Abstract
The prevalence of obesity, an established risk factor for several chronic diseases including cancer, has risen dramatically over the past four decades. Dietary change and/or increased physical activity are the most commonly recommended lifestyle-based strategies for preventing or reversing obesity. One of several physiological systems that may be enhanced by dietary change and exercise is the immune system. This study examines the effects of energy restriction (ER; 30% reduction relative to control energy intake) and/or EX (voluntary-wheel running) on systemic and mucosal immune function. Female C57BL/6 mice were randomized into four treatment conditions: 1) controls consuming food ad libitum (AL); 2) AL with access to running wheels (AL+EX); 3) 30% ER; and 4) 30% ER with access to running wheels (ER+EX). Both ER and EX reduced spleen weight and the number of splenic T and B lymphocytes (P<0.05). ER enhanced NK cell function, but significantly reduced Con A-induced T cell proliferation (P<0.05). In contrast, EX significantly enhanced Con A-induced proliferation and cytokine production from Peyer’s patch cells (P<0.05). These data suggest that ER and EX enhance some, but not all components of the immune system, and are likely working via different biological mechanisms to regulate NK and T cell function.
Introduction
Considerable evidence from both human and animal studies indicates that changes in energy balance can influence the risk of cancer and other chronic diseases (1, 2). In humans, diverse epidemiological studies have found that obesity and sedentary behavior increase the risk of cancer at numerous sites, particularly in the colon (3-6). In animal studies, energy restriction (ER)8 and exercise (EX) interventions delay tumorigenesis in spontaneous and chemically induced intestinal tumor models (7-10). The beneficial effects of ER and EX are likely to occur through a variety of mechanisms; however, the extent to which these overlap and the specific pathways associated with cancer prevention are poorly understood. Thus, gaining a better understanding of the effects of ER and EX, individually and combined, on a number of physiological systems is critical to elucidating the underlying biological mechanisms by which ER and EX reduce the risk of tumor formation.
One of several physiological systems that may be enhanced by ER and EX is the immune system (11-13), including both systemic and mucosal immunity. Improved systemic immune function correlates with a reduction in tumor growth in several transplantable tumor models (14, 15), as well as reduced intestinal polyp number and increased survival in a spontaneous intestinal tumor model (16). The mucosal immune system provides protection along the epithelial mucosal surfaces (i.e. respiratory, urogenital, and gastrointestinal tracts). The immune cells found in the Peyer’s patches, as well as other immunological sites in the gut, are in close proximity to the epithelial cells in the small intestine that become transformed during carcinogenesis. Thus, enhancement of mucosal immunity may provide selective protection from the growth and development of intestinal tumors (17). One study exploring the efficacy of probiotics on gut physiology has demonstrated a correlation between enhanced mucosal cytokine production, and a reduction in chemically-induced colon carcinogenesis (18). Together, these data suggest that improved immune function, either systemic or mucosal, is beneficial in the therapy of some established tumors and more importantly, can prevent the formation of neoplastic lesions in both spontaneous and carcinogen-induced models.
Changes in energy balance, ER and moderate EX enhance some components of systemic immunity such as T cell function in aged animals (19, 20) and NK cell function (21-28). However, the age of the animal at the onset, the duration and severity of ER influence immune responsiveness, reviewed in (29). Thus, additional studies are needed to fully characterize the effect of ER on immunity.
The impact of moderate, regular EX on immunity has not been well studied. Many studies have either examined the effect of an acute bout of EX or have studied the effect of high intensity, exhaustive EX on systemic immune function (30), both of which are important in understanding the physiological and immunological effects of training in athletes. However, these studies are less relevant to understanding the mechanism(s) by which moderate EX may impact immunity, and potentially serve as a cancer prevention strategy. Even less is known about the influence of ER and EX on mucosal immune function. To date, only two studies in humans have examined salivary IgA and both have demonstrated that salivary IgA is elevated with regular EX training (31, 32).
The purpose of this study was to investigate the effects of ER (30% energy restriction relative to control intake) and EX (6 weeks of voluntary running) on systemic and mucosal immune function in normal, non-tumor bearing mice. We hypothesized that negative energy balance induced by ER, EX, or the combination of both would enhance systemic and mucosal immune function. We chose to initially test this hypothesis in normal mice to characterize the effects of these interventions on immune function in the absence of tumor since it is well documented that tumors produce immunosuppressive factors (33) that may mask the relationship between ER, EX and immunity. The establishment of an ER- and/or EX-induced enhancement of immunity would provide the foundation for future studies to determine the role of immune function in the anti-cancer effects of ER and/or EX.
Materials and Methods
Animals and treatment regimens
Forty-eight 6-week-old female C57BL/6 mice were obtained from Charles River Breeding Laboratory (Frederick, MD). Upon receipt, mice were randomized to one of four treatment groups and housed individually at the National Cancer Institute-Frederick specific pathogen-free animal facility (Frederick, MD). Animal care was provided in accordance with the procedures outlined in the “Guide for the Care and Use of Laboratory Animals.” The four treatment groups included: 1) controls consuming food ad libitum (AL) (n=12); 2) AL-fed with access to running wheels (AL+EX) (n=11); 3) 30% ER (n=11); and 4) 30% ER with access to running wheels (ER+EX) (n=12). Mice were maintained on the ER and/or EX regimens for 6 weeks and then killed for collection of lymphoid organs. The AL control group was fed AIN-76A diet (34). The ER diet was formulated such that the reduction in calories was entirely from carbohydrates (35). All other components of the ER diet were isonutrient relative to the AL control group when administered in daily aliquots equivalent to 70% of the average daily intake of the AL control mice. Diets were manufactured by Bio-Serv, Inc. Access to running wheels was facilitated by fitting individual cages with a mouse running wheel apparatus (MiniMitter Co.). Wheel revolutions of individual mice were recorded and analyzed using the Vital View software (MiniMitter Co.). Movement was not monitored in mice that did not have access to running wheels. All mice were kept on a reverse 12 h dark (10:00-22:00)/light (22:00-10:00) cycle and provided with access to acidified distilled water ad libitum. Food intake and body weights were monitored weekly, and mice were observed daily for signs of ill health.
Body composition analysis
Mouse carcasses were scanned using a GE Lunar PIXImus Dual-Energy X-ray Absorptiometer (DEXA) to assess bone mineral density, lean mass, fat mass, and percent body fat, as previously described (35, 36).
Isolation of immune cells
Single cell suspensions of splenocytes were prepared from individual mice by mechanical dispersion as previously described (37). Peyer’s patches were excised from the wall of the small intestine and the lymphoid cells were dissociated as previously described (38). The number of Peyer’s patches per intestine from individual mice was counted and recorded. Single cell suspensions of splenocytes and Peyer’s patch cells were counted and the viability determined via trypan blue exclusion. The viability of splenocytes and Peyer’s patch cells from all treatment groups was greater than 95%. The lymphoid cells from the Peyer’s patches of three mice per treatment were pooled for use in the functional assays.
