Abstract
Perturbations of plasma IL-10, IL-12, IL-13, TNF-α, and IFN-γ were measured longitudinally in HIV-1 se-roconverting plasma donors and were compared to subjects with symptomatic primary HIV-1 infection. Control groups included uninfected patients with symptoms and risks for primary HIV-1, healthy controls, and asymptomatic plasma donors with primary HCV. IL-10, TNF-α, and IFN-γ rapidly rose in acute HIV-1 infection, while IL-13 predominated in acute HCV. Subjects with symptomatic primary HIV-1 had higher cytokine levels than asymptomatic subjects, statistically significant for TNF-α. Cytokine alterations occurred within 7 days of detectable HIV-1 viremia, emphasizing the need to study the earliest events of infection.
INTRODUCTION
Primary HIV-1 infection represents a period of intense viral replication followed by stabilization of viral load at a setpoint level that is maintained for months to years.1,2 Events occurring at the earliest points of infection may determine the rate at which infected subjects progress to AIDS. Study of the earliest stages of HIV-1 infection is difficult, as only those individuals who present with symptomatic primary HIV-1 are generally available for observation.
A number of investigators have studied cytokine levels in HIV-1 infection. Interleukin (IL)-6 is elevated at all phases of chronic infection.3 IL-10, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ are also elevated in chronic infection, while IL-2 and IL-4 are not.4-7 Individuals identified during symptomatic primary HIV-1 infection show elevations of IL-10, TNF-α, IFN-γ, IFN-α, and IL-1β.8-13 Normal or decreased levels of IL-2, IL-4, IL-6, IL-10, IL-12, tumor growth factor (TGF)-β, and TNF-α have been reported in primary HIV-1.8,9,11,13 Of note, data are conflicting as to whether or not IL-10 and TNF-α levels are elevated in primary HIV-1. Additionally, the macaque model has revealed early increases in IL-6 with most studies also documenting elevations in IL-1β, IL-10, IFN-γ, and TNF-α.14-18
To address the relationship between cytokine secretion and HIV-1 replication at the earliest point of infection, we identified a group of plasma donors at the time of conversion to HIV-1 RNA+ status who claimed to be asymptomatic and were documented to be afebrile at each visit. Cytokine profiles and viral loads were measured over 12-32 days after the first HIV-1 RNA+ specimen, and these parameters were compared with those of symptomatic primary HIV-1-infected subjects identified through the HIV Options project at the University of California, San Francisco (UCSF). Additional control populations included both symptomatic and asymptomatic HIV-1 seronegative subjects and plasma donors identified at the time of conversion to hepatitis C virus (HCV) RNA+ status. We found significant correlations between viral load and IL-10, TNF-α, and IFN-γ at the earliest phase of infection (in plasma donors), and higher levels of these cytokines in symptomatic compared to asymptomatic acutely HIV-1-infected subjects.
MATERIALS AND METHODS
Study subjects
Five groups of individuals were studied for correlation of cytokine profiles and disease status. For subjects whose gender was known (n = 32), all but two were male. These included plasma donors who became infected with either HIV-1 (n = 12) 1 or HCV (n = 6),19 symptomatic subjects presenting with primary HIV-1 infection (n = 10),20 and both symptomatic (n = 10) and asymptomatic (n = 2) HIV-1 seronegative individuals, with five temporally distinct samples drawn from each asymptomatic seronegative individual. Symptomatic patients with primary HIV-1 infection were all febrile at presentation, a median of 11 days (range 7-14) after onset of fever. Symptomatic, seronegative, HIV-1 RNA negative individuals were drawn from a cohort of men with risk factors and symptoms consistent with primary HIV-1 infection.
Viral load and antibody testing
Symptomatic subjects’ viral load was determined by bDNA amplification (Bayer Diagnostics, Tarrytown, NY), while plasma donors’ viral load was measured using National Genetics Institute (NGI, Los Angeles, CA) SuperQuant HIV-1 reverse transcriptase polymerase chain reaction (RT-PCR). HIV-1 antibody testing was performed with an HIV-1/2 third generation assay (Abbott Diagnostics, Abbott Park, IL or Genetic Systems/Bio-Rad, Hercules, CA). Detuned HIV-1 antibody testing was performed using a 20,000-fold dilution of plasma in a less-sensitive enzyme-linked immunosorbent assay (ELISA) format with the Vironostika kit (Biomerieux, Hazelwood, MO) as previously described.21 HCV viral load testing was performed by RT-PCR (UltraQual, NGI).
