Abstract
Three separate proteins, BchD, BchH, and BchI, together with ATP, insert magnesium into protoporphyrin IX. An analysis of ATP utilization by the subunits revealed the following: BchH catalyzed ATP hydrolysis at the rate of 0.9 nmol per min per mg of protein. BchI and BchD, tested individually, had no ATPase activity but, when combined, hydrolyzed ATP at the rate of 117.9 nmol/min per mg of protein. Magnesium ions were required for the ATPase activities of both BchH and BchI+D, and these activities were inhibited 50% by 2 mM o-phenanthroline. BchI additionally catalyzed a phosphate exchange reaction from ATP and ADP. We conclude that ATP hydrolysis by BchI+D is required for an activation step in the magnesium chelatase reaction, whereas ATPase activity of BchH and the phosphate exchange activity of BchI participate in subsequent reactions leading to the insertion of Mg2+ into protoporphyrin IX.
Keywords: chlorophyll, BchD, BchH, BchI, phosphorylation
The naturally occurring cyclic tetrapyrroles usually contain a metal atom chelated to the nitrogens at the center of the macrocyclic ring. Chlorophylls and bacteriochlorophylls have a chelated magnesium atom, with one reported exception of zinc chlorophyll in the bacterium Acidophilum rubrum (1). Hemes and siroheme contain chelated iron, whereas vitamin B12 has a centrally chelated cobalt atom. A nickel-containing cyclic tetrapyrrole, coenzyme F430, is found in methanogenic bacteria (2). These tetrapyrrole pigments participate in processes essential for life. Chlorophylls harvest solar energy and initiate its conversion into chemical energy. Hemes are involved in oxygen and electron transport. Vitamin B12 and coenzyme F430 participate as coenzymes in certain biochemical reactions.
In the biosynthetic pathways leading to chlorophyll and heme, the enzymes magnesium chelatase and ferrochelatase insert magnesium and iron, respectively, into protoporphyrin IX, whereas for vitamin B12 biosynthesis, cobaltochelatase inserts cobalt into hydrogenobyrinic acid (3). The tetrapyrrole substrate for the nickel chelatase has not yet been clarified. Ferrochelatase is a single monomeric protein of 40 kDa, and the enzymatic insertion of iron into protoporphyrin IX requires no other compounds (4). In contrast, magnesium and cobalt chelatases both require ATP for the enzymatic insertion of the metal atom. Cobaltochelatase consists of two separate proteins, a 140-kDa monomeric CobN protein and a heteropolymeric 450-kDa protein composed of the 37-kDa CobS and the 70-kDa CobT subunits. The enzymatic insertion of magnesium into protoporphyrin IX utilizes three completely separable proteins. These are referred to as subunits of the magnesium chelatase and are known from Rhodobacter as BchD, BchH, and BchI (5), from Synechocystis as ChD, ChH, and ChI (6), and from barley as Xantha-G, Xantha-F, and Xantha-H (7, 8). In these organisms, the magnesium chelatase subunits are soluble and probably exist separate from each other. The ATP requirement for the magnesium chelatase has been analyzed previously (5, 9), and the insertion of magnesium into protoporphyrin IX is thought to proceed in two stages. In the first stage, two of the subunits, BchD (ChD or Xan-G) and BchI (ChI or Xan-H), undergo in the presence of ATP an activation—probably formation—of a complex. Thereafter, Mg2+ is inserted into protoporphyrin IX, a step that also requires ATP and involves the third subunit, BchH (ChH or Xan-F). The non-hydrolyzable ATP analogue adenosine 5′-[β,γ-methylene]triphosphate cannot replace ATP, but the hydrolyzable analogue adenosine 5′-[γ-thio]triphosphate (ATP[γ-S]) can replace ATP in the first step but not in the second (9). These observations indicate that ATP plays a dual role in the magnesium chelatase reaction.
To clarify the role of ATP in the insertion of magnesium into protoporphyrin IX, we analyzed the ATPase activities associated with the purified subunits of the Rhodobacter sphaeroides magnesium chelatase. The subunits BchH and BchI+D are ATPases, whereas BchI has an ATP-to-ADP phosphate exchange activity. Specific inhibition of these activities with o-phenanthroline and NaF established that they are required for the insertion of magnesium into protoporphyrin IX.
MATERIALS AND METHODS
Production and Purification of Recombinant Rhodobacter BchD, BchH, and BchI.