Lymphocyte proliferation assays
1 × 106 lymphocytes from the spleen or Peyer’s patches were incubated in the presence of Con A as previously described (16). To adjust for potential ER and/or EX-induced changes in the percentage of T cells, the number of T cells per well was calculated based on the total number of cells per well (1 × 106) multiplied by the percentage of CD3+ cells in each tissue compartment as determined by flow cytometry in an effort to report changes in proliferation on a per T cell basis. Proliferation data are reported as stimulation indices (SI) per 1 × 106 T cells. SI were calculated by dividing the 3H-thymidine uptake in Becquerel (Bq) from lymphocytes incubated with Con A by the 3H-thymidine uptake from lymphocytes incubated with media alone. The efficiency of the beta counter was 57% for 3H. Data are reported as SI rather than Bq because immune experiments were performed on different experimental days and inter-assay variation by day existed. The proliferation of lymphocytes incubated with media alone ranged from 25-204 Bq. The proliferation of lymphocytes with 0.25, 0.5, 1.0, and 2.0 mg/L of Con A ranged from 0.1-2.3 kBq, 0.1-3.4 kBq, 0.6-3.8 kBq, and 0.7-3.6 kBq, respectively. Each assay was performed in triplicate.
Cytokine production assays
1 × 106 lymphocytes from the spleen or Peyer’s patch were incubated in flat-bottomed, 96-well plates in the presence of increasing concentrations of Con A. Supernatants were harvested after 48 h of incubation with Con A. TNFα, IL-6, IL-10, IL-12p70, and MCP-1 were measured using the Inflammation Cytokine Cytometric Bead Array kit (BD Biosciences) as per manufacturer instructions. IFNγ, IL-2, IL-4, and IL-5 were measured using the Th1/Th2 Cytokine Cytometric Bead Array kit (BD Biosciences) as per manufacturer instructions. Cytokine concentrations were adjusted per 5 × 106 cells in the spleen and 2 × 105 cells in the Peyer’s patches. Each assay was performed in triplicate.
Cytotoxicity assays
NKCC was assessed in standard 4 h chromium release assay as previously described (39), using 100:1, 50:1, 25:1, 12.5:1 E:T ratios. NKCC experiments were performed using 51Cr-labeled YAC-1 target cells. All NKCC measures were adjusted based on the percentage of NK cells (NK1.1+) in the spleen as quantified via flow cytometry. All experiments were performed in triplicate.
Flow cytometric analyses
Single cell suspensions of splenocytes and Peyer’s patch cells were washed once in PBS at 4° C. 1 × 106 cells were stained with saturating concentrations of conjugated antibodies for 30 min at 4° C as previously described (37). Following incubation with the conjugated antibodies, cells were washed twice in PBS and then fixed in 1% paraformaldehyde for flow cytometric analyses. Lymphoid and myeloid cells were gated on forward vs. side scatter and a total of 10,000 events were analyzed on a Becton Dickinson FACScan. Histograms of flow cytometric analyses were plotted and analyzed using Cell Quest software (BD Biosciences).
Statistical analyses
All data are presented as the mean ± SEM. Differences in the mean kilometers run per day between AL and ER mice were tested using Student’s t-test. Effects of ER and EX on body composition (e.g., body weight, lean mass, fat mass, percent body fat, and bone mineral density); lymphocyte proliferation; cytokine production and flow cytometric analyses were examined using two-way ANOVA. Body weight was included as a covariate in the analysis of bone mineral density and spleen weight; lean mass was included as a covariate for fat mass; and Con A level was included as a covariate for SI in the proliferation assays. The variances were unequal for fat mass and body weight. These data were log transformed which eliminated the unequal variances. Using the log transformed data and the untransformed data resulted in qualitatively identical results where diet had as strong effect and exercise had a moderate effect on fat mass and body weight. Therefore the untransformed data were presented to be consistent with the other 8 variables in Tables 1 and 2. Following determination of the ER and/or EX effects using two-way ANOVA, Tukey’s HSD post-hoc test was used to compare individual means among treatment groups. Statistical analyses were performed using SAS JMP. Statistical significance was accepted at the P ≤ 0.05 level.
TABLE 1.
Distance run and body composition measures (mean ± SEM)1 among mice exposed to AL, AL+EX, ER, or ER+EX treatment conditions for 6 weeks
| Treatment Group | Mean Kilometers Run per Day | Body Weight (grams) | Fat Mass2 (grams) | Lean Mass (grams) | Percent Body Fat | Bone Mineral Density3 (g/cm2 × 1000) |
|---|---|---|---|---|---|---|
| AL (n=12) | - | 24.1 ± 1.1 | 7.9 ± 1.1 | 16.1 ± 0.4 | 33.8 ± 3.1 | 49.9 ± 1.6 |
| AL+EX (n=11) | 4.2 ± 0.7 | 23.2 ± 0.8 | 7.9 ± 0.9 | 15.9 ± 0.4 | 32.4 ± 2.4 | 51.3 ± 2.5 |
| ER (n=11) | - | 18.9 ± 0.4 | 4.5 ± 0.4 | 14.8 ± 0.5 | 24.8 ± 1.8 | 45.6 ± 1.8 |
| ER+EX (n=12) | 1.4 ± 0.5 | 17.6 ± 0.5 | 3.9 ± 0.4 | 13.8 ± 0.3 | 24.7 ± 1.7 | 46.4 ± 1.4 |
| P (ER) | 0.004 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 |
| P (EX) | - | 0.117 | 0.210 | 0.254 | 0.576 | 0.004 |
Differences in mean kilometers run per day between groups tested with t-test, group differences remaining variables tested using two-way ANOVA.
Lean mass included as a covariate for fat mass.
Total body weight included as a covariate for bone mineral density.
TABLE 2.
Lymphoid characteristics (mean ± SEM) among mice exposed to AL, AL+EX, ER, or ER+EX treatments for 6 weeks
| Treatment Group | Spleen Weight (mg) | Spleen Weight as a Percentage of Body Weight1 | Total Splenocyte Cell Number (X 106) | Number of Peyer’s Patches per Intestine2 | Total Peyer’s Patch Cell Number2 (X 106) |
|---|---|---|---|---|---|
| AL | 73.9 ± 2.6 | 0.31 ± 0.02 | 109.8 ± 4.9 | 6.8 ± 0.4 | 18.1 ± 4.6 |
| AL+EX | 68.5 ± 3.5 | 0.29 ± 0.01 | 88.1 ± 5.1 | 6.7 ± 0.4 | 17.0 ± 6.7 |
| ER | 57.1 ± 3.1 | 0.30 ± 0.01 | 71.9 ± 5.5 | 7.0 ± 0.6 | 16.0 ± 3.5 |
| ER+EX | 43.1 ± 4.4 | 0.26 ± 0.02 | 57.6 ± 5.6 | 6.9 ± 0.4 | 10.8 ± 3.1 |
| P (ER) | <0.001 | 0.070 | <0.001 | 0.631 | 0.160 |
| P (EX) | 0.009 | 0.029 | 0.001 | 0.908 | 0.260 |
Total body weight included as a covariate for spleen weight.
These data represent the mean ± SEM of 4 pooled groups of 2-3 animals per group.
Results
Energy restriction impacts body composition to a greater extent than EX
ER significantly reduced body weight, fat mass, lean mass, percent body fat (Table 1; P <0.001), and bone mineral density (P=0.002). In contrast, EX only significantly increased bone mineral density (P=0.004). There were no interactive effects of ER and EX on any of the body composition measures shown in Table 1. There was heterogeneity in running activity among mice in both the AL+EX and ER+EX groups, with the distance run by individual mice ranging from 1.1 to 7.7 km/day in the AL+EX group and 0 to 5.2 km/day the ER + EX group.