Cytokine testing
Cytokine measurements were performed on previously frozen plasma samples using kits according to manufacturer’s instructions. IFN-γ was quantitated using Biotrak kits (Amersham Pharmacia, Piscataway, NJ), while IL-10, IL-12, IL-13, and TNF-α were measured using Quantikine HS kits (R&D Systems, Minneapolis, MN).
Statistical analysis
The mean change in each cytokine level for each 10-fold increase in viral load was calculated using generalized estimating equation regression for panel data within each infection cohort (Stata 8.0SE software, Stata Corp., College Station, TX). We used robust standard errors to account for multiple observations per subject. The p-value listed for each graph in Fig. 2 is a measure of the overall association between each cytokine and log of the viral load. We also compared the mean cytokine levels for the first observation of each subject in the HIV-1 symptomatic group to the last observation in the HIV-1 asymptomatic (plasma donors) group using unpaired t-tests for the equality of the means assuming unequal variances.
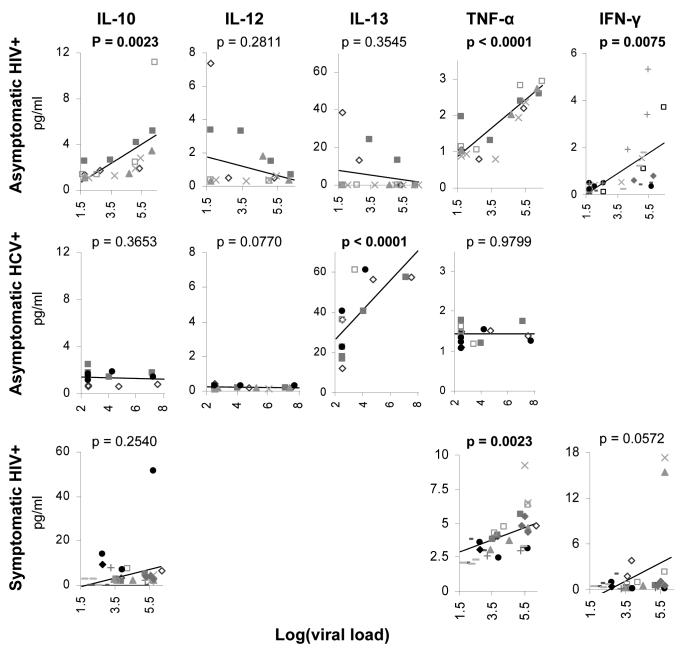
FIG. 2.
Correlation of cytokine levels with viral load during the ramp-up phase of HIV-1 (top row) and HCV (middle row) and during resolution of acute HIV-1 in symptomatic patients treated with HAART (bottom row). Only data points obtained before the peak of viremia are shown for HIV-1 and HCV in the ramp-up phase. p-values represent the overall association between each cytokine and log of the viral load. Significant p-values are highlighted in bold. Patient symbols are consistent with those shown in Fig. 1.
RESULTS
Plasma donors who developed either HIV-1 or HCV presented a unique opportunity to examine the dynamics of viral replication and cytokine evolution at the earliest stage of infection. In the plasma donor industry, collections are routinely held for 60 days before release to ensure that subsequent donations remain negative for viral pathogens such as HIV and HCV. If a donor becomes acutely HIV or HCV infected, the frozen plasma aliquots are not released for clinical use, though a limited number of such seroconversion panels have been aliquoted for research use. Plasma donors identified as HIV or HCV positive were informed of their diagnosis by the local plasma collection center and instructed to obtain medical follow-up with a physician. Follow-up samples from these patients were not available beyond the seroconversion period as plasma collection ceased at that point. Samples were obtained from 9 to 19 days prior to the first HIV-1 RNA+ sample. Consistent with the lack of symptoms or fever in this cohort, cytokine levels were low during the initial phase of infection. IL-10, TNF-α, and IFN-γ levels rose by 2 weeks (Fig. 1, top row). Three individuals exhibited delayed expansion of HIV-1 after the first detectable viremia. For these individuals only IFN-γ was measured, and the rise in IFN-γ levels lagged those with a more rapid rise in viremia. The results for acute HIV-1 contrasted with the course of acute HCV infection, where strong IL-13 secretion was noted (Fig. 1, middle row). Cytokine elevation in acute HCV infection preceded detectable viremia by 1-2 weeks, again in contrast to acute HIV-1 infection.