R. sphaeroides genes bchI and bchD in plasmid pETBCHID and bchH in plasmid pETBCHH were expressed in Escherichia coli BL21(DE3) as described by Gibson et al. (10), and the proteins BchD, BchH, and BchI were purified as described by Willows et al. (5). As previously described, BchH and BchI were obtained in pure form as judged by SDS/PAGE. The purified BchD protein preparation contained the E. coli chaperonin GroEL (70 kDa) as the major contaminant and two minor bands around 68 and 150 kDa.
Magnesium Chelatase Assays.
Enzymatic magnesium chelation was monitored either in a continuous assay or in a stopped assay at 30°C using a Perkin-Elmer Luminescence Spectrometer LS50B operated by Fl Winlab version 1.10 software (Perkin-Elmer). Deuteroporphyrin IX was used in the assays instead of protoporphyrin IX, as it is more water soluble. The incubation mixture in the stopped assay contained, in a total volume of 50 μl, the following: 20 mM Tricine-NaOH at pH 9.0, 1 mM DTT, 4 mM ATP, 20 mM creatine phosphate, 2.5 units of creatine kinase, 20 mM MgCl2, 5 μM deuteroporphyrin IX, and enzyme subunits as indicated. The mixture was incubated in the dark with shaking for 20 min at 30°C, and the reaction was stopped by adding 1 ml of acetone/water/25% ammonia (80:20:1, vol/vol) and 200 μl of hexane. The tubes were centrifuged for 2 min in an Eppendorf centrifuge at its top speed. The emission spectrum for the bottom acetone phase was recorded from 550 to 650 nm with an excitation wavelength of 408 nm. The amount of magnesium deuteroporphyrin formed was calculated from the fluorescence intensity at 580 nm by using a standard curve made with known amounts of the authentic compound. For the continuous assay, the components in the stopped assay mixture were scaled up 10-fold. All ingredients with the exception of one as indicated were placed in a fluorometer cuvette prewarmed to 30°C. The last ingredient was then added, and increase in fluorescence intensity at 580 nm with time was monitored using an excitation light at 408 nm.
Assays for ATPase and ATP-to-ADP Phosphate Exchange Activities.
The reaction mixture for the ATPase assay contained in a total volume of 60 μl, 20 mM Tricine-NaOH, pH 9.0, 1 mM DTT, 0.5 μCi of [8-14C]ATP (specific activity, 53.7 mCi/mmol; 1 Ci = 37 GBq), 1 mM ATP, 20 mM MgCl2, and enzyme subunits as indicated. The ADP-to-ATP phosphate exchange activities were also measured in 60-μl reaction mixtures, and they contained 20 mM Tricine-NaOH at pH 9.0, 1 mM DTT, 0.02 μCi of [8-14C]ADP (specific activity, 55 mCi/mmol), 4 mM ATP, 4 mM ADP, 20 mM MgCl2, and enzyme subunits as indicated. After incubation of the mixtures for 20 min at 30°C, tubes were transferred to ice, 4 μl of 7% perchloric acid was added to stop the reaction, and 2 μl each of 100 mM ATP, ADP, and AMP were added as carriers. Precipitated protein was removed by centrifugation, and the supernatant was loaded on to a PEI-cellulose column (1.5 × 10 cm) equilibrated with solution A (0.02 M LiCl with 0.76 ml of formic acid per liter of solution). Chromatography was performed at 5 ml/min using a 10-min column wash followed by a 20-min linear gradient from solution A to 100× concentrated solution A and a 5-min isocratic elution with the 100× solution A. Column eluate was monitored for UV light absorption at 260 nm. AMP did not bind to the column under these conditions, and ADP eluted approximately half way in the gradient. ATP, closely followed by ATP[γ-S], eluted toward the end of the gradient. The fractions of the eluate comprising the nucleotides were collected separately and analyzed for radioactivity in a liquid scintillation counter.
ATP Binding Assay.
Evidence for binding of ATP to the magnesium chelatase subunits was analyzed by using α- and γ-32P-labeled ATP. In a total volume of 50 μl, the assay mixture contained 20 mM Tricine-NaOH, pH 9.0, 1 mM DTT, 5 μCi of [32P]ATP (specific activities: [α-32P]ATP, 800 Ci/mmol; [γ-32P]ATP, 3000 Ci/mmol), 1 mM ATP, 20 mM MgCl2, and enzyme subunits as indicated. The mixtures were incubated for 20 min at 30°C and stopped by transferring the tubes to ice and adding 4 μl of 7% perchloric acid. The precipitated proteins were collected by transferring the stopped assay mixtures on to filter paper discs. The precipitated protein collected on the disc was washed with 5% ice-cold trichloroacetic acid followed by 96% ethanol. The discs were then dried under an infrared lamp and analyzed for radioactivity in a liquid scintillation counter.