Energy restriction and EX reduce spleen weight and cellularity
Both ER and EX significantly reduced spleen weight (Table 2; P<0.001 and P=0.009, respectively) and total splenocyte number (Table 2; P<0.001 and P=0.001, respectively). Body weight was significantly reduced by ER but not EX (Table 1); therefore, spleen weight was divided by body weight to adjust for differences in body size among mice in each of the treatment groups (Table 2). EX reduced spleen weight as a percentage of body weight (P=0.029), whereas ER was close but did not reach statistical significance (P=0.070). Although there were robust, statistically significant effects of either ER or EX on splenic weight and splenocyte number, there were no interactive effects of ER plus EX on these parameters (P=0.237 and P=0.478, respectively). In contrast to the splenic measurements, neither ER nor EX altered the number of Peyer’s patches per small intestine or the total number of cells in the Peyer’s patches (Table 2).
Since the total number of splenocytes was significantly reduced by both ER and EX (Table 2), we explored the distribution of leukocytes in the spleen among mice on each of the four treatment groups in an effort to identify the cell types most affected by each treatment (Table 3). ER significantly reduced the number of cells in the lymphoid compartment, including a reduction in the total number of T cells (CD3+) and B cells (CD19+) (P<0.001 and P=0.003, respectively). Within the T cell compartment, ER significantly reduced both the number of CD3+CD4+ (helper) and CD3+CD8+ (cytolytic) T cells (P<0.001 and P=0.019, respectively). However, ER did not alter the number of NK cells (NK1.1+), macrophages (CD11b+I-Ab+), or dendritic cells (CD11c+I-Ab+). In contrast, EX reduced the number of T cells (P=0.022), and in particular the number of CD3+CD8+ cytolytic T cells (P=0.028); but had no effect on the number of CD3+CD4+ helper T cells (P=0.133). There was a reduction in B cell number with EX; however, this effect did not reach statistical significance (P=0.069). EX reduced the number of macrophages in the spleen (P=0.019), but not the number of NK or dendritic cells.
TABLE 3.
Distribution of leukocytes in the spleen (mean ± SEM)1 among mice exposed to AL, AL+EX, ER, or ER+EX treatments for 6 weeks
| Treatment Group | Total T cells (CD3+) | Helper T cells (CD3+CD4+) | Cytolytic T cells (CD3+CD8+) | CD4:CD8 Ratio | B cells (CD19+) | NK cells (NK1.1+) | Macrophages (CD11b+I-Ab+) | Dendritic cells (CD11c+I-Ab+) |
|---|---|---|---|---|---|---|---|---|
| AL (n=12) | 60.7 ± 4.8 | 32.1 ± 2.3 | 24.4 ± 1.9 | 1.3 ± 0.04 | 43.9 ± 4.4 | 5.1 ± 0.3 | 1.0 ± 0.1 | 1.2 ± 0.2 |
| AL+EX (n=11) | 50.8 ± 2.8 | 30.1 ± 1.6 | 19.8 ± 0.9 | 1.5 ± 0.07 | 33.0 ± 5.8 | 4.5 ± 0.4 | 0.6 ± 0.1 | 1.1 ± 0.2 |
| ER (n=11) | 40.9 ± 4.6 | 19.3 ± 2.3 | 19.5 ± 1.9 | 1.0 ± 0.07 | 27.3 ± 4.6 | 4.5 ± 0.8 | 1.1 ± 0.2 | 1.2 ± 0.2 |
| ER+EX (n=12) | 31.7 ± 3.1 | 15.1 ± 1.6 | 16.1 ± 1.8 | 1.0 ± 0.05 | 21.7 ± 2.9 | 4.5 ± 0.8 | 0.7 ± 0.2 | 1.0 ± 0.2 |
| P (ER) | <0.001 | <0.001 | 0.019 | <0.001 | 0.003 | 0.655 | 0.672 | 0.653 |
| P (EX) | 0.022 | 0.133 | 0.028 | 0.136 | 0.069 | 0.660 | 0.019 | 0.618 |
Data shown are the total number of cells in each subset (X 106).
Unlike the robust effects of both ER and EX on the distribution of leukocytes in the spleen, neither ER nor EX significantly altered the number of T cells, B cells or macrophages in the Peyer’s patches (Table 4). ER, but not EX, significantly reduced the number of dendritic cells in the Peyer’s patches (P=0.021). Finally, there were no interactive effects of ER or EX on the distribution of leukocytes in the spleen (Table 3) or Peyer’s patches (Table 4).
TABLE 4.
Distribution of leukocytes in the Peyer’s patches (mean ± SEM)1,2 among mice exposed to AL, AL+EX, ER, or ER+EX treatments for 6 weeks
| Treatment Group | T cells (CD3+) | B cells (B220+) | Macrophages (CD11b+/I-Ab+) | Dendritic cells (CD11c+/I-Ab+) |
|---|---|---|---|---|
| AL | 3.5 ± 0.7 | 13.2 ± 1.8 | 0.8 ± 0.2 | 0.7 ± 0.1 |
| AL+EX | 2.8 ± 0.7 | 12.6 ± 2.4 | 0.9 ± 0.2 | 0.6 ± 0.1 |
| ER | 2.9 ± 0.3 | 11.5 ± 0.9 | 0.7 ± 0.3 | 0.4 ± 0.1 |
| ER+EX | 1.7 ± 0.3 | 8.3 ± 1.5 | 0.9 ± 0.3 | 0.3 ± 0.1 |
| P (ER) | 0.141 | 0.112 | 0.820 | 0.021 |
| P (EX) | 0.101 | 0.312 | 0.747 | 0.287 |
Data shown are the total number of cells in each subset (X 106).
These data represent the mean ± SEM of 4 pooled groups of 2-3 animals per group.
EX significantly enhances T cell proliferation and cytokine production in Peyer’s patch cells
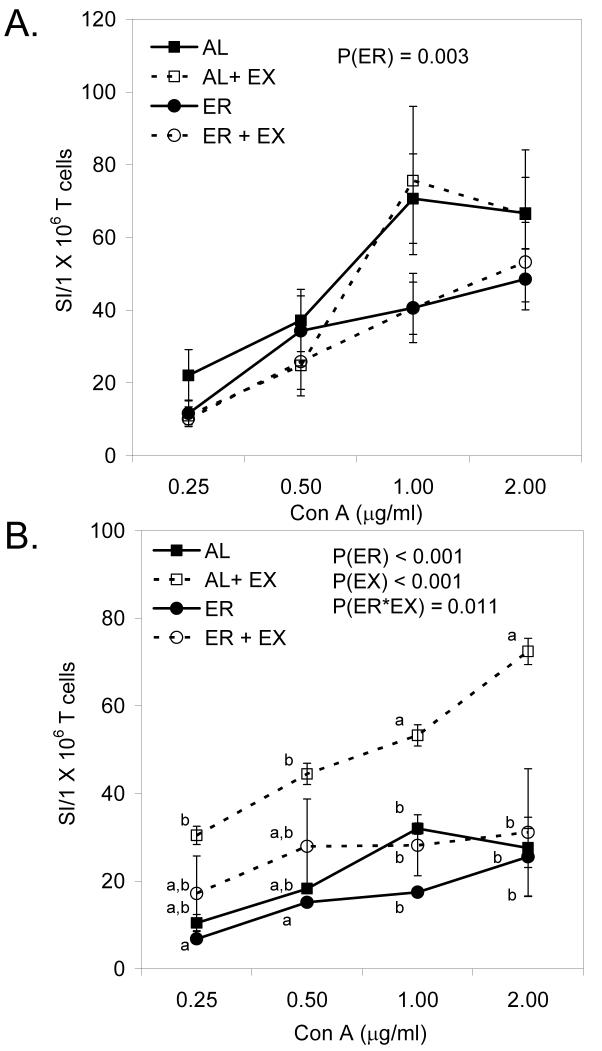
In splenocytes, ER had a significant inhibitory effect on Con A-induced T cell proliferation (Fig. 1A; P=0.003); however, EX had no effect on T cell proliferation (Fig. 1A; P=0.352). In contrast, there were significant main effects of both ER (P<0.001) and EX (P<0.001) on Con A-induced T cell proliferation in Peyer’s patch cells (Fig 1B), as well as a significant interaction between ER and EX on Peyer’s patch T cell proliferation (Fig. 1B; P=0.011). EX resulted in a much greater enhancement of T cell proliferation in Peyer’s patch cells in AL mice (AL+EX) than in mice that were energy restricted (ER+EX).