FIG. 1.
Cytokine levels were measured sequentially during the ramp-up phase of HIV-1 (top row) and HCV (middle row) and during resolution of acute HIV-1 in symptomatic patients treated with HAART (bottom row). Time is listed as days from first PCR-positive sample for HIV-1 and HCV ramp-up phases and days from onset of fever for HIV-1 resolution. The final HIV-1 ramp-up timepoint roughly correlates with the first timepoint of symptomatic patients in the bottom row. Notably, the scale for cytokine levels was changed for HIV-1-infected subjects in the resolution phase due to higher cytokine levels in this group. Patient symbols are consistent across rows, and not all cytokines were measured on every patient.
Symptomatic subjects with primary HIV-1 infection were recruited through the Options project at UCSF. Subjects are eligible for enrollment in the Options study if they present with a positive HIV-1 RNA test and are either seronegative on a standard HIV-1/2 antibody test or have a negative “detuned” anti-HIV-1 antibody test, indicative of recent infection with HIV-1.21 All of the selected subjects were febrile within 14 days of presentation. The most common additional symptoms were nonspecific and included anorexia, fatigue, headache, myalgias, and diarrhea, each present in more than 80% of the subjects enrolled in this study. Each of the symptomatic patients with primary HIV-1 infection was started on highly active antiretroviral therapy (HAART) soon after presentation. HAART led to diminution of cytokine levels over time in symptomatic subjects (Fig. 1, bottom row).
To determine the statistical significance of the longitudinal trends, cytokine levels were correlated to viral load. During the period of rising viral load in HIV-1, significant correlations were seen between viral load and IL-10, IFN-γ, and TNF-α (p = 0.0023, 0.0075, and <0.0001, respectively), with TNF-α associating most strongly (Fig. 2, top row). During acute HCV, IL-13 correlated strongly with viral load (p < 0.0001, Fig. 2, middle row). During the posttreatment phase of acute HIV-1, TNF-α significantly correlated with viral load (p = 0.0023, Fig. 2, bottom row), though not as strongly as earlier in infection.
We hypothesized that subjects with symptomatic primary HIV-1 infection would have higher levels of TNF-α secretion than asymptomatically infected subjects, potentially biasing the immune system to poorer control of HIV-1 replication. The latest timepoint for plasma donors was compared to the earliest measured timepoint for the symptomatic subjects. While not identical, these timepoints were at similar points in viral infection. Two of 11 plasma donors were past the peak viral load at the last timepoint measured and at least 1 of 10 symptomatic primary HIV-1-infected patients were caught prior to the peak of viral replication. Seven of 11 plasma donors at the last time-point and 5 of 10 symptomatic primary infection subjects at the first timepoint were HIV-1 antibody positive by third generation EIA testing. Mean IL-10, TNF-α, and IFN-γ levels (pg/ml) were calculated at the earliest timepoint for symptomatic subjects and at the last timepoint for asymptomatic subjects with acute HIV-1 infection, in HIV-1-seronegative and plasma HIV RNA-negative subjects with viral syndrome symptoms, and in healthy controls (Fig. 3). While there was a trend to higher levels of each of the cytokines measured in symptomatic vs. asymptomatic subjects with primary HIV-1 infection, the changes were only statistically significant for TNF-α (p = 0.02). Interestingly, IL-10, TNF-α, and IFN-γ levels were not statistically significantly different between HIV-1-infected subjects and subjects presenting with acute viral syndrome who were not HIV-1 infected. Cytokine levels were also measured in healthy controls for IL-10 (1.2 ± 0.4), IL-12 (0.2 ± 0), IL-13 (12.7 ± 13.1), and TNF-γ (1.0 ± 0.1). IFN-γ levels were not measured, though normal levels supplied by the kit manufacturer ranged from 0 to 2.6 pg/ml.
FIG. 3.