Other Methods.
Co(III)–ATP–o-phenanthroline was prepared as described (11). This inhibitor was mixed with protein fractions in 20 mM Tricine-NaOH buffer, pH 9.0, and incubated on ice for 5 min. The unreacted inhibitor was then removed by gel filtration on a NAP-5 column (Pharmacia Biotech) using the Tricine buffer. Protoporphyrin IX associated with the purified BchH subunit was removed by transferring the BchH to a buffer containing 20 mM Tricine-NaOH, pH 9.0, 1 mM DTT, and 0.5% Tween 20. The released porphyrin and Tween 20 was removed by passing through a SEP-PAK C18 cartridge (Waters/Millipore) preequilibrated with the above buffer without the detergent. SDS/PAGE was performed as described (12) using the Tris/Tricine running buffer system as described (13). Protein concentrations were determined by using the protein assay kit from Bio-Rad.
RESULTS
ATPase Activities of the R. sphaeroides Magnesium Chelatase Subunits.
Only BchH showed significant ATPase activity when apparently homogeneous BchH and BchI and partially purified BchD were analyzed individually for ATPase activity in assays containing 120 μM [8-14C]ATP (assays 1–3, Table 1). It hydrolyzed ATP at the rate of 0.90 nmol/min per mg of protein, and this activity was dependent on the presence of Mg2+ ions. But the addition of 5 mM deuteroporphyrin had no effect. Purified BchH has bound protoporphyrin IX. The bulk of this bound protoporphyrin can be removed by treatment with 0.5% Tween 20 and passing through a C18 column. Removal of the protoporphyrin also had no effect on the ATPase activity of BchH. However, prolonged exposure of BchH preparations to fluorescent room light resulted in a significant loss of the ATPase activity. This loss in activity was overcome by keeping the tubes containing the working solutions of BchH wrapped in aluminum foil in ice. When BchD and BchI are combined in the assays containing 120 μM [8-14C]ATP (no unlabeled ATP added), nearly 90% of the substrate was hydrolyzed to ADP (assay 4, Table 1). In similar assays containing unlabeled ATP (1 mM) together with [8-14C]ATP, the substrate hydrolysis was 50% (assay 9, Table 1), corresponding to the rate of 117.9 nmol/min per mg of protein. The chaperonin GroEL, known to have K+-dependent ATPase activity (14), is a major contaminant in the BchD preparations used in this study. However, combining the same amount of the BchD preparation (33 μg of protein) with BchH gave a rate of 0.49 nmol/min per mg of protein for ATP hydrolysis (assay 8, Table 1). The preparation of BchD (33 μg) by itself hydrolyzed ATP at the rate of 2.7 nmol/min per mg of protein (assay 5, Table 1). From these observations, it is concluded that the magnesium chelatase subunit BchH is a weak ATPase, whereas combining BchD and BchI results in a highly active ATPase.
Table 1.
ATPase activities associated with the R. sphaeroides magnesium chelatase subunits
| Assay | Subunits added | % radioactivity
|
ATPase activity, nmol of ATP hydrolyzed per min | ||
|---|---|---|---|---|---|
| AMP | ADP | ATP | |||
| 1 | BchD | 2.3 | 2.4 | 95.3 | 0.021 |
| 2 | BchH | 2.7 | 38.8 | 58.5 | 0.19 |
| 3 | BchI | 9.3 | 10.2 | 80.5 | 0.092 |
| 4 | BchD + BchI | 5.0 | 82.8 | 12.2 | 0.24 |
| 5 | BchD | 1.4 | 1.6 | 97.0 | 0.089 |
| 6 | BchH | 0.7 | 5.2 | 94.1 | 0.18 |
| 7 | BchI | 1.1 | 4.7 | 94.3 | 0.17 |
| 8 | BchD + BchH | 0.7 | 4.6 | 94.7 | 0.12 |
| 9 | BchD + BchI | 1.8 | 46.9 | 51.3 | 14.5 |
| 10 | BchH + BchI | 1.1 | 10.3 | 88.6 | 3.4 |
| 11 | BchD + BchH + BchI | 3.5 | 50.5 | 46.0 | 16.5 |
In assays 1–4, activity was determined in the presence of [8-14C]ATP with no unlabeled ATP added. In assays 5–11, activity was measured in the presence of [8-14C]ATP + 1 mM unlabeled ATP. Protein (μg per assay): BchD, 33; BchH, 210; BchI, 90.