FIGURE 1.
The effect of ER and EX on the proliferation of T cells collected from the spleen (A) and Peyer’s patches (B). Lymphocytes were stimulated with Con A to induce T cell proliferation at the concentrations indicated for 72 h. Data shown are means ± SEM (n=11-12/group). When the interaction between ER and EX was significant, Tukey’s post hoc test was done to compare individual means among treatment groups. Means without a common letter differ (P<0.05).
EX (P=0.014) but not ER (P=0.948) significantly enhanced interferon-γ (IFNγ) production by splenocytes in response to Con A stimulation (Fig. 2A). However, neither ER nor EX significantly altered Con A-induced IL-6 (Fig. 2B) and IL-5 (Fig. 2C) production from cultured splenocytes. Similar to the effects of EX on splenic IFNγ production, EX significantly enhanced IFNγ production from Peyer’s patch cells (Fig. 3A; P<0.001). In contrast, ER lowered IFNγ production in Peyer’s patch cells, although this did not reach statistical significance (Fig.3A; P=0.064). There was a significant main effect of EX (P<0.001) on Con A-induced IL-6 production in Peyer’s patch cells, as well as a significant interaction between ER and EX on Peyer’s patch T cell proliferation (Fig. 3B; P=0.010), with EX having a much greater effect in AL-fed mice as compared to ER mice. Finally, there were significant main effects of both ER (P<0.001) and EX (P<0.001) on Con A-induced IL-5 production in Peyer’s patch cells (Fig 3C), as well as a significant interaction between ER and EX on Peyer’s patch IL-5 production (Fig. 3C; P<0.001), with EX having only a stimulatory effect on IL-5 production in AL fed mice (Fig. 3C). No differences were observed in the production of IL-2, IL-4, IL-12, MCP-1 and TNFα in response to ER or EX from either splenocytes or cells collected from the Peyer’s patches (data not shown).
FIGURE 2.
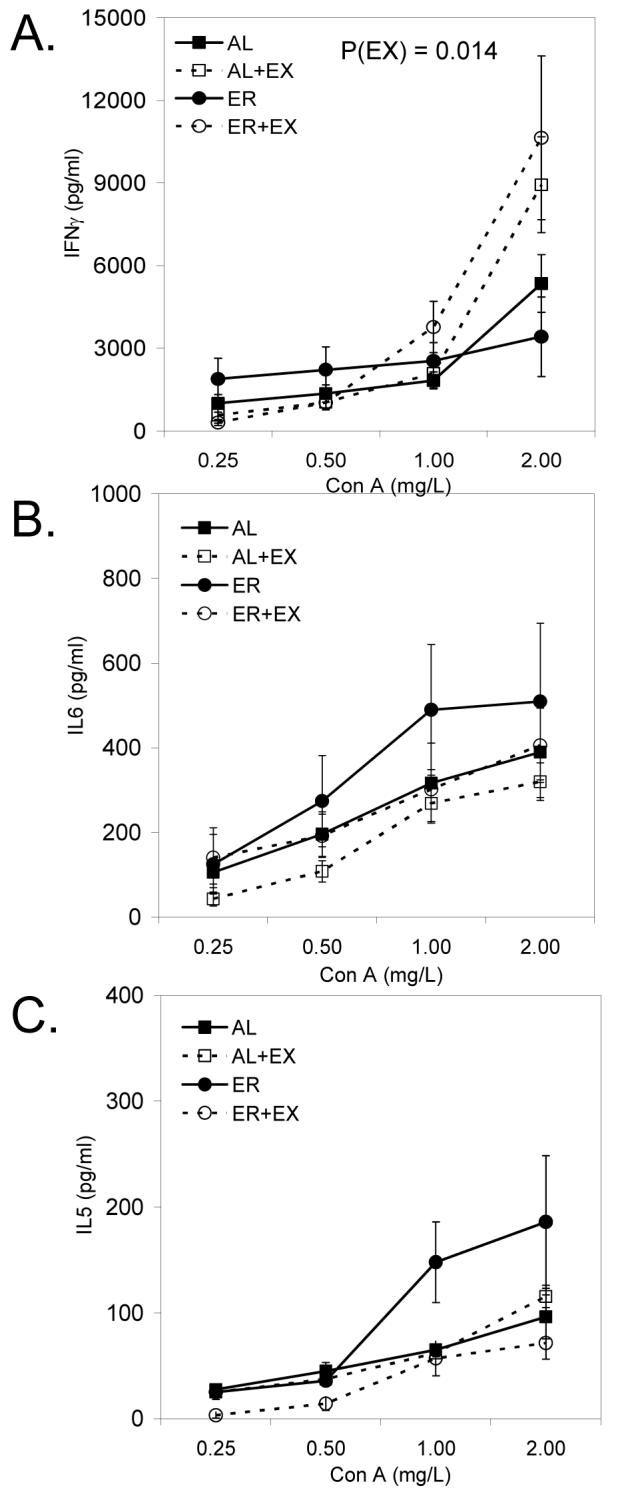
Con A-induced splenic IFNγ (A), IL-6 (B), and IL-5 (C) production from mice maintained on AL, AL+EX, ER, or ER+EX treatments. Lymphocytes were stimulated with Con A to induce cytokine production at the concentrations indicated for 48 h. Data shown are means ± SEM (n=11-12/group).
FIGURE 3.
