Abstract
Background
In recent years, resident cardiac progenitor cells have been identified in, and isolated from the rodent heart. These cells show the potential to form cardiomyocytes, smooth muscle cells, and endothelial cells in vitro and in vivo and could potentially be used as a source for cardiac repair. However, previously described cardiac progenitor cell populations show immature development and need co-culture with neonatal rat cardiomyocytes in order to differentiate in vitro. Here we describe the localisation, isolation, characterisation, and differentiation of cardiomyocyte progenitor cells (CMPCs) isolated from the human heart.
Methods
hCMPCs were identified in human hearts based on Sca-1 expression. These cells were isolated, and FACS, RT-PCR and immunocytochemistry were used to determine their baseline characteristics. Cardiomyogenic differentiation was induced by stimulation with 5-azacytidine.
Results
hCMPCs were localised within the atria, atrioventricular region, and epicardial layer of the foetal and adult human heart. In vitro, hCMPCs could be induced to differentiate into cardiomyocytes and formed spontaneously beating aggregates, without the need for co-culture with neonatal cardiomyocytes.
Conclusion
The human heart harbours a pool of resident cardiomyocyte progenitor cells, which can be expanded and differentiated in vitro. These cells may provide a suitable source for cardiac regeneration cell therapy. (Neth Heart J 2008;16:163-9.)
Keywords: human cardiac progenitor cell, cardiomyocytes, differentiation
Cardiovascular disease is an important cause of mortality in the Western world.1 The central cellular mechanism underlying the development of myocardial dysfunction is a decrease in the number of viable cardiomyocytes as a result of ischaemic injury or ongoing apoptosis, and the inability of remaining cardiomyocytes to compensate the cell loss by cardiomyocyte regeneration. Stem cells have been studied intensively as a source of new cardiomyocytes to ameliorate injured myocardium and improve cardiac function.2-4 The potential therapeutic benefit of stem cell transplantation has been investigated in animal models using bone marrow-derived cells,5-8 cardiac stem cells,5 embryonic stem (ES) cells9,10 and foetal cardiomyocytes11,12 by injecting them at the site of cardiac injury. The encouraging results reported in these animal studies led to the initiation of several clinical trials in which bone marrow derived cells and skeletal myoblasts were investigated.4,13-16 However, the developmental plasticity of bone marrow cells to differentiate into cardiomyocytes has been questioned17,18 and the predominant in vivo effect of bone marrow injection may be neoangiogenesis instead of muscle regeneration. Furthermore, autologous transplantation of skeletal myoblasts is confounded by the possible induction of life-threatening arrhythmias despite partial integration, survival and contribution to cardiac contractility.15 Another source of transplantable cardiomyocytes is human embryonic stem cell (hES) derived cardiomyocytes. Although hES cells can be directed into the cardiomyocyte lineage, with a foetal phenotype,19 their differentiation is not homogenous despite recent improvements in culture methods.20,21 Furthermore, immunogenic, arrhythmogenic and especially ethical problems will limit their clinical use. These obstacles underscore the need to search for new sources of autologous adult cells to generate cardiomyocytes for regeneration of the failing myocardium.
Among the potential candidates are several different cardiomyocyte progenitor cell populations that have been identified in the rodent and human heart.22 Cells expressing stem cell factor receptor c-Kit,5 stem cell antigen-1 (Sca-1),23 homeodomain transcription factor islet-1 (isl-1),24 side population cells (SP),25 and cells able to grow in cardiospheres26 have been suggested to be capable of differentiation into cardiomyocytes, either in vivo, or in vitro. However, recent reports indicate that in vivo, fusion of transplanted progenitor cells with resident adult cardiomyocytes can occur, which may lead to a misinterpretation of the cardiomyogenic differentiation of the stem cells.23 Until now, in vitro differentiation of stem cells into cardiomyocytes has only been achieved by co-culturing the cells with neonatal cardiomyocytes. To avoid misreading the in vitro differentiation capacity, other culture methods are needed to identify true cardiomyocyte generation in vitro.
In the present study, we isolated cardiomyocyte progenitor cells (CMPCs) from human heart tissue using an anti-Sca-1 antibody. Although an Sca-1 epitope in human cells is disputed, the cells we selected using the Sca-1 antibody from both foetal and adult human heart consistently proved to be a homogenous population and amenable to expansion in culture. We show that CMPCs are able to differentiate into mature cardiomyocytes in vitro after 5-azacytidine treatment, even after prolonged passage, thereby excluding artefacts that may result from co-culture. This report demonstrates the existence of human CMPCs in prenatal and postnatal human hearts and their capacity for cardiomyocyte differentiation in vitro.
