Abstract
Peptide growth factors were isolated from conditioned medium derived from rice (Oryza sativa L.) suspension cultures and identified to be a sulfated pentapeptide [H-Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln-OH] and its C-terminal-truncated tetrapeptide [H-Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-OH]. These structures were identical to the phytosulfokines originally found in asparagus (Asparagus officinalis L.) mesophyll cultures. The pentapeptide [phytosulfokine-α (PSK-α)] very strongly stimulated colony formation of rice protoplasts at concentrations above 10−8 M, indicating a similar mode of action in rice of phytosulfokines. Binding assays using 35S-labeled PSK-α demonstrated the existence of both high- and low-affinity specific saturable binding sites on the surface of rice cells in suspension. Analysis of [35S]PSK-α binding in differential centrifugation fractions suggested association of the binding with a plasma membrane-enriched fraction. The apparent Kd values for [35S]PSK-α binding were found to be 1 × 10−9 M for the high-affinity type and 1 × 10−7 M for the low-affinity type, with maximal numbers of binding sites of 1 × 104 sites per cell and 1 × 105 sites per cell, respectively. Competition studies with [35S]PSK-α and several synthetic PSK-α analogs demonstrated that only peptides that possesses mitogenic activity can effectively displace the radioligand. These results suggest that a signal transduction pathway mediated by peptide factors is involved in plant cell proliferation.
Keywords: growth factor, Oryza sativa
Plant cells cultured at low cell density, often at around 5 × 104 cells per ml, do not divide in response to any combinations of known plant hormones. However, proliferation can be induced by adding conditioned medium prepared from rapidly growing high-cell-density cultures (1). This phenomenon suggests that a secreted chemical factor(s) is essential for cell proliferation. The factor(s) need not be derived from the same species as the target cells because interspecific feeding effects have often been observed, even between very distant species (2–9). Recently, we isolated two factors having mitogenic activity from the conditioned medium of asparagus suspension cultures and determined their structures to be a disulfated pentapeptide, H-Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln-OH (phytosulfokine-α, PSK-α), and its C-terminal-truncated tetrapeptide (PSK-β) (10). Similar mitogenic activity for asparagus mesophyll cells was also detected in conditioned medium prepared from rice culture (10), an observation which suggests that PSK-α-like factors are distributed in several plant families.
In Gramineae, trials to isolate growth factor(s) have been conducted using bioassay systems based on protoplasts derived from cultured cells (7, 11), but only partial characterization resulted because of the relatively slow responses of protoplasts. We have therefore focused on the high sensitivity of asparagus mesophyll cells to factors contained in rice conditioned medium, which have the same or even greater mitogenic activity than those derived from suspension cultures of asparagus itself (10).
In this paper, we report the distribution of PSKs in rice cells as well as asparagus cells, and the identification of saturable high- and low-affinity binding on rice cells in suspension when 35S-labeled PSK-α is used as a ligand. Specific [35S]PSK-α binding was also detected in subcellular fractions prepared from rice suspension cells, with the highest binding activity in a plasma membrane-enriched fraction. Furthermore, a close correlation was found to exist between the effectiveness of several synthetic PSK-α analogs as competitors for the [35S]PSK-α binding and their mitogenic activities in a bioassay.
MATERIALS AND METHODS
Materials.
[35S]Sulfuric acid solution (1,500 Ci/mmol; 1 Ci = 37 GBq) was obtained from New England Nuclear. Pronase E was obtained from Sigma. DEAE-Sephadex A-25 was from Pharmacia. Bio-Gel P-2 was from Bio-Rad. The dialysis membrane, Spectra-Por with a molecular weight cut-off of 1,000, was from Spectrum (Houston, TX). A Sep-Pak C18 column was purchased from Waters and a reverse-phase HPLC column, Develosil ODS-UG-5, from Nomura Chemicals (Seto, Japan). Cellulase Onozuka RS and Pectriase were from Yakult (Tokyo). Cellulose and glass fiber filters were obtained from Advantec (Tokyo). The NCS-II scintillation mixture was from Amersham. All the other inorganic and organic chemicals were obtained from Wako Pure Chemicals (Osaka).
Plants.