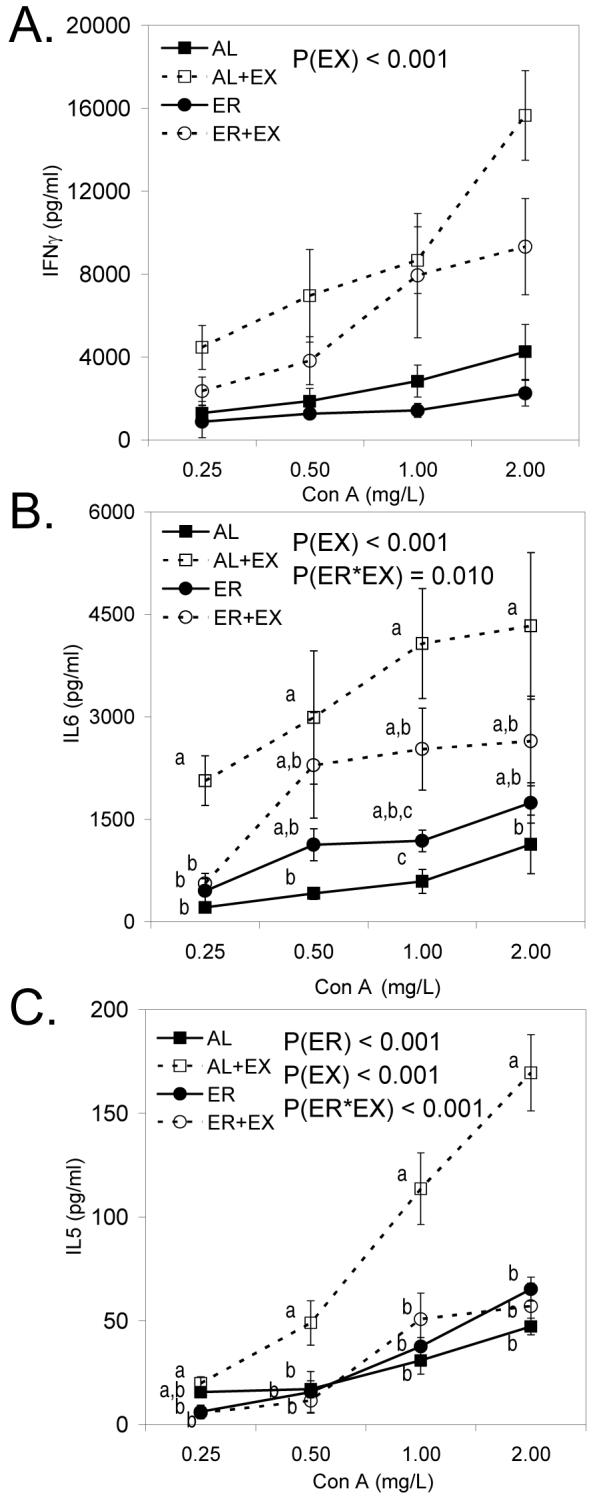
Con A-induced IFNγ (A), IL-6 (B), and IL-5 (C) production from cells collected from the Peyer’s patches in mice maintained on AL, AL+EX, ER, or ER+EX treatments. Lymphocytes were stimulated with Con A to induce cytokine production at the concentrations indicated for 48 h. Data shown are means ± SEM (n=11-12/group). When the interaction between ER and EX was significant, Tukey’s post hoc test was done to compare individual means among treatment groups. Means without a common letter differ (P<0.05).
Energy restriction enhances NK cell function
ER (P<0.001), but not EX (P=0.298), significantly enhanced splenic NK cell cytotoxicity over a range of effector to target (E:T) ratios (12.5:1 to 100:1) using Cr51-labeled YAC-1 target cells. There was no evidence of an interaction between ER and EX on NK cell function (P=0.368).
Discussion
To our knowledge, these results provide the first documentation of the differential effects of ER and EX on systemic and mucosal immune function. Both ER and EX reduced the size and cellularity of the spleen, which is comprised mainly of a loss of T and B lymphocytes. Despite these phenotypic similarities, the effects of ER and EX on the functional capacity of splenic and intestinal lymphocytes markedly differed. ER significantly impaired splenic and intestinal T cell proliferation, and had an inhibitory effect on cytokine production by intestinal lymphocytes. However, ER significantly enhanced splenic NK cell function. These data suggest that ER may be an effective intervention when enhanced NK cell function is a desired outcome. However, the inhibitory effects of ER on T cell proliferation and cytokine production may limit its use as an intervention strategy since adequate T cell function is an important component of anti-tumor immunity (40). In contrast to ER, EX enhanced both mucosal T cell proliferation and cytokine production, as well as IFNγ production in the spleen. Therefore, EX may be an effective intervention either alone, or potentially in combination with other cancer prevention strategies, to enhance the functional capabilities of lymphocytes, particularly those residing in the intestine.
Our study was designed to compare immunologic parameters in response to the two most commonly recommended weight control strategies, specifically ER and increased physical activity. ER significantly reduced body weight from both the lean and fat mass compartments; lowered percent body fat; and reduced bone mineral density, as previously reported (35, 41). In contrast, 6 weeks of voluntary EX did not significantly alter body weight or composition, but did signficantly increase bone mineral density, which is consistent with a moderate EX regimen (42, 43). The absence of a statistically significant effect of EX on body weight or composition is likely due to a relatively short training period (6 weeks). Subsequent studies in our laboratory have shown that 12 weeks of voluntary running results in signficant changes in body weight and composition (data not shown). Nevertheless, significant changes in bone mineral density in the sample of animals studied here clearly indicate that physiological changes are occurring in response to 6 weeks of EX.
Previous studies have also documented that ER and EX reduce the size and cellularity of the spleen (44-46). However, this study is the first to demonstrate that these effects are tissue specific, as the size and cellularity of the Peyer’s patches were not affected by either ER or EX. The reduction in the size of the spleen with both ER and EX can be explained by a loss of T and B lymphocytes. One hypothesis to explain these findings is that ER and EX induce apoptosis of lymphocytes in the spleen. ER has been shown to increase Fas/Fas-ligand expression on lymphocytes (47) and render T cells more sensitive to apoptotic signals (48). No studies to date have examined the role of regular, moderate EX on apoptosis of splenic lymphocytes; however, an acute bout of treadmill EX has been shown to induce apopotosis of lymphocytes in the thymus, spleen and intestine (49, 50). The biological mediator(s) of ER- and/or EX-mediated lymphocyte apoptosis is not known, but previous studies have shown that serum glucocorticoids, one possible candidate, are elevated in response to both ER (41, 51, 52) and EX (50, 53) and can induce apopotsis of T cells both in vitro (54) and in vivo (55).
ER significantly decreased Con A-induced lymphocyte proliferation of cells from both the spleen and intestine. We also found a statistically significant interaction between ER and EX on cytokine production from intestinal lymphocytes with ER inhibiting the EX-induced enhancement of IFNγ, IL-6, and IL-5 production from intestinal lymphocytes. The effects of ER on immune function are influenced by the duration of exposure to ER, with shorter term exposure (6-8 weeks) often resulting in impaired function and longer term exposure resulting in immune enhancement, reviewed in (29). For example, short term ER has been shown to reduce splenic T cell proliferation in normal mice (56). Additionally, in several rodent autoimmunity models, short term ER has been shown to decrease antigen specific proliferation of T cells, as well as decrease cytokine and autoantibody production (57-58). Together, these data suggest short term ER can suppress a number of T lymphocyte functions and may be beneficial in situations where T cell activation has induced a disease state. However, short term ER may not be beneficial to T cell function in young, healthy mice, as evidenced by the reduction in Con A proliferation following ER, as well as the diminution of the EX-induced enhancement of cytokine production by intestinal lymphocytes in this study.
