Su S, Su P, Yen RF, Tseng HM, Barnes A, Ma Y, Eidelberg D. Network modulation by the subthalamic nucleus in the treatment of Parkinson’s disease. Neuroimage. 2006;31:301–307. doi: 10.1016/j.neuroimage.2005.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitek JL, Bakay RA, Hashimoto T, Kaneoke Y, Mewes K, Zhang JY, Rye D, Starr P, Baron M, Turner R, DeLong MR. Microelectrode-guided pallidotomy: technical approach and its application in medically intractable Parkinson’s disease. J. Neurosurg. 1998;88:1027–1043. doi: 10.3171/jns.1998.88.6.1027. [DOI] [PubMed] [Google Scholar]
- Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov. Disord. 2002;17(Suppl 3):S69–72. doi: 10.1002/mds.10144. [DOI] [PubMed] [Google Scholar]
- Vitek JL, Giroux M. Physiology of hypokinetic and hyperkinetic movement disorders: model for dyskinesia. Ann. Neurol. 2000;47:S131–140. [PubMed] [Google Scholar]
- Walter BL, Vitek JL. Surgical treatment for Parkinson’s disease. Lancet Neurol. 2004;3:719–728. doi: 10.1016/S1474-4422(04)00934-2. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Bergman H, DeLong MR. The primate subthalamic nucleus. III. Changes in motor behavior and neuronal activity in the internal pallidum induced by subthalamic inactivation in the MPTP model of parkinsonism. J. Neurophysiol. 1994;72:521–530. doi: 10.1152/jn.1994.72.2.521. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J. Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wilbur WJ, Rinzel J. A theoretical basis for large coefficient of variation bimodality in neuronal interspike interval distributions. J. Theor. Biol. 1983;105:345–368. doi: 10.1016/s0022-5193(83)80013-7. [DOI] [PubMed] [Google Scholar]
- Xu W, Russo GS, Hashimoto T, Zhang J, Vitek JL. Subthalamic nucleus stimulation modulates thalamic neuronal activity in experimental parkinsonism. Society for Neurosciences Abstract. 2006 doi: 10.1523/JNEUROSCI.2027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]