Seeds of Asparagus officinalis L. cv. Mary Washington 500W (Takii Shubyo, Kyoto, Japan) were planted in moist sterile soil and kept in a growth room at 25°C ± 2°C under a daily 16-h light period as previously described (10). The cladodes of 40- to 60-day-old spears were used as the experimental material. Cell lines of Oryza sativa L. (gift from K. Shono, Dept. of Life Sciences, University of Tokyo) were maintained with weekly subculturing in Murashige and Skoog medium supplemented with 1.0 mg/liter 2,4-dichlorophenoxyacetic acid and 30 g/liter sucrose. Cultures were incubated at 25°C in the dark with rotary shaking at 120 rpm.
Conditioned Medium.
Aliquots of subcultured rice cells were suspended in 200 ml of culture medium and cultured in 500-ml Erlenmeyer flasks in the dark at 25°C with rotary shaking at 120 rpm for 10 days. Conditioned medium was separated from cultures by filtration (Advantec no. 2) and stored at −20°C.
Purification of the Factor(s).
Conditioned medium derived from rice suspension cultures (650 ml) was concentrated to 200 ml, adjusted to pH 8.0 with 6.0 M KOH, and then applied to a DEAE-Sephadex A-25 column (3.2 × 12 cm) that had been equilibrated with 20 mM Tris⋅HCl buffer, pH 8.0. The column was washed with 250 ml of equilibration buffer, and fractions were eluted successively with 250 ml of this buffer containing 400, 800, 1,200, or 1,600 mM KCl. Each fraction was desalted by dialysis (Spectra/Por 7 with molecular weight cut-off 1,000) and assayed. Mitogenic activities were detected by bioassay using asparagus mesophyll cells as previously described (10). Desalted active fractions recovered from the DEAE-Sephadex column (800 and 1,200 mM KCl fractions) were lyophilized, dissolved in 1.0 ml of 20 mM KH2PO4/KOH buffer, pH 5.8, and then applied to a Bio-Gel P-2 extra-fine column (1.7 × 42 cm) that had been equilibrated with the same buffer. Five-milliliter fractions were collected, and each was bioassayed. Active fractions recovered from the Bio-Gel column were lyophilized and further analyzed by liquid chromatography/mass spectrometry (LC/MS).
LC/MS Experiments.
Active fractions were dissolved in 200 μl of 0.1% trifluoroacetic acid (TFA) and separated on a reverse-phase HPLC column (4.6 × 250 mm) with 12% (vol/vol) acetonitrile containing 0.1% TFA at 0.5 ml/min. Mass spectra were obtained using a Fisons VG platform quadrupole mass spectrometer equipped to perform electrospray ionization, interfaced to a Jasco PU 980 HPLC system. The HPLC eluate was split 1:9 so that 50 μl/min flowed to the mass spectrometer during the separation. The source temperature was maintained at 70°C, and the range m/z 50–1000 was scanned over 1.9 s at unit resolution.
Amino Acid Sequence Analysis.
Amino acid sequences were determined by Edman degradation with an Applied Biosystems model 476A gas-phase sequencer. Phenylthiohydantoin derivatives of amino acids obtained at each cycle of the Edman degradation were determined by reverse-phase HPLC on an Applied Biosystems Brownlee C-18 column.
Agarose Block Assay.
The biological activities of PSK-α and -β for rice cells were bioassayed by using rice protoplasts and the agarose block technique (12). Rice cells (10-days culture) were suspended in 1.0% Cellulase Onozuka RS/0.1% Pectriase, in 0.55 M mannitol at pH 5.8, and incubated at 30°C for 2.0 h. Protoplasts were washed with 0.55 M mannitol and plated in thin layers of agarose (0.4%) in a 6-cm-diameter Petri dish at a density of 1.0 × 105 cells per ml. Agarose blocks were then cut into four pieces, transferred to 30 ml of Murashige and Skoog liquid medium containing 1.0 mg/liter 2,4-dichlorophenoxyacetic acid in the presence or absence of PSKs, and incubated at 25°C without shaking. After 4 weeks, total numbers of colonies (≥200-μm diameter) were scored under an inverted microscope.
Preparation of [35S]PSK-α.