In contrast to the inhibitory effect of ER on lymphocyte proliferation, EX significantly enhanced Con A-induced lymphocyte proliferation in intestinal lymphocytes, but had no effect on splenocyte proliferation. Previous reports in the literature have been inconsistent with some studies reporting an increase (59-62), a decrease (63), or no effect (53,64) of regular, moderate EX training on T cell proliferative responses. This heterogeneity in proliferative responses may be due to timing of lymphocyte collection with respect to the last EX bout, and varying intensity and duration of EX interventions. Finally, it appears that lymphocytes isolated from different lymphoid tissue may be differentially impacted by EX training. For example, moderate EX has been shown to enhance the Con A-induced proliferation of T cells collected from the peripheral blood but not the spleen of hamsters (53). Additionally, treadmill EX in rats resulted in an increase in Con A-induced lymphocyte proliferation in the mesenteric lymph nodes, but not the spleen (65). These results are consistent with our findings in which proliferative responses were increased following EX in intestinal lymphocytes but not splenocytes. These data suggest that EX selectively enhances T cell proliferation in some, but not all lymphoid organs.
In addition to documenting the EX-induced enhancement of intestinal T cell proliferation, this study is the first to demonstrate an EX-induced enhancement of mucosal cytokine production, which may improve cellular and humoral immune responses in the mucosa. To date, the only mucosal immune endpoint examined in response to moderate EX has been the humoral response, specifically the mucosal-associated antibody, IgA. Several studies in humans have shown that salivary secretory IgA levels were enhanced with moderate EX training (31, 32). However, no studies have examined the effect of EX on cell-mediated immunity in the mucosal compartment. In the present study, the novel findings that EX led to an increase in Con A-stimulated lymphocyte proliferation in conjuction with an increase in the in vitro production of IL-5, IL-6, and IFNγ from Peyer’s patch cells suggest that regular, moderate EX enhances cell-mediated and potentially, downstream humoral responses in the mucosal immune system. Since the Peyer’s patches are the inductive site in the mucosal immune system where immune cells first encounter antigen and initiate IgA production and mucosal T cell responses (66), an EX-induced enhancement of T cell reponses in the Peyer’s patches may result in improved immunosurveillance aganst ingested pathogens and preneoplastic and/or neoplastic cell growth in the gastrointestinal tract. However, additional studies in appropriate animal models are needed to address this question.
Finally, in our study ER significantly enhanced NK cell cytotoxicity, as previously reported (58). NK cells are important in controlling viral infections (67) and some neoplasias (39,68). The increase in NK cell cytotoxicity as a result of ER may be one mechanism by which ER may reduce tumor formation in spontaneous and chemically-induced tumor models, as it is well documented that NK cells are an important component of anti-tumor immunity (69,70). In previous studies, moderate EX has also been shown to enhance NK cell activity (21-28). The lack of a statistically significant effect of EX on NK cell cytotoxicity in these studies may be due to a relatively short training period (6 weeks). Other studies in the literature reporting an EX-induced enhancement of NK cell function have utilized a training protocol of several months.
In summary, we have documented that ER and EX, two lifestyle-based interventions known to prevent obesity and inhibit tumorigenesis in rodent models, differentially modulate components of systemic and mucosal immunity. ER enhanced NK cell function in the spleen, whereas EX mainly enhanced proliferation and cytokine production from intestinal lymphocytes. These results demonstrate that ER and EX are likely working through different mechanisms, at least with respect to regulating immune function. Additionally, these data demonstrate that moderate EX can enhance lymphocyte proliferation and cytokine production in the absence of signficant changes in body composition, suggesting that the immune enhancing effects of EX in humans may be achieved in a relatively short period of time (6 weeks) without large decreases in body weight or fat mass. These findings also suggest that EX may be an important intervention to couple with ER (diet) in humans to prevent the immune inhibition that may result from short term ER. Finally, the results from this study suggest that moderate EX may be a viable intervention strategy to test in combination with other cancer prevention or therapeutic agents where an enhancement of cytokine and proliferative capabilities of lymphocytes may be beneficial. Future studies are aimed at evaluating the role of EX on antigen-specific immune function to determine if the EX-induced enhancement of immunity observed in this study impacts adaptive immune responses (i.e. response to vaccination) and influences anti-tumor immunity.
Acknowledgements
The authors are grateful for the technical assistance of Lisa Riffle, Garland Davis, and Donald Hill. The authors thank Debra Weingarten for her assistance in the preparation of this manuscript
Footnotes
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and by the National Cancer Institute Cancer Prevention Fellowship Program. This work was also supported by funding to S. Hursting from NIEHS # P30 ES007784 and the Breast Cancer Research Foundation.
Author disclosures: C. J. Rogers, D. Berrigan, D. A. Zaharoff, K. W. Hance, A. C. Patel, S. N. Perkins, J. Schlom, J. W. Greiner, and S. D. Hursting, no conflicts of interest.
- AL
- consumed food ad libitum
- AL+EX
- consumed food ad libitum plus given access to voluntary running wheels
- Con A
- concanavalin A
- E:T
- effector:target
- ER
- 30% energy restriction
- EX
- exercise
- ER+EX
- 30% energy restriction plus given access to voluntary running wheels
- IL
- interleukin
- km
- kilometer
- mAb
- monoclonal antibodies
- MCP-1
- monocyte chemoattractant protein-1
- NK
- natural killer
- NKCC
- NK cell cytotoxicity
- SI
- stimulation index
No supplemental online material has been submitted.