It was observed that certain BchI preparations, after passing through a fast desalting column (Sephadex G-25) in the SMART system, lose the ability to hydrolyze ATP when combined with BchD. These preparations contained 6 mg of protein per ml and had been through desalting columns during purification. Furthermore, they had been kept frozen for about 2 years in 50 mM Tricine-NaOH buffer at pH 7.9 containing 25 mM MgCl2, 1 mM DTT, and 15% glycerol and had been subjected to several freeze-thaw cycles before passing through the column. Gel exclusion chromatography showed the presence of low molecular weight, 280-nm light-absorbing material in purified BchI preparations. The ability to reconstitute the ATPase with BchD was restored by adding the column fraction with the low molecular weight compounds to desalted BchI.
ATP-to-ADP Phosphate Exchange Activity of BchI.
The sequence 36GDRGTGKSTAVRALA50 is present in the deduced amino acids of the bchI gene of the R. sphaeroides magnesium chelatase. This sequence has the motif GX4GKSX6A found in the primary structure of a number of ATP-binding proteins. The ATPase activity of BchI was low, however, compared with that in assays where BchI and BchD were combined. These observations indicated that the subunit BchI may participate in an ATP-utilizing reaction other than ATP hydrolysis. When BchI was incubated in the presence of 4 mM ADP containing [8-14C]ADP and 4 mM ATP, about 40% of the radioactivity appeared in ATP (Table 2). Omitting ATP from the incubation mixture resulted in a drastic reduction in the formation of radioactive ATP. From this and the absence of radioactivity in AMP, it is evident that radioactive ATP was formed by the transfer of phosphate from unlabeled ATP to [8-14C]ADP. BchI showed high phosphate exchange activity (576 nmol/min per mg of protein) compared with the other subunits of the magnesium chelatase, and Mg2+ ions were required for this activity. The ATP-to-ADP phosphate exchange activity was not affected in BchI preparations that had been chromatographed through fast desalting columns and that had lost their ability to reconstitute the ATPase with BchD. The thiophosphoryl group of ATP[γ-S] was not transferred to ADP when BchI was incubated with 4 mM ATP[γ-S], 4 mM ADP (with [8-14C]ADP), and 20 mM MgCl2. However, ATP[γ-S] could replace ATP as a substrate in the ATPase activities observed with BchH and BchD + BchI.
Table 2.
ATP-to-ADP phosphate exchange activity of BchI
| Assay | Subunits added and components missing | % radioactivity
|
nmol of ATP formed by phosphate transfer per min | ||
|---|---|---|---|---|---|
| AMP | ADP | ATP | |||
| 1 | BchD + BchH + BchI | 6.7 | 60.6 | 32.7 | 43.6 |
| 2 | BchD | 1.8 | 92.4 | 5.8 | 7.3 |
| 3 | BchH | 7.1 | 81.9 | 11.0 | 14.7 |
| 4 | BchI | 1.6 | 59.5 | 38.9 | 51.9 |
| 5 | BchI − ATP | 2.2 | 90.0 | 7.8 | 10.4 |
| 6 | BchI − Mg2+ | 1.5 | 91.5 | 7.0 | 9.3 |
Protein (μg per assay): BchD, 33; BchH, 210; BchI, 90.
Inhibition of the ATPase Activities of Magnesium Chelatase Subunits.