The partially protected peptide-resin, Boc-Tyr(OH)-Ile-Tyr(OH)-Thr(t-Bu)-Gln(Trt)-linker-resin, was synthesized by Fastmoc chemistry with a peptide synthesizer (Applied Biosystems model 433A) (13). [35S]Sulfuric acid solution (925 MBq, 1,500 Ci/mmol) was lyophilized and diluted with 1.3 μl of unlabeled H2SO4. Partially protected peptide-resins (5.0 μmol) in dimethylformamide/pyridine (4:1, 0.5 ml) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (10 mg) were added to this preparation and stirred at room temperature. After 5 days, an additional 1.3 μl of unlabeled H2SO4 was added and the reaction mixture was stirred for 2 days further. The sulfated peptide-resin was collected by filtration, washed with distilled water, and cleaved in 1.0 ml of 95% aqueous TFA at room temperature for 30 min. The cleaved and deprotected peptides were precipitated with ice-cold diethyl ether (10 ml), dissolved in 1.0% NH4OH (1.0 ml), and purified by HPLC on a Develosil ODS-UG-5 column (8.0 × 250 mm) by isocratic elution of 10% acetonitrile containing 0.1% ammonium acetate at a flow rate of 2.0 ml/min. The total yield of [35S]PSK-α was 60 μg (1.4%) and its specific activity was 780 mCi/mmol.
Preparation of Rice Membrane Fractions.
Rice cells (100 g fresh weight) were homogenized in a blender in 150 ml of 25 mM Tris⋅HCl, pH 7.0/10 mM MgCl2/2 mM dithiothreitol/2.0 μM leupeptin/2 mM phenylmethylsulfonyl fluoride/250 mM sucrose at 4°C. The slurry was filtered through Miracloth (Calbiochem) and successively centrifuged at 1,000, 8,000, 13,000, and 40,000 × g. The membrane pellet obtained at each centrifugation step was suspended in 5.0 ml of 10 mM 2-(N-morpholino)ethanesulfonic acid (Mes), pH 5.8/10 mM MgCl2/100 mM sucrose. Vanadate-sensitive ATPase activity was determined according to O’Neill and Spanswick (14) except that the reaction mixture contained 0.02% Triton X-100. The released Pi was determined with the Pi assay kit (Phospha C-Test, Wako). Vanadate inhibition was achieved with 0.5 mM orthovanadate. Cytochrome-c oxidase was assayed spectrophotometrically at 25°C by following the oxidation of cytochrome c at 550 nm. The assay buffer contained 20 mM KH2PO4/KOH (pH 7.0), 50 μM cytochrome c reduced with sodium dithionite, and 0.1% Triton X-100. NADPH–cytochrome-c reductase was assayed by the method of Yoshida (15).
Binding Assay.
For binding assays using suspension cells, rice cells derived from 10-day suspension culture were filtered through 500-μm stainless steel meshes to remove large cell clusters, collected by centrifugation (100 × g), suspended in assay buffer (10 mM Mes/10 mM MgCl2/3% mannitol, pH 5.8) for 1 h, and washed twice with assay buffer. In the standard assay, 5.0-ml aliquots of cell suspension (0.5 ml of packed cells (≈2 × 106 cells) per 5.0 ml of buffer) in assay buffer containing 32 nM [35S]PSK-α (≈2 × 105 cpm) were incubated for 240 min with shaking at 80 rpm at 4°C in the absence or presence of 100-fold excess of unlabeled PSK-α. After incubation, the reaction mixture was filtered through a glass fiber filter (Advantec GC 50) and the cells were washed twice with the buffer (5.0 ml each) within 20 sec. Radioactivity of bound [35S]PSK-α was determined with a liquid scintillation counter (Beckman LS-6500) in 5.0 ml of scintillation mixture (Amersham NCS-II) at 95% counting efficiency.
For binding assays using membrane fractions, aliquots (400 μl) of membrane suspensions were incubated with 32 nM [35S]PSK-α for 240 min at 4°C in the absence or presence of 100-fold excess of unlabeled PSK-α. After incubation, the reaction mixture was overlaid onto 1.0 ml of assay buffer containing 0.5 M sucrose and centrifuged at 86,000 × g for 5.0 min at 4°C. The supernatant was discarded and the pellet was analyzed for radioactivity. Protein concentration was measured as described by Bradford (16) with BSA as a reference.
RESULTS
Identification of PSK-α in Rice Conditioned Medium.