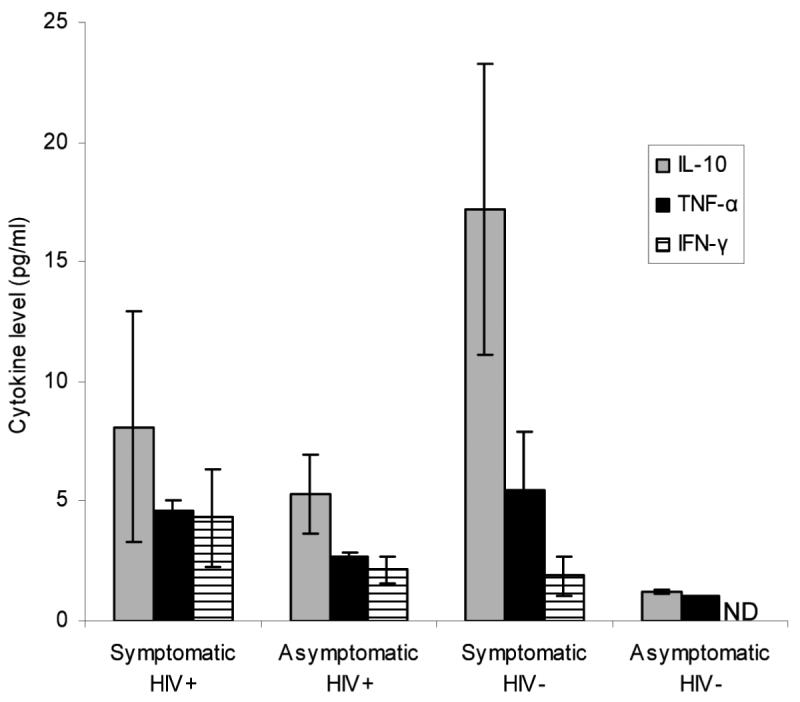
Cytokine levels at the first timepoint for symptomatic HIV+ subjects were compared to those at the last timepoint for plasma donors. Only TNF-α showed a statistically significant elevation in symptomatic compared to asymptomatic subjects. Levels were also measured in high-risk HIV-1 seronegative and RNA negative subjects with viral syndrome symptoms and in normal, healthy controls. ND = not done.
DISCUSSION
These studies of patients at the earliest timepoints of HIV-1 infection confirmed the high levels of IL-10, TNF-α, and IFN-γ seen later in infection22 and contradict studies that have shown normal or low levels of IL-108 and TNF-α9 in primary infection. The levels of these cytokines correlated with temporal evolution of viremia. Surprisingly, a comparison arm following primary HCV infection revealed a strong predominance of IL-13 secretion early in infection without elevated levels of IL-10 or TNF-α.
In the preseroconversion phase of HIV-1, TNF-α levels showed the tightest correlation with viral load, possibly consistent with the proinflammatory, HIV-1-inducing effect of TNF-α.22-25 IL-10 and IFN-γ levels also correlated with viral load during the same period. IFN-γ has synergistic HIV-1 inducing activity with TNF-α in vitro.26 HIV-1 and TNF-α may exert a positive feedback loop on each other, leading to elevated levels of infection during primary infection. This notion is supported by massive levels of CD4+ T cell infection in primary SIV.27,28 IL-12 levels were very low during the earliest period of HIV-1 infection, consistent with a prior report.13 The influence of Th1 (e.g., IL-12) vs. Th2 (e.g., IL-10) biasing cytokines would appear to favor Th2 development at the earliest phase of HIV-1. Also of particular interest were the three plasma donors who showed delayed ramp-up in HIV-1 after the first detectable viremia. The viral dynamics of these subjects have been characterized,1 and the demonstration that IFN-γ secretion is also delayed in these patients implies that the initial low level viremia may be immunosilent. The relatively small number of cytokines measured in the current study precludes a definitive conclusion, and these data will need to be extended using a larger panel of cytokine analyses.