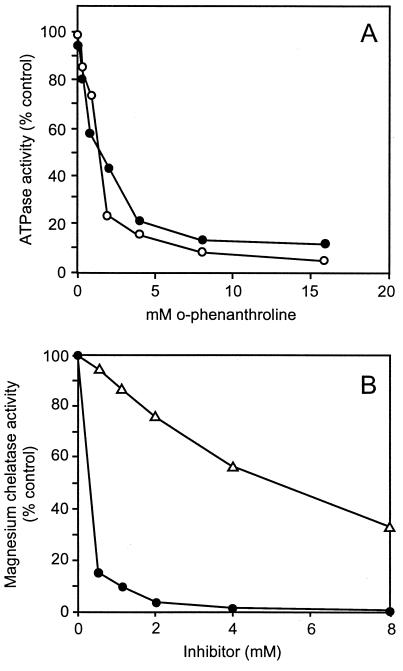
o-Phenanthroline inhibited the ATPase activities of both BchI + BchD and BchH. Fifty% inhibition was observed with 0.2 mM inhibitor in both cases. (Fig. 1A). Co(III)–ATP–o-phenanthroline, a reagent that labels the active site of bona fide ATPases (15), also inhibited the ATPase activity associated with the magnesium chelatase subunits of R. sphaeroides. This complex reagent was not as potent as o-phenanthroline, and for 50% inhibition, 5 mM was required. Unlike o-phenanthroline, it was possible to use this reagent for labeling and specifically inactivate single subunits of the magnesium chelatase. The subunits were separately treated with 10 mM Co(III)–ATP–o-phenanthroline, and the unbound reagent was removed by passing through a NAP-5 column using 20 mM Tricine-NaOH, pH 9.0, and 1 mM DTT. As illustrated in Table 3, this treatment inactivated BchD and BchH subunits. After Co(III)–ATP–o-phenanthroline treatment, BchD was deep brown and BchI was pale brown, indicating that BchD has a higher affinity for ATP than does BchI. It is concluded that both BchD and BchI subunits contribute to the catalytic site required for ATP binding and subsequent hydrolysis. Sodium fluoride and sodium metavanadate, which are known to inhibit several other ATPases (16), had no effect on the ATPase associated with the magnesium chelatase subunits.
Figure 1.
Inhibition of ATPase and magnesium chelatase activities by o-phenanthroline and Co(III)–ATP–o-phenanthroline. (A) o-Phenanthroline inhibition of the ATPase activities of BchH (○) and BchD + BchI (•). (B) Inhibition of magnesium chelatase activity by o-phenanthroline (•) and Co(III)–ATP–o-phenanthroline (▵). The inhibitory effect of these compounds was tested in stopped assays containing (in μg of protein): BchD, 33; BchH, 210; and BchI, 90.
Table 3.
Inhibition of ATPase activities of the magnesium chelatase subunits with Co(III)–ATP–o-phenanthroline
| Assay | Subunits added | ATPase activity, % control |
|---|---|---|
| 1 | BchD + BchI | 100 |
| 2 | BchDt + BchI | 45.9 |
| 3 | BchD + BchIt | 99.2 |
| 4 | BchDt + BchIt | 45.1 |
| 5 | BchH | 100 |
| 6 | BchHt | 41.1 |
The subunits in a buffer containing 20 mM Tricine-NaOH at pH 9.0 and 1 mM DTT were incubated for 5 min on ice with the inhibitor (10 mM), and excess inhibitor was removed by passing through a NAP-5 column. These treated proteins (BchDt, BchIt, and BchHt) were analyzed against the untreated proteins for ATPase activity. Protein (μg per assay): BchD, 33; BchDt, 33; BchH, 210; BchHt, 210; BchI, 90; BchIt, 90.
The Activation Step of the Magnesium Chelatase Reaction.
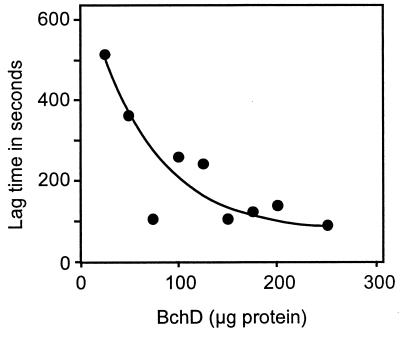
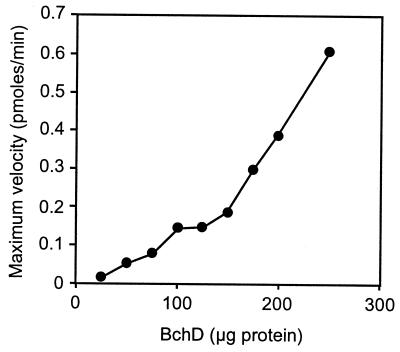
By monitoring the formation of magnesium deuteroporphyrin continuously, it has been shown previously that there is a lag before the reaction reaches its maximum velocity (9). This lag was eliminated when BchD and BchI were preincubated with ATP and Mg2+ for 1 min at 30°C before the addition of BchH to the assay (5). The lag phase represents an activation step. In continuous assays, the lag time decreased with increasing BchD concentrations (Fig. 2). However, increasing the amount of BchI had no effect on the length of the lag phase. The BchD subunit therefore must play a key role in the activation step, probably initiating the activation process by first binding to ATP. None of the three chelatase subunits were labeled when they were incubated with [γ-32P]ATP and Mg2+ either separately or in different combinations. Activation by protein phosphorylation was thus excluded. The maximum velocity of the magnesium chelatase reaction (Vmax after the lag) increased with increasing amounts of BchD or BchI in the assay (Fig. 3).