Rice conditioned medium was first fractionated by stepwise elution from a DEAE-Sephadex column. The active factor(s) was strongly adsorbed on the column and was found in 800 mM and 1,200 mM KCl fractions. These fractions were desalted by dialysis and purified on a gel permeation column of Bio-Gel P-2. The factor(s) was eluted in the 40–45 ml fraction. The apparent Mr of this factor(s) was estimated as approximately 1,000 by comparison with the size markers. Active fractions from the gel permeation column were further purified on a reverse-phase HPLC column, with 10% of the eluate directly flowing to the mass spectrometer during separation. Two major peaks that possessed high specific biological activity with Mr of 846 and 718 were obtained (Fig. 1 A and B). The eluted materials had chemical properties of oligopeptides, that is, total loss of activity in the presence of Pronase E (data not shown). Amino acid sequence analysis of the active components revealed primary sequences of Tyr-Ile-Tyr-Thr-Gln and Tyr-Ile-Tyr-Thr. The physicochemical properties and primary amino acid sequences of the active principles are identical with those of PSK-α [Tyr(SO3H)-Ile-Tyr(SO3H)-Thr-Gln] and PSK-β [Tyr(SO3H)-Ile-Tyr(SO3H)-Thr] originally isolated from conditioned medium prepared from asparagus mesophyll cultures (10). PSK-α and -β chemically synthesized by solid-phase methods (13) cochromatographed with their natural counterparts on reverse-phase HPLC and showed symmetrical peaks, confirming the structures described above. Thus the active principles responsible for the “cross-feeding effect” between asparagus and rice cells were identified as PSKs.
Figure 1.
HPLC profile and LC/MS spectra of the active principles. (A) Active fractions eluted from the Bio-Gel P-2 column were dissolved in 200 μl of 0.1% TFA and separated on a reverse-phase column [Develosil ODS-5 (4.6 × 250 mm)] with 12% acetonitrile containing 0.1% TFA at 0.5 ml/min. (B and C) Mass spectra were obtained using a Fisons VG platform quadrupole mass spectrometer equipped to perform electrospray ionization, interfaced to an HPLC system. The HPLC eluate was split 1:9 so that 50 μl/min flowed to the mass spectrometer during separation. (B) Mass spectrum of the active compound eluted at 15.1 min. (C) Mass spectrum of the active compound eluted at 24.4 min.
Mitogenic Activities of PSKs for Rice Protoplasts.
The biological activities of PSK-α and -β for rice cells were bioassayed using protoplasts and the agarose block technique (12). Rice protoplasts cultured at suboptimal density in the presence of PSK-α and -β in the 10−8 to 10−6 M range gave rise to many microcalli after 4 weeks of culture (Fig. 2). Colony formation frequencies observed under the inverted microscope were as follows: 66.8% ± 7.5% (PSK-α, 1.0 × 10−6 M), 39.4% ± 2.1% (PSK-α, 1.0 × 10−8 M), 48.4% ± 3.0% (PSK-β, 1.0 × 10−6 M), 16.4% ± 0.8% (PSK-β, 1.0 × 10−8 M), and 13.7% ± 2.3% (control). These results indicated that PSKs act as growth factors for rice cells as well as asparagus cells. The concentrations of PSK-α and -β in partially purified rice conditioned medium analyzed by LC/MS were 3.4 × 10−8 M and 3.2 × 10−7 M, respectively, sufficient for stimulation of rice cell division. Although the biosynthetic mechanisms of PSK-α and -β have not been clarified, it is likely that PSK-β is an enzymatically degraded product of PSK-α because conversion occurred in the cell-free conditioned medium within 3 days (data not shown).
Figure 2.
Micrographs of microcalli derived from rice protoplasts. Rice protoplasts were plated in thin layers of agarose (0.4%) in a Petri dish at a density of 1.0 × 105 cells per ml. Agarose blocks were then cut into four pieces, transferred to Murashige and Skoog liquid medium containing 1.0 mg/liter 2,4-dichlorophenoxyacetic acid in the presence or absence of PSKs, and incubated at 25°C without shaking. After 4 weeks, total numbers of colonies (≥200-μm diameter) were scored under an inverted microscope. Colony formation frequency was as follows: 66.8% ± 7.5% [PSK-α, 1.0 × 10−6 M (A)], 39.4% ± 2.1% [PSK-α, 1.0 × 10−8 M (B)], 48.4% ± 3.0% [PSK-β, 1.0 × 10−6 M], 16.4% ± 0.8% [PSK-β, 1.0 × 10−8 M], and 13.7% ± 2.3% [control (C)]. (Bar = 500 μm.)