Material and methods
Isolation and culture of cardiomyocyte progenitor cells from human hearts
To collect human foetal tissue and atrial biopsies, individual permission was obtained using standard informed consent procedures and prior approval of the ethics committee of the University Medical Center Utrecht. Foetal hearts were collected after elective abortion followed by Langendorff perfusion with Tyrode’s solution containing collagenase and protease. Atrial biopsies were minced into small pieces followed by collagenase treatment. After cardiomyocyte depletion of the cell suspension, cardiomyocyte progenitor cells were isolated by magnetic cell sorting (MACS, Miltenyl Biotec, Sunnyvale, CA) using Sca-1-coupled magnetic beads, according to the manufacturer’s protocol. Sca-1+ cells were eluted from the column by washing with PBS supplemented with 2% foetal calf serum (FCS) and cultured on 0.1% gelatin-coated dishes in M199 (Gibco)/EGM (3:1) supplemented with 10% FCS (Gibco), 10 ng/ml basic fibroblast growth factor (bFGF), 5 ng/ml epithelial growth factor (EGF), 5 ng/ml insulin-like growth factor (IGF-1) and 5 ng/ml hepatocyte growth factor (HGF).
To induce differentiation, cells were treated with 5 μM 5-azacytidine (Sigma) for 72 hours in differentiation medium (Iscove’s Modified Dulbecco’s Medium/Ham’s F12 (1:1) (Gibco)) supplemented with L-glutamine (Gibco), 2% horse serum, nonessential amino acids, insulin-transferrin-selenium supplement, and 10-4 M ascorbic acid (Sigma). After induction, the medium was changed every three days.
RNA isolation and RT-PCR
RNA was isolated using TriPure (Roche) as described by the manufacturer. cDNA was synthesised with the iScript cDNA synthesis kit (Biorad). Primers for quantitative reverse transcriptase polymerase chain reaction (RT-PCR) were designed with Beacon Designer 4.0 (Premier Biosoft International). Primer sequences and annealing temperatures are available on request. Quantitative expression of genes was normalised for expression of β-actin. Results were analysed on 10% acrylamide gel stained with ethidium bromide.
Flow cytometric analysis
Cultured CMPCs (passage 7) were trypsinised and 200,000 cells per sample were used for fluorescenceactivated cell sorting (FACS) analysis. The cells were washed twice in wash-buffer (wb: 1% FCS/PBS/ 0.05M azide) and resuspended in 100 μl wb containing antibody. The cells were incubated on ice in the dark for 30 minutes, washed four times with cold wb, resuspended in 250 μl wb and analysed using a Beckman Coulter Cytomics FC500 FACS. Antibodies used were fluorescein isothiocyanate (FITC) or phycoerthrin conjugated against CD14, CD34, CD45, CD133, CD105 (endoglin), Sca-1, and isotype control IgGs, all from Pharmingen BD.
Immunocytochemistry
For immunocytochemistry, coverslips with cultured cells were fixed in 4% paraformaldehyde at room temperature or methyl alcohol at -20 °C. Cells were permeabilised (0.2% Triton X-100/PBS) and blocked (2% bovine serum albumin (BSA), 15-30 minutes). Subsequently, coverslips were incubated overnight at 4 °C with primary antibody in PBS/10% normal goat serum (NGS). The antibodies used recognised Cx40 (Chemicon), Cx43 (Zymed), α-actinin (Sigma), troponin I (Chemicon), and phospho-histone 3 (Abcam). The following day, coverslips were blocked again and incubated with secondary antibody in PBS/10% NGS for two hours. Immunolabelling was performed using Texas Red (TR)- or FITC-conjugated secondary antibodies (Jackson Laboratories). Hoechst dye was used to localise nuclei. All incubation steps were performed at room temperature and in between all incubation steps cells were washed with PBS. Finally, coverslips were mounted in Vectashield (Vector Laboratories) and examined with a Nikon Optiphot- 2 light microscope equipped for epifluorescence.