Figure 2.
Effect of increasing the BchD concentration on the lag phase of the magnesium chelatase reaction. All of the ingredients in a total volume of 500 μl (20 mM Tricine-NaOH at pH 9.0, 1 mM DTT, 4 mM ATP, 20 mM creatine phosphate, 25 units of creatine kinase, 20 mM MgCl2, 5 μM deuteroporphyrin IX, 0.72 mg of BchH, and 0.42 mg of BchI) except BchD were prewarmed to 30°C in a fluorometer cuvette. BchD was added, and increase in fluorescence emission at 580 nm was monitored continuously for 30 min with excitation light at 408 nm.
Figure 3.
Effect of increasing the BchD concentration on the Vmax. Magnesium chelatase activity was measured in continuous assays as described in Fig. 2. Vmax is the maximum slope obtained after the lag phase.
o-Phenanthroline and Co(III)–ATP–o-Phenanthroline Inhibition of Magnesium Chelatase Activity.
o-Phenanthroline at 0.2 mM caused 50% inhibition of the chelatase activity observed with purified BchD, BchH, and BchI (Fig. 1B). Previously, it was shown that o-phenanthroline inhibited magnesium chelatase activity in intact cucumber chloroplasts with 50% inhibition at 0.6 mM (17). The Co(III)–ATP–o-phenanthroline, the reagent that labels the active site of ATPases, also inhibited the R. sphaeroides chelatase with 50% inhibition at 5 mM. The ability to reconstitute the magnesium chelatase of the subunits after they were individually treated with Co(III)–ATP–o-phenanthroline is shown in Table 4: Treatment of the BchD with Co(III)-ATP-o-phenanthroline reduced drastically its ability to reconstitute the magnesium chelatase activity. Treatment of BchH and BchI gave 55% and 20% reduction, respectively, in chelatase activity compared with the control with untreated subunits. Because ATPase activities of BchI + BchD and BchH are similarly inhibited by o-phenanthroline and Co(III)–ATP–o-phenanthroline, it is concluded that the ATPase activities of the subunits participate in the insertion of magnesium into protoporphyrin IX.
Table 4.
Inhibition of the magnesium chelatase activity by separately treating BchD, BchH, and BchI with Co(III)–ATP–o-phenanthroline
| Subunits added to assay | Activity, % control |
|---|---|
| BchD + BchH + BchI | 100 |
| BchD + BchH + BchIt | 80.5 |
| BchDt + BchH + BchI | tr |
| BchD + BchHt + BchI | 45.8 |
| BchD + BchIt + BchHt | 34.7 |
| BchDt + BchH + BchIt | tr |
| BchDt + BchHt + BchI | tr |
| BchDt + BchHt + BchIt | tr |
The chelatase subunits were treated with the inhibitor as described in Table 3. Magnesium chelatase activity was analyzed in stopped assays. Protein (μg per assay): BchD, 33; BchDt, 33; BchH, 210; BchHt, 210; BchI, 90; BchIt, 90. The control assay contained untreated BchD, BchH, and BchI, and the test assays contained different combinations of the treated subunits. tr, Trace.
Sodium Fluoride Effect.
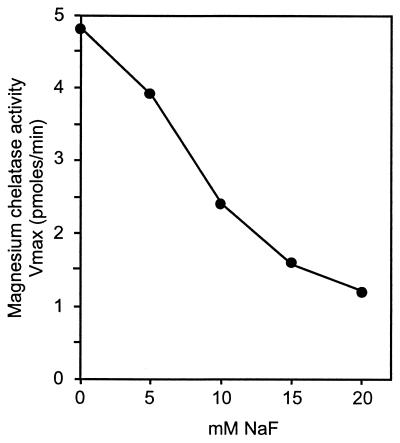
Millimolar concentrations of NaF inhibited magnesium chelatase activity with 50% inhibition at 10 mM NaF (Fig. 4). The same relative inhibition by NaF was obtained regardless if an ATP regenerating system was used in the assay or not. This excluded an indirect inhibition of magnesium chelatase by inhibition of the ATP regenerating system. It was also not a salt effect, because 40 mM NaCl had no effect on the magnesium chelatase activity. Furthermore, tested up to 40 mM, NaF had no effect on the ATPase or the ATP-to-ADP phosphate exchange activities of the chelatase subunits. However, in the ATPase assays of BchH performed in the presence of 20 mM NaF or more, it was noted that approximately 50% of the [8-14C]ATP added bound to the protein and precipitated upon addition of 7% perchloric acid. Both α-32P- and γ-32P-labeled ATP bound to BchH and were not released by washing the protein precipitate with ice-cold 10% trichloroacetic acid. ATP binding was dependent on the presence of Mg2+ in the assay (Table 5). However, on SDS/PAGE the 32P-labeled ATP was released from the protein. We conclude that NaF inhibition of magnesium chelatase is due to binding of ATP to BchH.