PSK-α Binding.
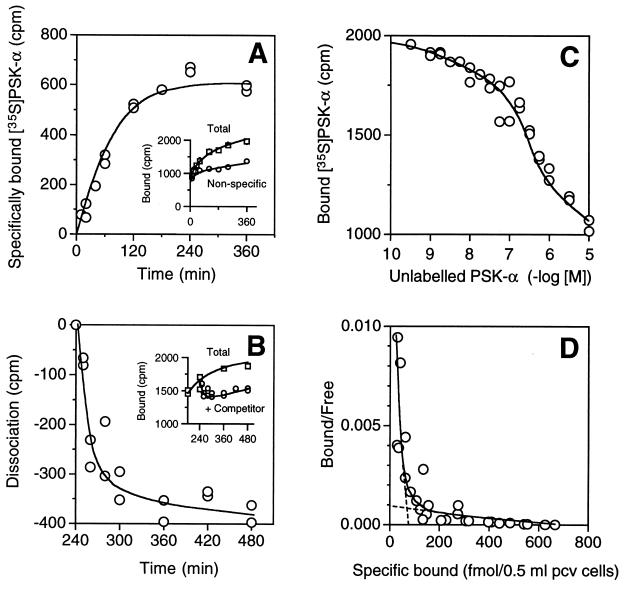
Because of the presence of highly hydrophilic tyrosine O-sulfates in PSK molecules, it is unlikely that they pass through plasma membranes and directly interact with target molecules inside cells. We therefore prepared radiolabeled PSK-α to determine whether cell surface receptor(s) exist. The radioisotope was introduced at the tyrosine sulfate residues; that is, H-Tyr(35SO3H)-Ile-Tyr(35SO3H)-Thr-Gln-OH was prepared at a specific activity of 780 mCi/mmol. When 10-day rice suspension cells were incubated with [35S]PSK-α, 0.7–1.0% of the radioactivity sedimented with the cell pellets (total binding). The specific binding of [35S]PSK-α was time-dependent with a t1/2 of 60 min, and maximal binding was achieved by 240 min, although nonspecific binding gradually increased thereafter (Fig. 3A). Addition of excess unlabeled ligand led to the loss of specifically bound radioactivity, and this displacement was virtually compete within 240 min, confirming the binding to be reversible (Fig. 3B). The specific binding of [35S]PSK-α was decreased by increasing the concentration of unlabeled PSK-α (Fig. 3C), and a Scatchard plot of the specific binding values gave a curvilinear profile, indicating that rice cells possess two types of PSK-α receptors (Fig. 3D): a high-affinity type (Kd1 = 1 × 10−9 M; 1 × 104 sites per cell) and a low-affinity type (Kd2 = 1 × 10−7 M; 1 × 105 sites per cell).
Figure 3.
(A) Specific binding of [35S]PSK-α to rice suspension cells as a function of incubation time. Rice cells were incubated at 4°C in the presence of 32 nM [35S]PSK-α and radioactivity of bound [35S]PSK-α was determined at the indicated time. Specific [35S]PSK-α binding is the binding displaceable by excess (100-fold) unlabeled ligand, obtained from total binding minus nonspecific binding (Inset). (B) Kinetics of [35S]PSK-α dissociation after addition of 3.2 μM unlabeled PSK-α. Dissociation was started 240 min after initial incubation with [35S]PSK-α. (Inset) Total binding curve after addition of the competitor. (C) Competitive displacement of [35S]PSK-α by unlabeled PSK-α. Rice cells were incubated for 240 min at 4°C in the presence of unlabeled PSK-α at the indicated concentrations. (D) Scatchard plot of specifically bound [35S]PSK-α. Nonspecific binding was subtracted from each value in C, and the resulting data were used for the Scatchard plot.
Displacement by Various PSK-α Analogs.