Literature Cited
- 1.National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. Obes Res. 1998 Sep;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 2.Vainio H, Bianchini F, editors. Weight Control and Physical Activity. IARC Press; Lyon: 2002. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TK, Calle EE, Rodriguez C, Kahn HS, Thun MJ. Body mass index and colon cancer mortality in a large prospective study. Am J Epidemiol. 2000 Nov 1;152:847–54. doi: 10.1093/aje/152.9.847. [DOI] [PubMed] [Google Scholar]
- 4.Slattery ML. Physical activity and colorectal cancer. Sports Med. 2004;34:239–52. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005 May;7:204–13. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004 Aug 23;23:6365–78. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 7.Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–52. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- 8.Basterfield L, Reul JM, Mathers JC. Impact of physical activity on intestinal cancer development in mice. J Nutr. 2005 Dec;135:3002S–8S. doi: 10.1093/jn/135.12.3002S. [DOI] [PubMed] [Google Scholar]
- 9.Colbert LH, Mai V, Tooze JA, Perkins SN, Berrigan D, Hursting SD. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis. 2006 Oct;27:2103–7. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- 10.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005 Jun;98:2219–25. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 11.Campbell KL, McTiernan A. Exercise and biomarkers for cancer prevention studies. J Nutr. 2007 Jan;137:161S–9S. doi: 10.1093/jn/137.1.161S. [DOI] [PubMed] [Google Scholar]
- 12.Hursting SD, Rogers CJ, Mahabir S, Nunez NP, Barrett JC, Perkins SN, Forman MR. Energy Balance and Cancer. In: Heber D, Blackburn G, Go V, Milner J, editors. Nutritional Oncology. Academic Press; Amsterdam: 2006. pp. 69–83. [Google Scholar]
- 13.Rogers CJ, Berrigan D, Colbert LH, Greiner JW, Perkins SD, Hursting SD. Mechanisms linking physical activity and cancer prevention. Sports Medicine. 2007 doi: 10.2165/00007256-200838040-00002. In press. [DOI] [PubMed] [Google Scholar]
- 14.Garnett CT, Greiner JW, Tsang KY, Kudo-Saito C, Grosenbach DW, Chakraborty M, Gulley JL, Arlen PM, Schlom J, Hodge JW. TRICOM vector based cancer vaccines. Curr Pharm Des. 2006;12:351–61. doi: 10.2174/138161206775201929. [DOI] [PubMed] [Google Scholar]
- 15.Palena C, Abrams SI, Schlom J, Hodge JW. Cancer vaccines: preclinical studies and novel strategies. Adv Cancer Res. 2006;95:115–45. doi: 10.1016/S0065-230X(06)95004-0. [DOI] [PubMed] [Google Scholar]
- 16.Zeytin HE, Patel AC, Rogers CJ, Canter D, Hursting SD, Schlom J, Greiner JW. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA.Tg/MIN mice. Cancer Res. 2004 May 15;64:3668–78. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]
- 17.Revaz V, Nardelli-Haefliger D. The importance of mucosal immunity in defense against epithelial cancers. Curr Opin Immunol. 2005 Apr;17:175–9. doi: 10.1016/j.coi.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Perdigon G, de Moreno de LeBlanc A, Valdez J, Rachid M. Role of yoghurt in the prevention of colon cancer. Eur J Clin Nutr. 2002 Aug;56(Suppl 3):S65–8. doi: 10.1038/sj.ejcn.1601490. [DOI] [PubMed] [Google Scholar]
- 19.Pahlavani MA. Caloric restriction and immunosenescence: a current perspective. Front Biosci. 2000 Jun 1;5:D580–7. doi: 10.2741/pahlavani. [DOI] [PubMed] [Google Scholar]
- 20.Hirokawa K, Utsuyama M. Animal models and possible human application of immunological restoration in the elderly. Mech Ageing Dev. 2002 Apr 30;123:1055–63. doi: 10.1016/s0047-6374(01)00389-x. [DOI] [PubMed] [Google Scholar]
- 21.Crist DM, Mackinnon LT, Thompson RF, Atterbom HA, Egan PA. Physical exercise increases natural cellular-mediated tumor cytotoxicity in elderly women. Gerontology. 1989;35:66–71. doi: 10.1159/000213001. [DOI] [PubMed] [Google Scholar]
- 22.DiPenta JM, Johnson JG, Murphy RJ. Natural killer cells and exercise training in the elderly: a review. Can J Appl Physiol. 2004 Aug;29:419–43. doi: 10.1139/h04-027. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman-Goetz L, Arumugam Y, Sweeny L. Lymphokine activated killer cell activity following voluntary physical activity in mice. J Sports Med Phys Fitness. 1994 Mar;34:83–90. [PubMed] [Google Scholar]
- 24.Hoffman-Goetz L, May KM, Arumugam Y. Exercise training and mouse mammary tumour metastasis. Anticancer Res. 1994 Nov-Dec;14:2627–31. [PubMed] [Google Scholar]
- 25.MacNeil B, Hoffman-Goetz L. Chronic exercise enhances in vivo and in vitro cytotoxic mechanisms of natural immunity in mice. J Appl Physiol. 1993 Jan;74:388–95. doi: 10.1152/jappl.1993.74.1.388. [DOI] [PubMed] [Google Scholar]
- 26.Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsen-Cannarella SL. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993 Jul;25:823–31. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Nieman DC, Nehlsen-Cannarella SL, Markoff PA, Balk-Lamberton AJ, Yang H, Chritton DB, Lee JW, Arabatzis K. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990 Dec;11:467–73. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 28.Yan H, Kuroiwa A, Tanaka H, Shindo M, Kiyonaga A, Nagayama A. Effect of moderate exercise on immune senescence in men. Eur J Appl Physiol. 2001 Dec;86:105–11. doi: 10.1007/s004210100521. [DOI] [PubMed] [Google Scholar]
- 29.Hance KW, Rogers CJ, Hursting SD, Greiner JW. Combination of physical activity, nutrition, or other metabolic factors and vaccine response. Front Biosci. 2007;12:4997–5029. doi: 10.2741/2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005 Apr;98:1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 31.Akimoto T, Kumai Y, Akama T, Hayashi E, Murakami H, Soma R, Kuno S, Kono I. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med. 2003 Feb;37:76–9. doi: 10.1136/bjsm.37.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol. 2002 Jun;87:153–8. doi: 10.1007/s00421-002-0609-1. [DOI] [PubMed] [Google Scholar]
- 33.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007 Jan;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 34.Clinton SK, Mulloy AL, Li SP, Mangian HJ, Visek WJ. Dietary fat and protein intake differ in modulation of prostate tumor growth, prolactin secretion and metabolism, and prostate gland prolactin binding capacity in rats. J Nutr. 1997 Feb;127:225–37. doi: 10.1093/jn/127.2.225. [DOI] [PubMed] [Google Scholar]
- 35.Berrigan D, Lavigne JA, Perkins SN, Nagy TR, Barrett JC, Hursting SD. Phenotypic effects of calorie restriction and insulin-like growth factor-1 treatment on body composition and bone mineral density of C57BL/6 mice: implications for cancer prevention. In Vivo. 2005 Jul-Aug;19:667–74. [PubMed] [Google Scholar]
- 36.Nagy TR, Clair AL. Precision and accuracy of dual-energy X-ray absorptiometry for determining in vivo body composition of mice. Obes Res. 2000 Aug;8:392–8. doi: 10.1038/oby.2000.47. [DOI] [PubMed] [Google Scholar]
- 37.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007 Mar 1;25:2085–94. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spalding DM, Koopman WJ, Eldridge JH, McGhee JR, Steinman RM. Accessory cells in murine Peyer’s patch. I. Identification and enrichment of a functional dendritic cell. J Exp Med. 1983 May 1;157:1646–59. doi: 10.1084/jem.157.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochsenbein AF. Principles of tumor immunosurveillance and implications for immunotherapy. Cancer Gene Ther. 2002 Dec;9:1043–55. doi: 10.1038/sj.cgt.7700540. [DOI] [PubMed] [Google Scholar]