Immunohistochemistry
Cryo sections (7 μm) were blocked with 1.2% hydrogen peroxide in methanol for 15 min, air dried, and after washing with PBS, blocked with 2% BSA in PBS for 30 minutes. The sections were incubated with the anti-Sca-1 antibody (Pharmingen), diluted 1:100 in blocking solution, o/n at 4°C. PowerVision Poly- HRP-Conjugates (ImmunoVision Technologies) was used as secondary antibody with the Fast 3,3’- diaminobenzidine tablet set (DAB, SIGMA). The sections were counterstained with Meyer’s haematoxylin and mounted in Entellan.
Western blot analysis
Western blot analysis was performed as described previously.27 Detection was by ECL (Amersham). P-H3 and H3 antibodies that specifically recognise phosphorylated histone 3 or total histone 3, respectively, were used 1:5000. Beta-actin detection (1:10,000, Chemicon) was used as a loading control.
Results
Localisation and characterisation of human cardiomyocyte progenitor cells
To identify CMPCs in foetal and adult human heart, we used an anti-Sca-1 antibody that has been shown to recognise mouse cardiac progenitor cells.23 Human CMPCs identified on this basis were found within the atrium, the intra-atrial septum, the atrium-ventricular boundary, and scattered within the epicardial layer (figure 1). To isolate CMPCs, cardiac tissue was enzymatically dissociated, followed by cardiomyocyte depletion. Using a Ferro coupled anti Sca-1 antibody, a cell fraction with a diameter of <50 μm was isolated and subsequently characterised by flow cytometry (figure 2). Foetal CMPCs were negative for CD45, CD34, CD133, and CD14, and positive for CD105 and Sca-1. After isolation, foetal and adult progenitor cells were able to proliferate in vitro as spindle-shaped cells with a high nucleus-to-cytoplasm ratio (figure 3A and B). RT-PCR analysis of foetal progenitor cells revealed that they do not express Oct4, a marker for pluripotent ES cells (figure 3C). However, they did show expression of early cardiac transcription factors Gata4 and Nkx 2.5, while cardiomyocyte-specific genes were not expressed. Adult progenitor cells showed a similar expression pattern (not shown).
Figure 1.
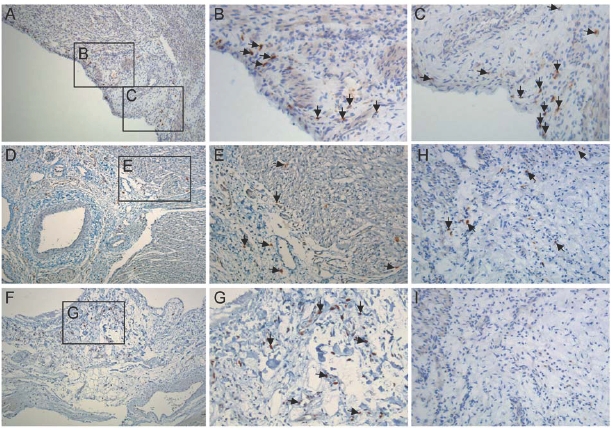
hCMPC in the human heart. (A-G) Immunohistochemistry for Sca-1 in foetal and adult heart. (B, C) High power magnification of areas in A. (D) Atrial ventricular boundary. (E) High power magnification of area in D. (F, H) hCMPCs in biopsy from adult patient. (G) High power magnification of area in F. (I) IgG control. Arrows designate some of the hCMPCs. Magnification: A, D, F, H, I: 100x, B, C, E, G: 200x.
Figure 2.
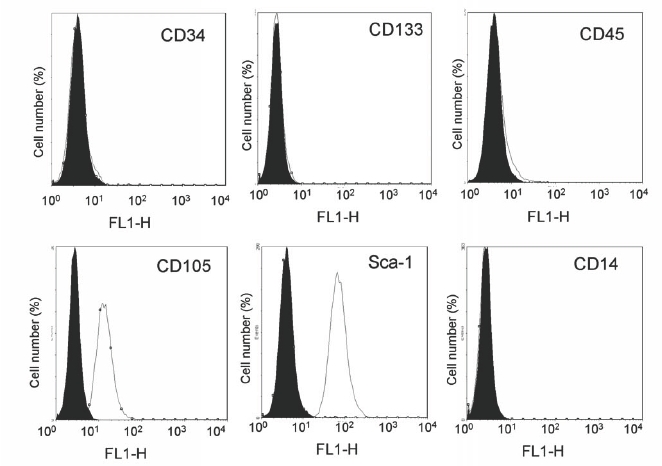
Flow cytometric analysis of (stem) cell marker expression on cultured foetal hCMPCs. Histogram plots are shown with the isotype control in black and the specific signal in white.