The possible significance of PSK-α binding to rice cells in the stimulation of cell proliferation was assessed by comparing the binding affinity and mitogenic activity of various PSK-α analogs. Of those tested, only the disulfated peptides effectively competed with the [35S]PSK-α for binding (Table 1). The differential effects of the various PSK-α analogs on radiolabeled ligand binding corresponded closely with their ability to induce cell proliferation in the asparagus mesophyll assay system described previously (13). The known plant hormones naphthaleneacetic acid and N6-benzyladenine, at a concentration of 1.0 mg/liter, did not affect [35S]PSK-α binding. Thus, the binding sites detected by this radioligand assay system were concluded to be PSK-α-specific receptor molecules that mediate physiological events inside the target cells triggered by binding PSK-α.
Table 1.
Competitive displacement of [35S]PSK-α by various PSK analogs
| Competitor | Bound [35S]PSK-α, relative value | Mitogenic activity (ED50), nM* |
|---|---|---|
| PSK-α | 0 | 4 |
| PSK-β | 58.2 | 50 |
| PSK-α-(1–3) | 26.2 | 20 |
| PSK-α-(2–5) | 118.1 | >1,000 |
| PSK-α-(1–2) | 103.6 | >1,000 |
| [OH]PSK-α | 97.6 | >1,000 |
| Naphthaleneacetic acid | 114.5 | — |
| N6-Benzyladenine | 102.2 | — |
| Control | 100.0 | — |
Rice cells were incubated with 32 nM [35S]PSK-α in the presence of 3.2 μM various synthetic PSK analogs or 1.0 mg/liter naphthaleneacetic acid or N6-benzyladenine. After 240 min, cells were collected by filtration and analyzed for radioactivity. Abbreviations: PSK-α-(1–3), Tyr(SO3H)-Ile-Tyr(SO3H); PSK-α-(2–5), Ile-Tyr(SO3H)-Thr-Gln; PSK-α-(1–2), Tyr(SO3H)-Ile; [OH]PSK-α, Tyr-Ile-Tyr-Thr-Gln.
See ref. 13.
Localization of Specific PSK-α Binding Sites.
Preliminary attempts to determine the location of PSK-α binding sites were made by differential centrifugation of rice cell homogenates. The results showed that [35S]PSK-α binding activity sedimented over a wide range of centrifugal forces, but the highest specific binding activity on a protein basis was observed with the fraction that precipitated at 40,000 × g (Table 2). The separation of plasma membrane from mitochondria and endoplasmic reticulum was analyzed by measuring the distribution in each fraction of the marker enzyme activities vanadate-sensitive ATPase (plasma membrane), cytochrome-c oxidase (mitochondria), and NADPH–cytochrome-c reductase (endoplasmic reticulum). As shown in Table 2, the 40,000 × g fraction was relatively rich in plasma membranes, although it was partially contaminated with lighter mitochondrial membranes. Specific [35S]PSK-α binding activity correlated well with the distribution of vanadate-sensitive ATPase activity, indicating an association of the [35S]PSK-α binding sites with a plasma membrane-enriched fraction.
Table 2.
Binding of [35S]PSK-α to various centrifugation fractions of rice cell membranes
| Centrifugation
|
Specific binding, fmol/mg protein | Marker enzyme activity, nmol/ min per mg protein
|
|||
|---|---|---|---|---|---|
| Force | Time, min | Vanadate-sensitive ATPase | Cyt-c oxidase | NADPH–Cyt-c reductase | |
| 1,000 × g | 10 | 8.4 | 9.4 | 2.7 | 2.0 |
| 8,000 × g | 15 | 27.2 | 13.9 | 15.7 | 2.4 |
| 13,000 × g | 15 | 44.7 | 28.9 | 23.0 | 2.1 |
| 40,000 × g | 30 | 60.1 | 33.3 | 22.0 | 2.9 |
Rice cell homogenate was filtered and successively centrifuged at various forces. The membrane pellets were suspended in binding buffer and aliquots (400 μl) of membrane suspensions were incubated with 32 nM [35S]PSK-α for 240 min at 4°C in the absence or presence of 100-fold excess of unlabeled PSK-α. After incubation, the reaction mixture was overlaid onto 1.0 ml of binding buffer containing 0.5 M sucrose and centrifuged at 86,000 × g for 5.0 min at 4°C. The supernatant was discarded and the pellet was analyzed for radioactivities. Marker enzymes used were as follows: vanadate-sensitive ATPase for plasma membrane, cytochrome-c oxidase for mitochondria, and NADPH–cytochrome-c reductase for endoplasmic reticulum.