Figure 4.
The effect of NaF on the maximal velocity of the magnesium chelatase reaction. NaF effect was tested in continuous assays as described in Fig. 2.
Table 5.
Labeling of BchH with [α-32P]ATP and [γ-32P]ATP
| Assay | Composition | Radioactivity, dpm |
|---|---|---|
| 1 | [α-32P]ATP | 22,200 |
| 2 | 1 + 40 mM NaF | 2,758,200 |
| 3 | [γ-32P]ATP | 4,300 |
| 4 | 3 + 40 mM NaF | 1,402,900 |
| 5 | 2 − MgCl2 | 3,900 |
In addition to labeled ATP, the assays contained 20 mM Tricine-NaOH at pH 9.0, 1 mM DTT, 0.5 mM ATP, 20 mM MgCl2, and 0.24 mg of BchH. MgCl2 was omitted in assay 5. After incubation, BchH was precipitated with perchloric acid, loosely associated ATP was removed by washing with trichloroacetic acid, and bound radioactivity was determined.
DISCUSSION
Three separate proteins, the subunits of the magnesium chelatase together with ATP, insert magnesium into protoporphyrin IX, mesoporphyrin IX, or deuteroporphyrin IX. Protoporphyrin IX is the natural substrate for this complex enzyme, but in assays in vitro, more water-soluble deuteroporphyrin is preferred. Previous studies have established an absolute requirement for ATP and indicated that enzymatic magnesium chelation proceeded by a two-step reaction. The ATP requirement suggests that energy is used during magnesium chelation, in contrast to the ATP-independent Fe2+ insertion catalyzed by the ferrochelatase. In this paper, Mg2+ dependent ATPase activities are shown with the subunits BchH and BchI + BchD. Because addition of deuteroporphyrin had no effect on these ATPase activities, it is unlikely that they are directly coupled to magnesium chelation. However, inhibition of the ATPase activities by treatment of BchD and BchH with Co(III)–ATP–o-phenanthroline destroyed their ability to reconstitute the magnesium chelatase activity. It is thus evident that ATP hydrolysis is required for reactions preceding the insertion of Mg2+ into protoporphyrin IX. One such reaction suggested from earlier work is an activation where only BchD and BchI participate in the presence of ATP and Mg2+. High rates of ATP hydrolysis (117.9 nmol/min per mg of protein) under these conditions indicate that energy is required to maintain BchD and BchI in an activated state to function in the metal chelation. It cannot be excluded that a part of this hydrolysis is by GroEL, present in the BchD preparation. A small fraction of BchI molecules could exist in the unfolded state, and some ATP hydrolysis could occur during chaperonin activity of GroEL. The activation step in the chelatase reaction must involve a reaction other than protein phosphorylation because none of the subunits were labeled with 32P after incubation with [γ-32P]ATP. The activation step probably involves a formation of a BchD + BchI protein complex.
Compared with BchD + BchI, the subunit BchH showed low ATPase activity. This activity was also inhibited by o-phenanthroline. Furthermore, the affinity ligand, Co(III)–ATP–o-phenanthroline, binds to BchH, whereas NaF traps ATP on BchH and disables its function in the chelatase reaction. NaF inhibited the magnesium chelatase activity catalyzed by BchD, BchH, and BchI without inhibiting their ATPase or ATP-to-ADP exchange activities. However, in the presence of NaF, ATP remains bound to BchH and is not easily released. BchH is therefore likely to have two sites for ATP recognition, one sensitive to NaF and the other insensitive. NaF will prevent the release of ATP molecules bound at the sensitive site whereas molecules entering the other site are hydrolyzed. It remains to be seen whether these reactions lead to conformational changes in BchH and are required for the insertion of Mg2+ into protoporphyrin IX.