Figure 3.
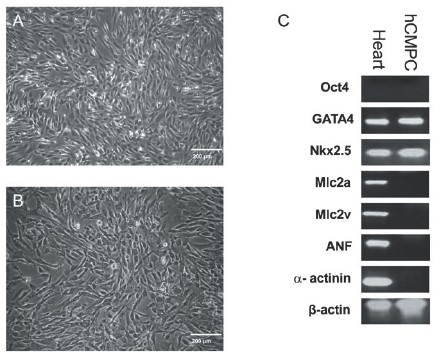
Bright field images of foetal (A) and adult (B) hCMPCs. (C) Semiquantitative RT-PCR on RNA isolated from undifferentiated hCMPCs probed for the expression of the indicated genes.
Cardiomyogenic differentiation of human CMPCs
To initiate differentiation of hCMPCs towards a cardiomyogenic lineage, the proliferation of the cells should be arrested. In P19 embryonal carcinoma cells and mouse Sca-1+ cardiac progenitor cells, cardiomyogenic differentiation can be induced by stimulation with the demethylating agent 5-azacytidine.23,28 5-azacytidine inhibited cell proliferation of CMPCs, as shown by the reduced number of mitotic figures, staining positive for phospho-histone 3 (ser10) (figure 4A), and by Western blot of the same samples (figure 4B). We subsequently found a strongly increased expression of the cardiomyogenic transcription factors Gata4 and Nkx 2.5 (figure 4C).
Figure 4.
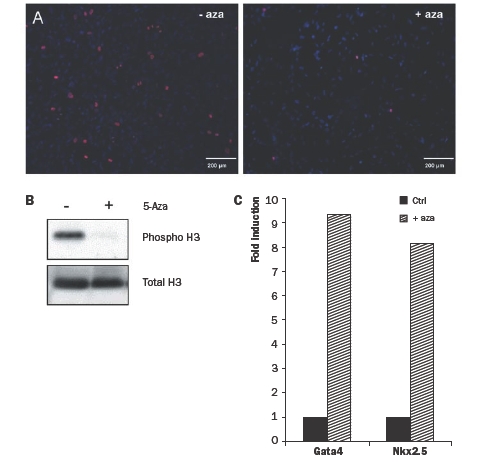
(A) Immunocytochemistry on hCMPCs before (left) and after (right) 5-azacytidine stimulation. Phosphorylated-histone 3 is indicated in red, nuclei in blue. (B) Western blot analysis for phospho-H3 and total H3 on protein from hCMPCs with or without 5-azacytidine stimulation. (C) Quantitative RTPCR on RNA from hCMPCs with (striped bars) or without (black bars) 5-azacytidine stimulation. Expression was normalised for β-actin and fold expression was calculated compared with control.
After stimulation with 5-azacytidine and culture in differentiation medium for several weeks, hCMPCs developed into spontaneously beating aggregates. RT-PCR showed increased expression of several cardiomyocyte-specific genes (figure 5A). Differentiated cells also showed expression of troponin I and α-actinin (figure 5B), indicating that they had become cardiomyocytes. The gap junctional proteins connexin 40 and 43 were expressed at the cell membrane border of hCMPC-derived cardiomyocytes (figure 5C and D), suggesting that these cells are able to functionally couple with each other and other cardiomyocytes, which is necessary to form a functional syncytium.
Figure 5.
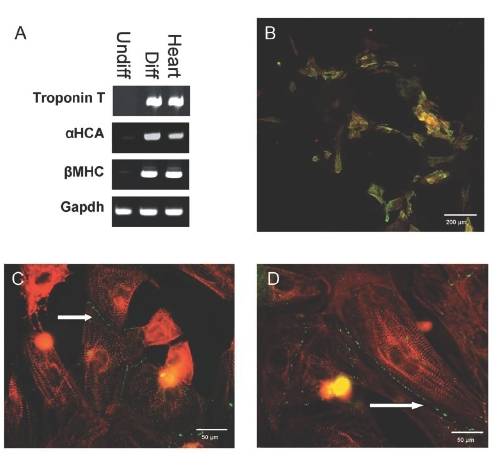
(A) Semiquantitative RT-PCR on RNA isolated from differentiated hCMPCs. (B) Immunolabelling against troponin I (green) and α-actinin (red) in hCMPCs differentiated into cardiomyocytes after 5-azacytidine stimulation. (C and D) Immunolabelling against the connexion isoforms Cx40 and Cx43 (green) and α-actinin (red). Arrows indicate cell membrane localisation gof connexin isoforms.