Early HCV infection showed a strong correlation of IL-13 with viral load and time of infection. IL-13 levels began rising as early as 18 days before viremia was detected, likely reflecting both the relatively high threshold of 600 copies/ml plasma for the RNA detection assay and potential local replication in the liver prior to dissemination in the bloodstream.19 The strong elevation of IL-13 in all the observed acute HCV subjects is interesting given the propensity of HCV to cause chronic, fibrotic liver disease. IL-13 has been implicated in the pathogenesis of fibrosis in a schistosomiasis model of hepatic injury.29 Blockade of IL-13 prevents hepatic fibrosis without increasing parasite or egg burden when given during acute or chronic infection.30,31 The finding that IL-13 is selectively elevated in early HCV infection provides a potential clinical target to prevent one of the most troubling outcomes of chronic HCV infection.
To measure whether cytokine levels correlated with presence of symptoms in primary HIV-1 infection, we compared the cytokine profiles for the final timepoint of the HIV-1 plasma donor panels with the first available timepoint of the symptomatic primary HIV-1 infection subjects. These timepoints were similar, though a higher proportion of asymptomatic subjects was preseroconversion and still in the ramp-up phase of viremia. IL-10, TNF-α, and IFN-γ levels were all higher in the symptomatic subjects, with the differences statistically significant only for TNF-α. The lack of statistical significance for IL-10 and IFN-γ may have been due to the relatively small numbers of subjects in the current study. Symptomatic HIV-1 infection has been linked with a worsened prognosis.32-35 It was not possible to determine if the elevated cytokine levels seen in symptomatic patients independently affect viral load in the current study; this would require larger cohorts. Given the positive effect of activation on viral replication, it is tempting to speculate that early elevated cytokine levels, particularly TNF-γ, could provide a target for immune therapy during acute HIV-1 infection.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (U01 AI-41531) and the Doris Duke Charitable Foundation (P.J.N.).
REFERENCES
- 1.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 2.Markowitz M, Mohri H, Mehandru S, et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: A case report. Lancet. 2005;365:1031–1038. doi: 10.1016/S0140-6736(05)71139-9. [DOI] [PubMed] [Google Scholar]
- 3.Breen EC, Rezai AR, Nakajima K, et al. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990;144:480–484. [PubMed] [Google Scholar]
- 4.Graziosi C, Pantaleo G, Gantt KR, et al. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi S, Hamamoto Y, Kobayashi N, Yamamoto N. Serum level of TNF alpha in HIV-infected individuals. AIDS. 1990;4:169–170. [PubMed] [Google Scholar]
- 6.Lahdevirta J, Maury CP, Teppo AM, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1988;85:289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- 7.Navikas V, Link J, Persson C, et al. Increased mRNA expression of IL-6, IL-10, TNF-alpha, and perforin in blood mononuclear cells in human HIV infection. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:484–489. [PubMed] [Google Scholar]
- 8.Barcellini W, Rizzardi GP, Poli G, et al. Cytokines and soluble receptor changes in the transition from primary to early chronic HIV type 1 infection. AIDS Res Hum Retroviruses. 1996;12:325–331. doi: 10.1089/aid.1996.12.325. [DOI] [PubMed] [Google Scholar]
- 9.Biglino A, Sinicco A, Forno B, et al. Serum cytokine profiles in acute primary HIV-1 infection and in infectious mononucleosis. Clin Immunol Immunopathol. 1996;78:61–69. doi: 10.1006/clin.1996.0009. [DOI] [PubMed] [Google Scholar]
- 10.Gaines H, von Sydow MA, von Stedingk LV, et al. Immunological changes in primary HIV-1 infection. AIDS. 1990;4:995–999. doi: 10.1097/00002030-199010000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Graziosi C, Gantt KR, Vaccarezza M, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Sydow M, Sonnerborg A, Gaines H, Strannegard O. Interferon-alpha and tumor necrosis factor-alpha in serum of patients in various stages of HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7:375–380. doi: 10.1089/aid.1991.7.375. [DOI] [PubMed] [Google Scholar]