DISCUSSION
Recently, there has been a growing awareness that oligopeptides play an important role as signaling molecules in plants (10, 17, 18). PSK-α, one of these biologically active peptides first isolated from conditioned medium derived from asparagus mesophyll culture, is considered to be the main chemical factor responsible for “conditioning” or “nursing”—i.e., the growth-promoting effects triggered by culture media previously used for cell culture or by physically separated “feeder” cells. Interestingly, such effects are often observed between heterologous plant species as well as homologous ones, indicating that the responsible mitogenic factor(s) is species-unspecific (cross-feeding effect) (2–9). In this paper, we have demonstrated that PSK-α is distributed in at least two plant families and actually acts as a growth factor for rice as well as asparagus cells. LC/MS analysis of partially purified rice conditioned medium revealed concentrations of PSKs to be similar or slightly higher than those found for asparagus conditioned medium (10) and sufficient for stimulation of rice cell division. We have also identified PSKs in conditioned medium of maize cultures (data not shown). However, we could not detect PSKs’ activity in conditioned medium derived from cell cultures of dicotyledonous plants, such as tobacco and Zinnia elegans, suggesting that the growth factor(s) of dicots have different structure(s) from PSKs. This is in line with cross-feeding experiments, which also pointed out incompatibility of factors between monocots and dicots (8, 9).
Using [35S]PSK-α, we have been able to identify binding sites on intact rice suspension cells and plasma membrane-rich fraction prepared by differential centrifugation. It is noteworthy that [35S]PSK-α does not demonstrate a modified chemical structure, in contrast to many 125I-labeled peptide ligands and, therefore, shows full biological activity (data not shown). [35S]PSK-α binding is reversible and is characterized by equilibrium binding constants (Kd) of 1 × 10−9 M (Kd1) and 1 × 10−7 M (Kd2), suggesting the presence of two types of binding sites in terms of their affinity. Expression of two classes of mammalian cell receptor binding sites has been reported for nerve growth factor (19), interleukin-2 (20), insulin-like growth factor (21), and epidermal growth factor (22). The relative affinity of high-affinity binding sites for [35S]PSK-α was here found to be the same or a little lower than those observed for such mammalian growth factors (10−11 to 10−9 M for high-affinity type), but 4 to 10,000 times higher than those for plant signaling molecules, such as the fungal glucan phytoalexin elicitor (23–25) and lipo-oligosaccharidic NodRm factors (26).
One remarkable result of the present study was the high ligand specificity of the binding. It is quite clear that displacement of the [35S]PSK-α from binding requires that the competitor fulfills stringent structural requirements (Table 2). The significance of the presence of two sulfated tyrosine residues should be emphasized. The total numbers of [35S]PSK-α binding sites (≥1 × 105 sites per cell) were also remarkably high and comparable to those for mammalian epidermal growth factor receptors found in human carcinoma cells and vulva epidermoid carcinoma cells (105 to 106 sites per cell) (27). Asparagus mesophyll cells were also found to have specific [35S]PSK-α binding sites on their surfaces, but the apparent binding was relatively low (≤2,000 sites per cell) and the binding constants could not be estimated. It is not clear whether hyperproduction of [35S]PSK-α binding sites found in rice suspension cells is related to their rapid growth as compared with asparagus mesophyll cells.
Another important observation is the distribution of [35S]PSK-α binding in subcellular fractions from rice suspension cells. Analysis of [35S]PSK-α binding in differential centrifugation fractions suggested that the binding is associated with a plasma membrane-enriched fraction, although its affinity type could not be determined because of the relatively low amount of specific binding. Trials to prepare the radiolabeled ligand with higher specific activities without changing the chemical structure of PSK-α are now in progress.
In conclusion, our present studies demonstrated that the sulfated pentapeptide PSK-α is distributed in at least two plant families and that it is involved in plant cell proliferation mediated by specific membrane-associated binding. Because this PSK-α binding is not affected by auxin or cytokinin competition, the results indicate the existence of novel signal transduction pathways that activate genes responsible for cell proliferation in plants.
