Abstract
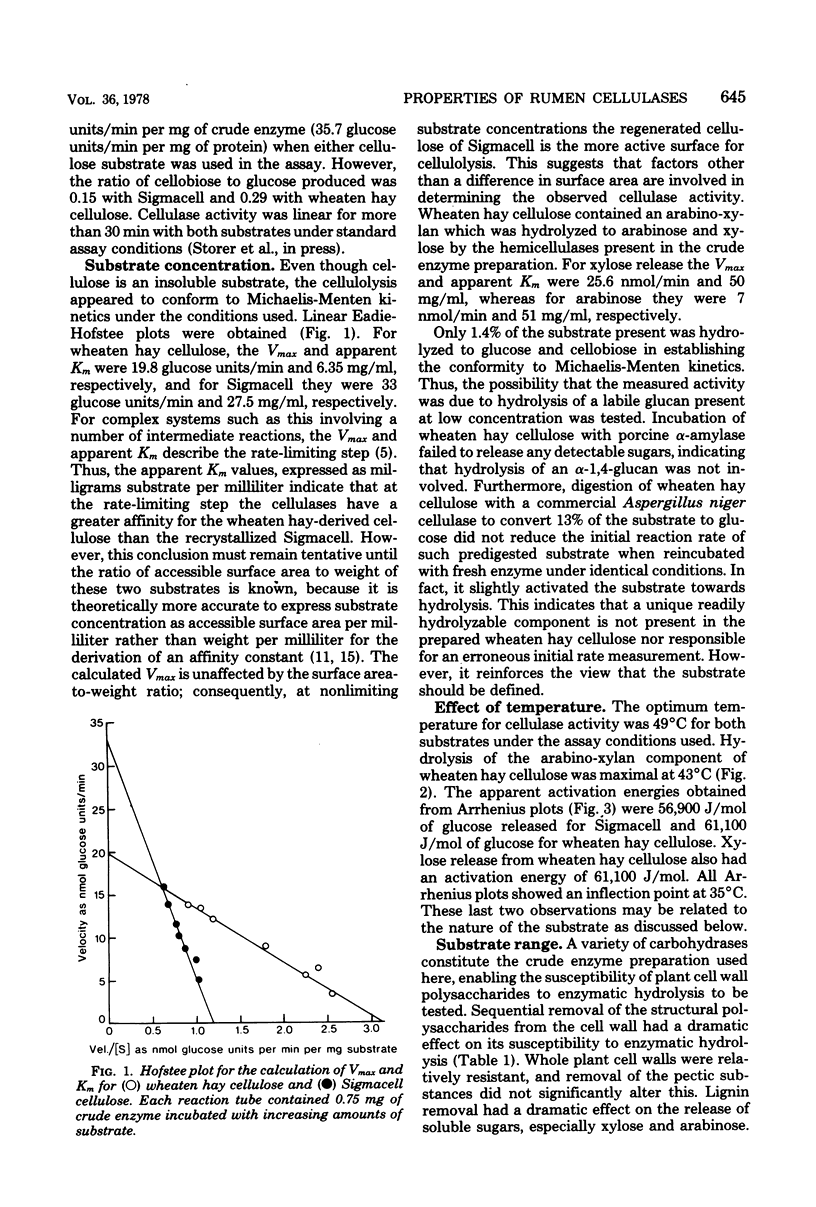
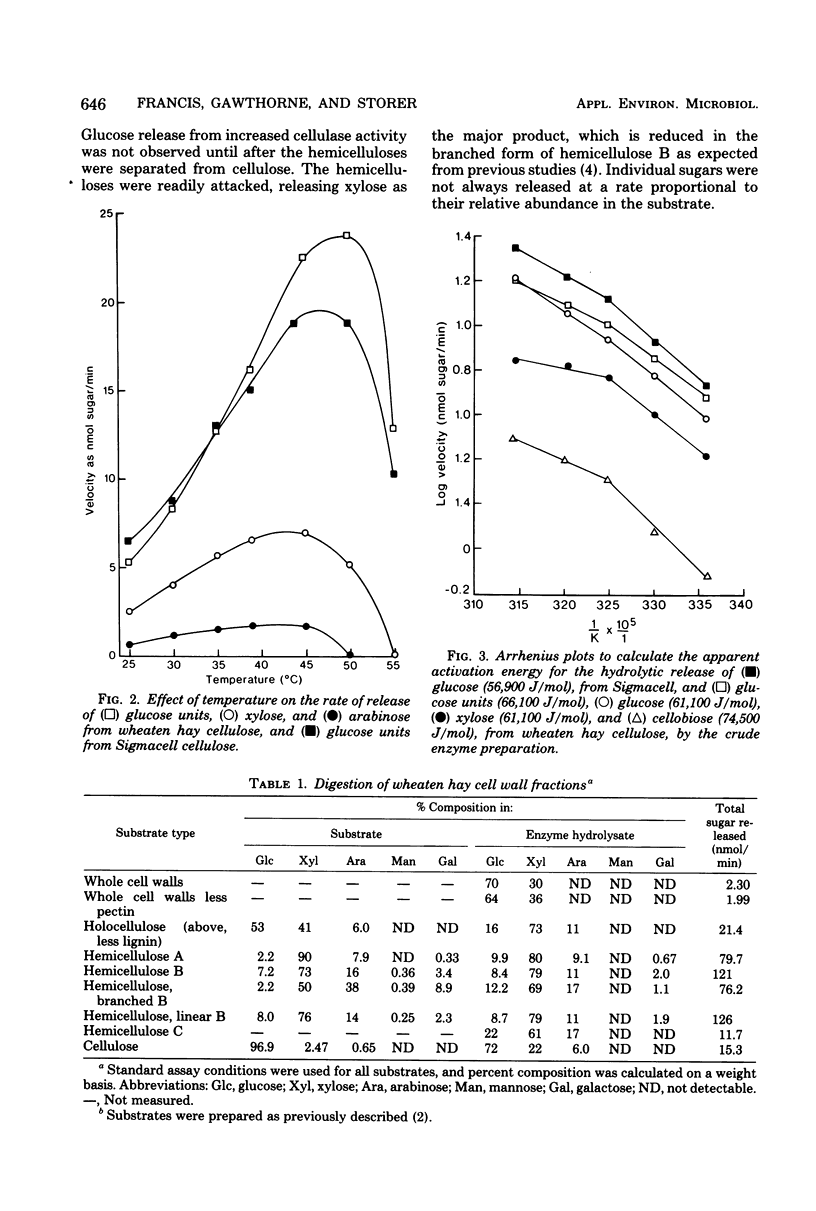
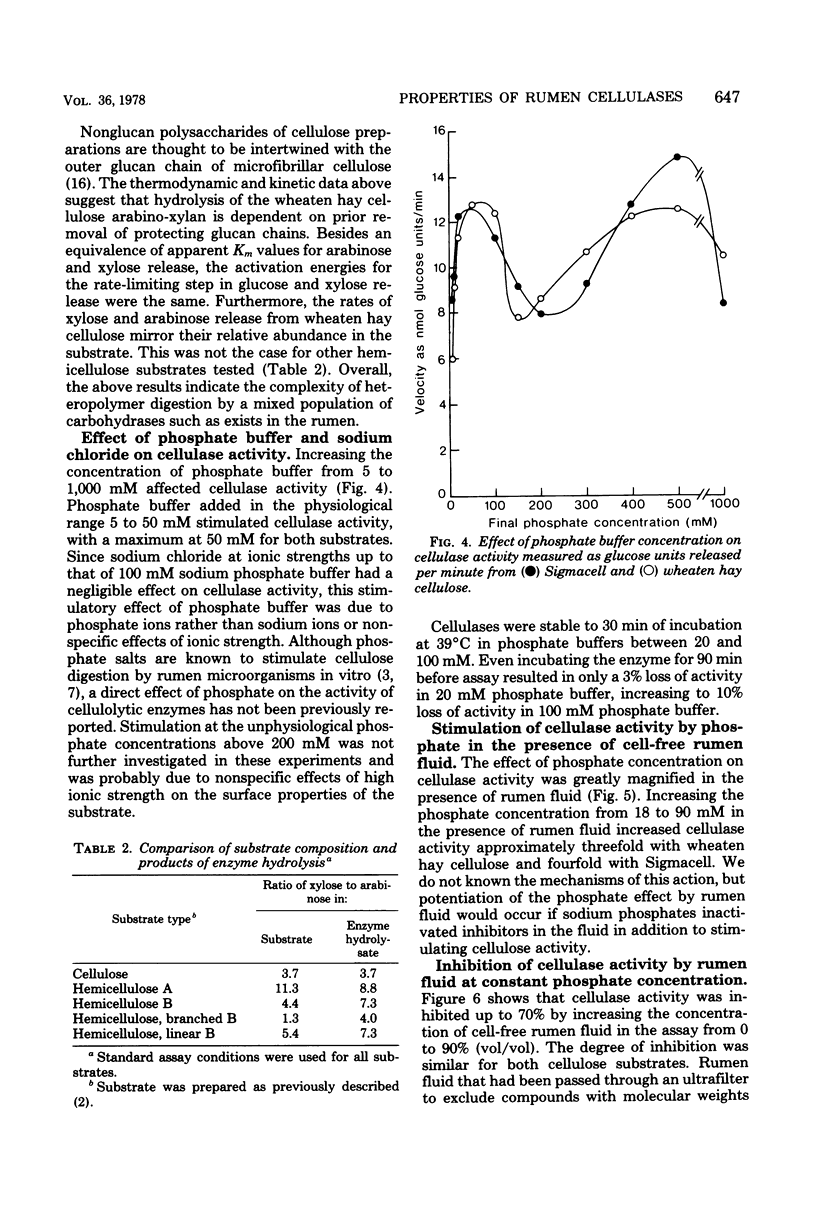
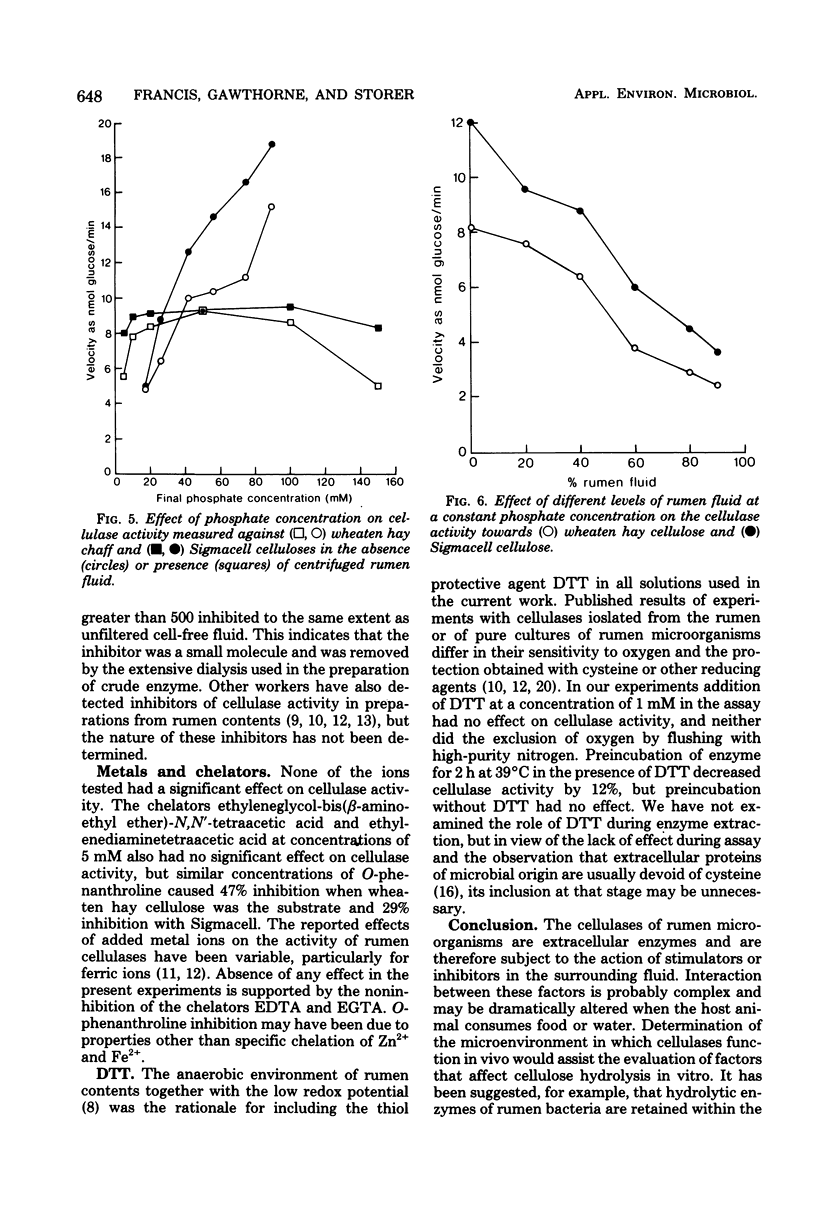
Sodium phosphate buffer was used to extract cellulases from the plant solids fraction of rumen contents. The mixed cellulase preparation had maximal activity at pH 6.9 and 49°C. The Vmax and the apparent Km for wheaten hay cellulose were 19.8 glucose units/min and 6.35 mg/ml, respectively, and for microcrystalline cellulose (Sigmacell) at the same enzyme concentration, they were 33 glucose units/min and 27.5 mg/ml, respectively. For these assays a glucose unit was defined as nanomoles of glucose plus twice the nanomoles of cellobiose. Consideration of thermodynamic and kinetic data suggested that the hydrolysis of a relatively labile arabino-xylan comprising 3% of the wheaten hay cellulose was dependent