Discussion
In this study, we report the isolation and cardiomyogenic differentiation of resident cardiac progenitor cells. We show that, upon isolation, these human CMPCs are already committed to the cardiac lineage, as shown by their expression of early cardiac transcription factors. These cells can be efficiently propagated in vitro and differentiated into spontaneously beating cardiomyocytes after 5-azacytidine stimulation, excluding the need for co-culture with neonatal cardiomyocytes.
In the past years, several groups have reported the identification of rodent and human cardiac progenitor cells.29 Due to different methods for isolation and subsequent culture it is difficult to make a direct comparison between these progenitor cell populations. It is very likely, however, that they have been derived from a common mesodermal precursor,30,31 and that current isolation methods result in cell populations that are at a different developmental stage. These cells were shown to differentiate in vitro into cardiomyocytes; however, none of these populations showed spontaneous beating without co-culture with rat neonatal cardiomyocytes. Moreover, they were shown to differentiate towards the endothelial and smooth muscle lineage. In vivo, cardiac progenitor cells also show the capacity to form different cardiac cell types. It still remains unknown, however, which signals are required to drive differentiation. Furthermore, in vivo differentiation remains inefficient, indicating the need to elucidate the fate of cardiac progenitor cells under normal and pathological circumstances. Possibly, the reactivation of the foetal gene expression programme after myocardial infarction or the release of growth factors play an important role in guiding these cells towards their optimal potential. In a separate study, we show that growth factor addition during differentiation greatly enhances cardiomyocyte formation and maturation in vitro.32 The potential of hCMPCs to differentiate into endothelial cells and smooth muscle cells as well, greatly enhances their putative clinical application.
The unexpectedly high frequency with which we were able to isolate and culture hCMPCs from atrial biopsies of adult patients undergoing cardiac surgery opens perspectives for autologous transplantation at a later date than the initial surgery if cultures were carried out under clinically compatible conditions.
Analysis of the differentiation potential of the foetalderived hCMPCs showed that addition of the demethylating agent 5-azacytidine induced the expression of cardiac and contractile genes and spontaneous beating. Expression of gap junctional proteins is almost exclusively found on the sarcolemma of CMPCderived cardiomyocytes. It should, however, be confirmed whether these gap junction channels are functional, e.g. by testing metabolic and electrical coupling. Especially since transplantation of poorly coupled skeletal myoblasts in human hearts in a clinical trial resulted in ventricular tachyarrhythmias in some patients.15 Proper intercellular coupling with host heart cells will therefore be necessary in order to preserve conduction characteristics and will be among the most important criteria for determining whether hCMPCs can be taken forward to clinical trials. A detailed electrophysiological characterisation of hCMPC-derived cardiomyocytes may be required to predict their behaviour after transplantation and integration into host tissue. Human CMPCs, characterised in this study, provide a useful tool to study human cardiomyocyte differentiation and could be used for drug screening. Eventually they may serve as a suitable source for cellular therapy in failing hearts.
Acknowledgements
This work was supported by a VIDI grant (016.056.319, MJG) and a VENI grant (916.36.012, TvV) from the Netherlands Organisation for Scientific Research (NWO), the BSIK programme (Dutch Programme for Tissue Engineering) UGT.6746 (JS, TvV), the Netherlands Heart Foundation (JS, 2003B07304), a ISHR/Servier Fellowship (MR), a European Society of Cardiology Research Grant (MR), and the Van Ruyven Foundation.