- 13.Sinicco A, Biglino A, Sciandra M, et al. Cytokine network and acute primary HIV-1 infection. AIDS. 1993;7:1167–1172. doi: 10.1097/00002030-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Benveniste O, Vaslin B, Le Grand R, et al. Interleukin 1 beta, interleukin 6, tumor necrosis factor alpha, and interleukin 10 responses in peripheral blood mononuclear cells of cynomolgus macaques during acute infection with SIVmac251. AIDS Res Hum Retroviruses. 1996;12:241–250. doi: 10.1089/aid.1996.12.241. [DOI] [PubMed] [Google Scholar]
- 15.Cheret A, Le Grand R, Caufour P, et al. Cytokine mRNA expression in mononuclear cells from different tissues during acute SIVmac251 infection of macaques. AIDS Res Hum Retroviruses. 1996;12:1263–1272. doi: 10.1089/aid.1996.12.1263. [DOI] [PubMed] [Google Scholar]
- 16.Cheret A, Le Grand R, Caufour P, et al. RANTES, IFN-gamma, CCR1, and CCR5 mRNA expression in peripheral blood, lymph node, and bronchoalveolar lavage mononuclear cells during primary simian immunodeficiency virus infection of macaques. Virology. 1999;255:285–293. doi: 10.1006/viro.1998.9558. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1 specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. see comments. [DOI] [PubMed] [Google Scholar]
- 18.Zou W, Lackner AA, Simon M, et al. Early cytokine and chemokine gene expression in lymph nodes of macaques infected with simian immunodeficiency virus is predictive of disease outcome and vaccine efficacy. J Virol. 1997;71:1227–1236. doi: 10.1128/jvi.71.2.1227-1236.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glynn SA, Wright DJ, Kleinman SH, et al. Dynamics of viremia in early hepatitis C virus infection. Transfusion. 2005;45:994–1002. doi: 10.1111/j.1537-2995.2005.04390.x. [DOI] [PubMed] [Google Scholar]
- 20.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory tests and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16:1119–1129. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 21.Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA. 1998;280:42–48. doi: 10.1001/jama.280.1.42. [DOI] [PubMed] [Google Scholar]
- 22.Kedzierska K, Crowe SM. Cytokines and HIV-1: Interactions and clinical implications. Antiviral Chem Chemother. 2001;12:133–150. doi: 10.1177/095632020101200301. [DOI] [PubMed] [Google Scholar]
- 23.Folks TM, Clouse KA, Justement J, et al. Tumor necrosis factor alpha induces expression of human immunodeficiency virus in a chronically infected T-cell clone. Proc Natl Acad Sci USA. 1989;86:2365–2368. doi: 10.1073/pnas.86.7.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Israel N, Hazan U, Alcami J, et al. Tumor necrosis factor stimulates transcription of HIV-1 in human T lymphocytes, independently and synergistically with mitogens. J Immunol. 1989;143:3956–3960. [PubMed] [Google Scholar]
- 25.Kinter AL, Poli G, Fox L, Hardy E, Fauci AS. HIV replication in IL-2-stimulated peripheral blood mononuclear cells is driven in an autocrine/paracrine manner by endogenous cytokines. J Immunol. 1995;154:2448–2459. [PubMed] [Google Scholar]
- 26.Han X, Becker K, Degen HJ, Jablonowski H, Strohmeyer G. Synergistic stimulatory effects of tumour necrosis factor alpha and interferon gamma on replication of human immunodeficiency virus type 1 and on apoptosis of HIV-1-infected host cells. Eur J Clin Invest. 1996;26:286–292. doi: 10.1046/j.1365-2362.1996.116271.x. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 28.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 29.Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–2591. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- 30.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273–282. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 31.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–785. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keet IP, Krijnen P, Koot M, et al. Predictors of rapid progression to AIDS in HIV-1 seroconverters. AIDS. 1993;7:51–57. doi: 10.1097/00002030-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Lindback S, Brostrom C, Karlsson A, Gaines H. Does symptomatic primary HIV-1 infection accelerate progression to CDC stage IV disease, CD4 count below 200 × 10(6)/l, AIDS, and death from AIDS? BMJ. 1994;309:1535–1537. doi: 10.1136/bmj.309.6968.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedersen C, Lindhardt BO, Jensen BL, et al. Clinical course of primary HIV infection: Consequences for subsequent course of infection. BMJ. 1989;299:154–157. doi: 10.1136/bmj.299.6692.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanhems P, Hirschel B, Phillips AN, et al. Incubation time of acute human immunodeficiency virus (HIV) infection and duration of acute HIV infection are independent prognostic factors of progression to AIDS. J Infect Dis. 2000;182:334–337. doi: 10.1086/315687. [DOI] [PubMed] [Google Scholar]