on prior removal of the protecting β-1,4-glucose chains at the outer surface of the cellulose preparation. Sequential removal of structural polysaccharides from the plant cell wall rendered the latter more susceptible to cellulase activity. Cellulase activity was stimulated by increasing the concentration of phosphate from 5 to 50 mM. The stimulation was magnified in the presence of cell-free rumen fluid. Cellulase activity was not stimulated by calcium, magnesium, iron, zinc, manganese, copper, or cobalt ions and was unaffected by the chelators ethylenediaminetetraacetic acid and ethyleneglycol-bis (β-aminoethyl ether)-N,N′-tetraacetic acid. O-phenanthroline inhibited activity by 30 to 50%, but this may have been due to nonchelate properties. Anaerobic conditions or thiol protective agents were not essential for either the activity or stability of the cellulases during assay. An ultrafiltrable inhibitor of cellulase activity was detected in cell-free rumen fluid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON K. D. True and apparent activation energies of enzymic reactions. Biochim Biophys Acta. 1953 Feb;10(2):221–229. doi: 10.1016/0006-3002(53)90246-7. [DOI] [PubMed] [Google Scholar]
- King K. W. Enzymic degradation of crystalline hydrocellulose. Biochem Biophys Res Commun. 1966 Aug 12;24(3):295–298. doi: 10.1016/0006-291x(66)90153-7. [DOI] [PubMed] [Google Scholar]
- Krishnamurti C. R., Kitts W. D. Preparation and properties of cellulases from rumen microorganisms. Can J Microbiol. 1969 Dec;15(12):1373–1379. doi: 10.1139/m69-248. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leatherwood J. M. Cellulase from Ruminococcus albus and mixed rumen microorganisms. Appl Microbiol. 1965 Sep;13(5):771–775. doi: 10.1128/am.13.5.771-775.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLAREN A. D. ENZYME REACTIONS IN STRUCTURALLY RESTRICTED SYSTEMS. IV. THE DIGESTION OF INSOLUBLE SUBSTRATES BY HYDROLYTIC ENZYMES. Enzymologia. 1963 Nov 15;26:237–246. [PubMed] [Google Scholar]
- Sloneker J. H. Determination of cellulose and apparent hemicellulose in plant tissue by gas-liquid chromatography. Anal Biochem. 1971 Oct;43(2):539–546. doi: 10.1016/0003-2697(71)90285-5. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Marston H. R. Production, absorption, distribution and excretion of vitamin B 12 in sheep. Br J Nutr. 1970 Dec;24(4):857–877. doi: 10.1079/bjn19700092. [DOI] [PubMed] [Google Scholar]
- Smith W. R., Yu I., Hungate R. E. Factors affecting cellulolysis by Ruminococcus albus. J Bacteriol. 1973 May;114(2):729–737. doi: 10.1128/jb.114.2.729-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