Acknowledgments
We thank Dr. K. Shono (Department of Life Sciences, University of Tokyo) for providing the O. sativa (Oc) cell lines. This research was supported in part by the Program for Promotion of Basic Research Activities for Innovative Biosciences and by Research Fellowships of the Japan Society for the Promotion of Science for Young Scientists.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: PSK, phytosulfokine; LC/MS, liquid chromatography/mass spectrometry; TFA, trifluoroacetic acid.
References
- 1.Stuart R, Street H E. J Exp Bot. 1969;20:556–571. [Google Scholar]
- 2.Benbadis A. Les Cultures de Tissus de Plantes. Paris: Colloq. Nat. Centre National de la Recherche Scientifique; 1968. pp. 121–130. [Google Scholar]
- 3.Vardi A, Raveh D. Z Pflanzenphysiol. 1976;78:350–359. [Google Scholar]
- 4.Cella R, Galun E. Plant Sci Lett. 1980;19:243–252. [Google Scholar]
- 5.Schäffler E, Koop H U. J Plant Physiol. 1990;137:95–101. [Google Scholar]
- 6.Bellincampi D, Morpurgo R, Morpurgo G. Physiol Plant. 1993;88:99–104. [Google Scholar]
- 7.Somers D A, Birnberg P R, Petersen W L, Brenner M L. Plant Sci (Limerick) 1987;53:249–256. [Google Scholar]
- 8.Hahne B, Lörz H, Hahne G. Plant Cell Rep. 1990;8:590–593. doi: 10.1007/BF00270060. [DOI] [PubMed] [Google Scholar]
- 9.Smith J A, Green C E, Gengenbach B G. Plant Sci Lett. 1984;36:67–72. [Google Scholar]
- 10.Matsubayashi Y, Sakagami Y. Proc Natl Acad Sci USA. 1996;93:7623–7627. doi: 10.1073/pnas.93.15.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birnberg P R, Somers D A, Brenner M L. J Plant Physiol. 1988;132:316–321. [Google Scholar]
- 12.Shillito R D, Paszkowski J, Potrykus I. Plant Cell Rep. 1983;2:244–247. doi: 10.1007/BF00269151. [DOI] [PubMed] [Google Scholar]
- 13.Matsubayashi Y, Hanai H, Hara O, Sakagami Y. Biochem Biophys Res Commun. 1996;225:209–214. doi: 10.1006/bbrc.1996.1155. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill S D, Spanswick R M. Plant Physiol. 1984;75:586–591. doi: 10.1104/pp.75.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida S. Plant Physiol. 1979;64:241–246. doi: 10.1104/pp.64.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.Pearce G, Strydom D, Johnson S, Ryan C A. Science. 1991;253:895–898. doi: 10.1126/science.253.5022.895. [DOI] [PubMed] [Google Scholar]
- 18.van de Sande K, Pawlowski K, Czaja I, Wieneke U, Schell J, Schmidt J, Walden R, Matvienko M, Wellink J, van Kammen A, Franssen H, Bisseling T. Science. 1996;273:370–373. doi: 10.1126/science.273.5273.370. [DOI] [PubMed] [Google Scholar]
- 19.Sutter A, Riopelle R J, Harris-Warrick R M, Scooter E M. J Biol Chem. 1979;254:5972–5982. [PubMed] [Google Scholar]
- 20.Wang H, Smith K A. J Exp Med. 1987;166:1055–1069. doi: 10.1084/jem.166.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tollefsen S E, Thompson K, Petersen D J. J Biol Chem. 1987;262:16461–16469. [PubMed] [Google Scholar]
- 22.Gamou S, Kim Y S, Shimizu N. Mol Cell Endocrinol. 1984;37:205–213. doi: 10.1016/0303-7207(84)90053-4. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa M, Keen N T, Wang M C. Plant Physiol. 1983;73:497–506. doi: 10.1104/pp.73.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walter E, Ebel J. Proc Natl Acad Sci USA. 1987;84:4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cosio E G, Pöpperl H, Schmidt W E, Ebel J. Eur J Biochem. 1988;175:309–315. doi: 10.1111/j.1432-1033.1988.tb14198.x. [DOI] [PubMed] [Google Scholar]
- 26.Bono J, Riond J, Nicolaou K C, Bockovich N J, Estevez V A, Cullimore J V, Ranjeva R. Plant J. 1995;7:253–260. doi: 10.1046/j.1365-313x.1995.7020253.x. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter G, Cohen S. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]