- 41.Mai V, Colbert LH, Berrigan D, Perkins SN, Pfeiffer R, Lavigne JA, Lanza E, Haines DC, Schatzkin A, Hursting SD. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res. 2003 Apr 15;63:1752–5. [PubMed] [Google Scholar]
- 42.Vainionpaa A, Korpelainen R, Vihriala E, Rinta-Paavola A, Leppaluoto J, Jamsa T. Intensity of exercise is associated with bone density change in premenopausal women. Osteoporos Int. 2006;17:455–63. doi: 10.1007/s00198-005-0005-x. [DOI] [PubMed] [Google Scholar]
- 43.Wu J, Wang XX, Higuchi M, Yamada K, Ishimi Y. High bone mass gained by exercise in growing male mice is increased by subsequent reduced exercise. J Appl Physiol. 2004 Sep 97;:806–10. doi: 10.1152/japplphysiol.01169.2003. [DOI] [PubMed] [Google Scholar]
- 44.Kubo C, Day NK, Good RA. Influence of early or late dietary restriction on life span and immunological parameters in MRL/Mp-lpr/lpr mice. Proc Natl Acad Sci U S A. 1984 Sep;81:5831–5. doi: 10.1073/pnas.81.18.5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Otto AC, Rona du Toit DJ, Pretorius PH, Lotter MG, van Aswegen A. The effect of exercise on normal splenic volume measured with SPECT. Clin Nucl Med. 1995 Oct;20:884–7. doi: 10.1097/00003072-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Utsuyama M, Ichikawa M, Konno-Shirakawa A, Fujita Y, Hirokawa K. Retardation of the age-associated decline of immune functions in aging rats under dietary restriction and daily physical exercise. Mech Ageing Dev. 1996 Nov 13;91:219–28. doi: 10.1016/s0047-6374(96)01792-7. [DOI] [PubMed] [Google Scholar]
- 47.Reddy Avula CP, Muthukumar A, Fernandes G. Calorie restriction increases Fas/Fas-ligand expression and apoptosis in murine splenic lymphocytes. FEBS Lett. 1999 Sep 17;458:231–5. doi: 10.1016/s0014-5793(99)01163-1. [DOI] [PubMed] [Google Scholar]
- 48.Luan X, Zhao W, Chandrasekar B, Fernandes G. Calorie restriction modulates lymphocyte subset phenotype and increases apoptosis in MRL/lpr mice. Immunol Lett. 1995 Sep;47:181–6. doi: 10.1016/0165-2478(95)00091-5. [DOI] [PubMed] [Google Scholar]
- 49.Hoffman-Goetz L, Quadrilatero J. Treadmill exercise in mice increases intestinal lymphocyte loss via apoptosis. Acta Physiol Scand. 2003 Nov;179:289–97. doi: 10.1046/j.1365-201X.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman-Goetz L, Zajchowski S, Aldred A. Impact of treadmill exercise on early apoptotic cells in mouse thymus and spleen. Life Sci. 1999;64:191–200. doi: 10.1016/s0024-3205(98)00551-7. [DOI] [PubMed] [Google Scholar]
- 51.Chacon F, Esquifino AI, Perello M, Cardinali DP, Spinedi E, Alvarez MP. 24-hour changes in ACTH, corticosterone, growth hormone, and leptin levels in young male rats subjected to calorie restriction. Chronobiol Int. 2005;22:253–65. doi: 10.1081/cbi-200053522. [DOI] [PubMed] [Google Scholar]
- 52.Stewart JW, Koehler K, Jackson W, Hawley J, Wang W, Au A, Myers R, Birt DF. Prevention of mouse skin tumor promotion by dietary energy restriction requires an intact adrenal gland and glucocorticoid supplementation restores inhibition. Carcinogenesis. 2005 Jun;26:1077–84. doi: 10.1093/carcin/bgi051. [DOI] [PubMed] [Google Scholar]
- 53.Peters BA, Sothmann M, Wehrenberg WB. Blood leukocyte and spleen lymphocyte immune responses in chronically physically active and sedentary hamsters. Life Sci. 1989;45:2239–45. doi: 10.1016/0024-3205(89)90065-9. [DOI] [PubMed] [Google Scholar]
- 54.Yang Y, Bailey J, Vacchio MS, Yarchoan R, Ashwell JD. Retinoic acid inhibition of ex vivo human immunodeficiency virus-associated apoptosis of peripheral blood cells. Proc Natl Acad Sci U S A. 1995 Mar 28;92:3051–5. doi: 10.1073/pnas.92.7.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalo JA, Gonzalez-Garcia A, Martinez C, Kroemer G. Glucocorticoid-mediated control of the activation and clonal deletion of peripheral T cells in vivo. J Exp Med. 1993 May 1;177:1239–46. doi: 10.1084/jem.177.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Christadoss P, Talal N, Lindstrom J, Fernandes G. Suppression of cellular and humoral immunity to T-dependent antigens by calorie restriction. Cell Immunol. 1984 Oct 1;88:1–8. doi: 10.1016/0008-8749(84)90046-7. [DOI] [PubMed] [Google Scholar]
- 57.Abe T, Nakajima A, Satoh N, Ohkoshi M, Sakuragi S, Koizumi A. Suppression of experimental autoimmune uveoretinitis by dietary calorie restriction. Jpn J Ophthalmol. 2001 Jan-Feb;45:46–52. doi: 10.1016/s0021-5155(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 58.Shibolet O, Alper R, Avraham Y, Berry EM, Ilan Y. Immunomodulation of experimental colitis via caloric restriction: role of Nk1.1+ T cells. Clin Immunol. 2002 Oct;105:48–56. doi: 10.1006/clim.2002.5260. [DOI] [PubMed] [Google Scholar]
- 59.Coleman KJ, Rager DR. Effects of voluntary exercise on immune function in rats. Physiol Behav. 1993 Oct;54:771–4. doi: 10.1016/0031-9384(93)90090-3. [DOI] [PubMed] [Google Scholar]
- 60.Hoffman-Goetz L, Thorne RJ, Houston ME. Splenic immune responses following treadmill exercise in mice. Can J Physiol Pharmacol. 1988 Nov;66:1415–9. doi: 10.1139/y88-230. [DOI] [PubMed] [Google Scholar]
- 61.Shinkai S, Kohno H, Kimura K, Komura T, Asai H, Inai R, Oka K, Kurokawa Y, Shephard R. Physical activity and immune senescence in men. Med Sci Sports Exerc. 1995 Nov;27:1516–26. [PubMed] [Google Scholar]
- 62.Tharp GD, Preuss TL. Mitogenic response of T-lymphocytes to exercise training and stress. J Appl Physiol. 1991 Jun;70:2535–8. doi: 10.1152/jappl.1991.70.6.2535. [DOI] [PubMed] [Google Scholar]
- 63.Lin YS, Jan MS, Chen HI. The effect of chronic and acute exercise on immunity in rats. Int J Sports Med. 1993 Feb;14:86–92. doi: 10.1055/s-2007-1021151. [DOI] [PubMed] [Google Scholar]
- 64.Jonsdottir IH, Asea A, Hoffmann P, Dahlgren UI, Andersson B, Hellstrand K, Thoren P. Voluntary chronic exercise augments in vivo natural immunity in rats. J Appl Physiol. 1996 May;80:1799–803. doi: 10.1152/jappl.1996.80.5.1799. [DOI] [PubMed] [Google Scholar]
- 65.Dos Santos Cunha WD, Giampietro MV, De Souza DF, Vaisberg M, Seelaender MC, Rosa LF. Exercise restores immune cell function in energy-restricted rats. Med Sci Sports Exerc. 2004 Dec;36:2059–64. doi: 10.1249/01.mss.0000147626.32295.38. [DOI] [PubMed] [Google Scholar]
- 66.Beagley KW, Elson CO. Cells and cytokines in mucosal immunity and inflammation. Gastroenterol Clin North Am. 1992 Jun;21:347–66. [PubMed] [Google Scholar]
- 67.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 68.Barlozzari T, Leonhardt J, Wiltrout RH, Herberman RB, Reynolds CW. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985 Apr;134:2783–9. [PubMed] [Google Scholar]
- 69.Vujanovic NL, Yasumura S, Hirabayashi H, Lin WC, Watkins S, Herberman RB, Whiteside TL. Antitumor activities of subsets of human IL-2-activated natural killer cells in solid tissues. J Immunol. 1995 Jan 1;154:281–9. [PubMed] [Google Scholar]
- 70.Whiteside TL, Herberman RB. The role of natural killer cells in immune surveillance of cancer. Curr Opin Immunol. 1995 Oct;7:704–10. doi: 10.1016/0952-7915(95)80080-8. [DOI] [PubMed] [Google Scholar]