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008;117:e25-146. [DOI] [PubMed] [Google Scholar]
- 2.Hassink RJ, Passier R, Goumans MJ, Mummery CL, Doevendans PA. New and viable cells to replace lost and malfunctioning myocardial tissue. Minerva Cardioangiol 2004;52:433-45. [PubMed] [Google Scholar]
- 3.Smits AM, van Vliet P, Hassink RJ, Goumans MJ, Doevendans PA. The role of stem cells in cardiac regeneration. J Cell Mol Med 2005;9:25-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Laake LW, Van Hoof D, Mummery CL. Cardiomyocytes derived from stem cells. Ann Med 2005;37:499-512. [DOI] [PubMed] [Google Scholar]
- 5.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763-76. [DOI] [PubMed] [Google Scholar]
- 6.Fukuda K, Yuasa S. Stem cells as a source of regenerative cardiomyocytes. Circ Res 2006;98:1002-13. [DOI] [PubMed] [Google Scholar]
- 7.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodelling and restore performance of infarcted hearts. Nat Med 2003;9:1195-201. [DOI] [PubMed] [Google Scholar]
- 8.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, et al. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci U S A 2001;98:10344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menard C, Hagege AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet 2005;366:1005-12. [DOI] [PubMed] [Google Scholar]
- 10.Behfar A, Zingman LV, Hodgson DM, Rauzier JM, Kane GC, Terzic A, et al. Stem cell differentiation requires a paracrine pathway in the heart. FASEB J 2002;16:1558-66. [DOI] [PubMed] [Google Scholar]
- 11.Rubart M, Pasumarthi KB, Nakajima H, Soonpaa MH, Nakajima HO, Field LJ. Physiological coupling of donor and host cardiomyocytes after cellular transplantation. Circ Res 2003;92:1217-24. [DOI] [PubMed] [Google Scholar]
- 12.Muller-Ehmsen J, Krausgrill B, Burst V, Schenk K, Neisen UC, Fries JW, et al. Effective engraftment but poor mid-term persistence of mononuclear and mesenchymal bone marrow cells in acute and chronic rat myocardial infarction. J Mol Cell Cardiol 2006;41:876-84. [DOI] [PubMed] [Google Scholar]
- 13.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 2006;113:1287-94. [DOI] [PubMed] [Google Scholar]
- 14.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI). Circulation 2002;106:3009-17. [DOI] [PubMed] [Google Scholar]
- 15.Menasche P. Skeletal myoblast transplantation for cardiac repair. Expert Rev Cardiovasc Ther 2004;2:21-8. [DOI] [PubMed] [Google Scholar]
- 16.Smits JM, De Meester J, Deng MC, Scheld HH, Hummel M, Schoendube F, et al. Mortality rates after heart transplantation: how to compare center-specific outcome data? Transplantation 2003;75:90-6. [DOI] [PubMed] [Google Scholar]
- 17.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature 2004;428:668-73. [DOI] [PubMed] [Google Scholar]
- 18.Laflamme MA, Myerson D, Saffitz JE, Murry CE. Evidence for cardiomyocyte repopulation by extracardiac progenitors in transplanted human hearts. Circ Res 2002;90:634-40. [DOI] [PubMed] [Google Scholar]
- 19.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation 2003;107:2733-40. [DOI] [PubMed] [Google Scholar]
- 20.Passier R, Oostwaard DW, Snapper J, Kloots J, Hassink RJ, Kuijk E, et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells 2005;23:772-80. [DOI] [PubMed] [Google Scholar]
- 21.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res 2002;91:501-8. [DOI] [PubMed] [Google Scholar]
- 22.Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation 2006;113:1451-63. [DOI] [PubMed] [Google Scholar]
- 23.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, et al. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 2003;100:12313-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature 2005;433:647-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, et al. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 2005;97:52-61. [DOI] [PubMed] [Google Scholar]
- 26.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, et al. Isolation and Expansion of Adult Cardiac Stem Cells From Human and Murine Heart. Circ Res 2004;95:911-21. [DOI] [PubMed] [Google Scholar]
- 27.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J 2002;21:1743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohtsu Y, Johkura K, Ito K, Akashima T, Asanuma K, Ogiwara N, et al. Stimulation of P19CL6 with multiple reagents induces pulsating particles in vivo. Curr Med Res Opin 2005;21:795-803. [DOI] [PubMed] [Google Scholar]
- 29.van Vliet P, Sluijter JP, Doevendans PA, Goumans MJ. Isolation and expansion of resident cardiac progenitor cells. Expert Rev Cardiovasc Ther 2007;5:33-43. [DOI] [PubMed] [Google Scholar]
- 30.Laugwitz KL, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development 2008;135:193-205. [DOI] [PubMed] [Google Scholar]
- 31.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 2006;127:1137-50. [DOI] [PubMed] [Google Scholar]
- 32.Goumans MJ, de Boer TP, Smits AM, van Laake W, van Vliet P, Metz CH, et al. TGFb1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res 2008. In press. [DOI] [PubMed] [Google Scholar]