It was established in a previous study (9) that ATP[γ-S] can replace ATP in the activation step of the pea magnesium chelatase reaction. This hydrolyzable analogue of ATP, however, was ineffective in the subsequent step or steps that involve the insertion of Mg2+ into protoporphyrin IX. In the present study, ATP[γ-S] was shown to be ineffective in the ATP-to-ADP phosphate exchange reaction catalyzed by BchI. It is suggested that ATP-to-ADP phosphate exchange activity of BchI is a partial reaction of the magnesium chelatase subsequent to the activation step.
Acknowledgments
We thank Nina Rasmussen and Ann-Sofi Steinholtz for the preparation of the figures and Inge Sommer for composing the manuscript. We also thank Dr. Robert Willows for helpful discussions. A fellowship from the European Molecular Biology Organization (to M.H.) is gratefully acknowledged.
ABBREVIATION
- ATP[γ-S]
adenosine 5′-[γ-thio]triphosphate
References
- 1.Wakao N, Yokoi N, Isoyama N, Hiraishi A, Shimada K, Kobayashi M, Kise H, Iwaki M, Itoh S, Takaichi S, Sakurai Y. Plant Cell Physiol. 1996;37:889–893. [Google Scholar]
- 2.Thauer R K, Bonacker L G. In: The Biosynthesis of the Tetrapyrrole Pigments, Ciba Foundation Symposium 180. Chadwick D J, Ackrill K, editors. Chichester, England: Wiley; 1994. pp. 210–227. [DOI] [PubMed] [Google Scholar]
- 3.Debussche L, Couder M, Thibaut D, Cameron B, Crouzet J, Blanche F. J Bacteriol. 1992;174:7445–7451. doi: 10.1128/jb.174.22.7445-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Labbe-Bois R, Camadro J-M. In: Metal Ions in Fungi. Winkelman G, Winge D R, editors. New York: Dekker; 1994. pp. 413–453. [Google Scholar]
- 5.Willows R D, Gibson L C, Kannangara C G, Hunter C N, von Wettstein D. Eur J Biochem. 1996;235:438–443. doi: 10.1111/j.1432-1033.1996.00438.x. [DOI] [PubMed] [Google Scholar]
- 6.Jensen P E, Gibson L C D, Henningsen K W, Hunter C N. J Biol Chem. 1996;271:16662–16667. doi: 10.1074/jbc.271.28.16662. [DOI] [PubMed] [Google Scholar]
- 7.Kannangara C G, Vothknecht U C, Hansson M, von Wettstein D. Mol Gen Genet. 1997;254:85–92. doi: 10.1007/s004380050394. [DOI] [PubMed] [Google Scholar]
- 8.Jensen P E, Willows R D, Peterson B L, Vothknecht U C, Stumann B M, Kannangara C G, Henningsen K W, von Wettstein D. Mol Gen Genet. 1996;250:383–394. doi: 10.1007/BF02174026. [DOI] [PubMed] [Google Scholar]
- 9.Walker C J, Weinstein J D. Biochem J. 1994;299:277–284. doi: 10.1042/bj2990277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson L C, Willows R D, Kannangara C G, von Wettstein D, Hunter C N. Proc Natl Acad Sci USA. 1995;92:1941–1944. doi: 10.1073/pnas.92.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber M M, Danchin A. Methods Enzymol. 1977;46:315–321. [Google Scholar]
- 12.Fling S P, Gregerson D S. Anal Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- 13.Schägger H, von Jagow G. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 14.Vitanen P V, Lubben T H, Reed J, Goloubinoff P, O’Keefe D P, Lorimer G H. Biochemistry. 1990;29:5665–5671. doi: 10.1021/bi00476a003. [DOI] [PubMed] [Google Scholar]
- 15.Granot J, Weber M M, Danchin A. Bioinorg Chem. 1978;9:81–92. doi: 10.1016/s0006-3061(00)82007-4. [DOI] [PubMed] [Google Scholar]
- 16.Morris M B, Monteith G, Roufogalis B D. J Cell Biochem. 1992;48:356–366. doi: 10.1002/jcb.240480404. [DOI] [PubMed] [Google Scholar]
- 17.Walker C J, Weinstein J D. Plant Physiol. 1990;95:1189–1196. doi: 10.1104/pp.95.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]