Abstract
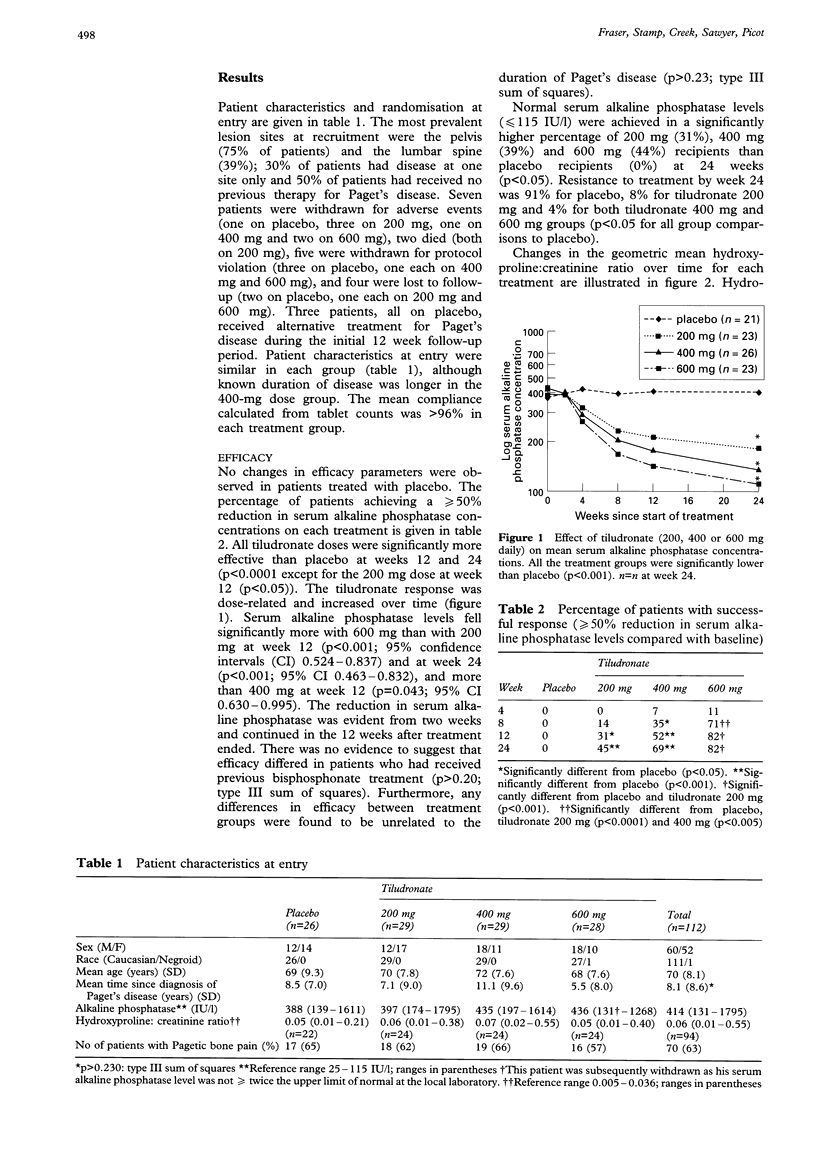
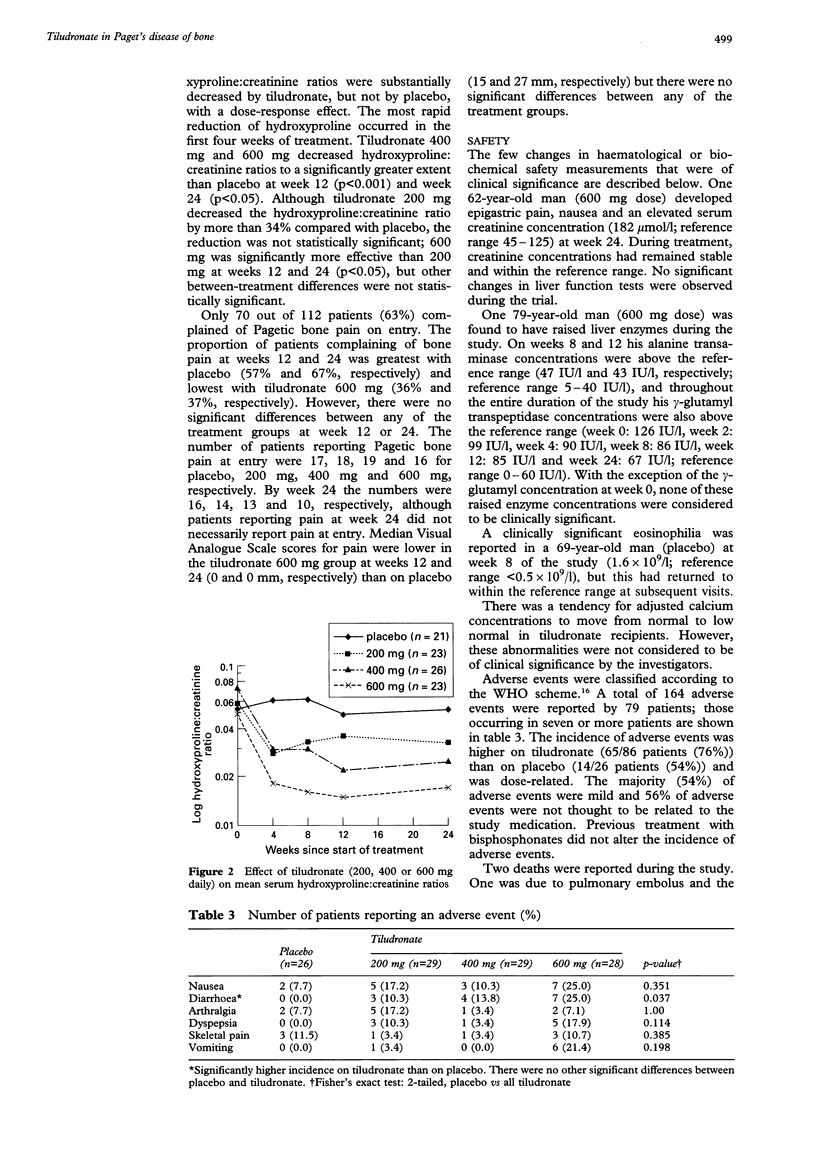
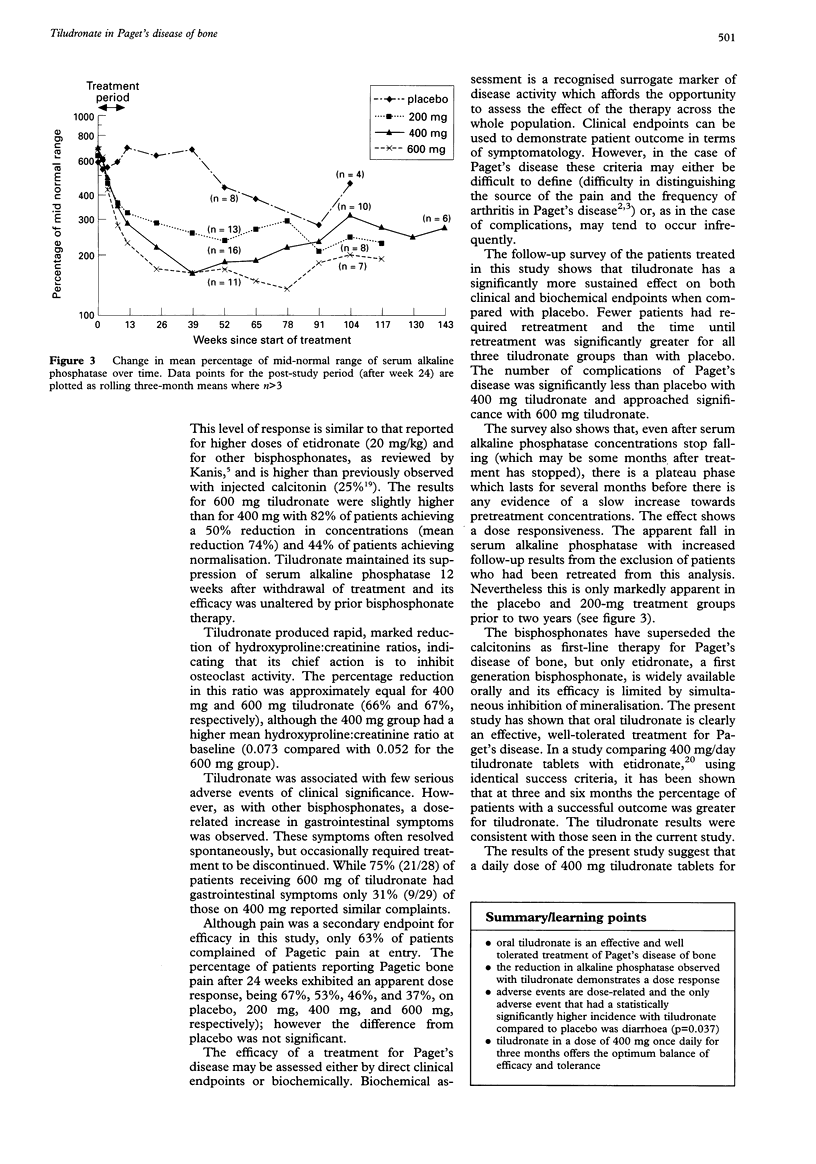
A multicentre, randomised, placebo-controlled, dose-ranging study was conducted to investigate the therapeutic activity and sustained efficacy of tiludronate (200 mg, 400 mg and 600 mg once daily) taken orally for 12 weeks in patients with Paget's disease. Serum alkaline phosphatase concentrations were compared with baseline at weeks 12 and 24; treatment success was defined as a 50% reduction compared with baseline. Changes in the hydroxyproline: creatinine ratio were also measured. Pain was assessed using the Huskisson Visual Analogue Scale and by questionnaire. Patients completing at least 11 weeks of treatment were followed-up 18 months later by postal questionnaire. Significantly greater numbers of patients in the tiludronate groups successfully responded to treatment compared with the placebo group. A dose-response was observed; the percentage of patients responding to treatment being 31% (200 mg), 52% (400 mg) and 82% (600 mg) at week 12 and 45% (200 mg), 70% (400 mg) and 82% (600 mg) at week 24. Tiludronate treatment also significantly reduced hydroxyproline: creatinine ratios compared with placebo, again showing a dose response. Dose-related gastrointestinal symptoms were the commonest adverse events, occurring in 2.4%, 11.0%, 5.5% and 18.9% of patients receiving placebo and tiludronate 200, 400 and 600 mg daily, respectively. The response to oral tiludronate was sustained for more than 18 months in some patients and there was evidence of a reduction in the longer term complications of the disease. These results show that oral tiludronate is an effective, well-tolerated treatment for Paget's disease; the 400 mg once daily dose appears to offer the optimum balance of efficacy and tolerance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyce B. F., Smith L., Fogelman I., Johnston E., Ralston S., Boyle I. T. Focal osteomalacia due to low-dose diphosphonate therapy in Paget's disease. Lancet. 1984 Apr 14;1(8381):821–824. doi: 10.1016/s0140-6736(84)92272-4. [DOI] [PubMed] [Google Scholar]
- Cantrill J. A., Anderson D. C. Treatment of Paget's disease of bone. Clin Endocrinol (Oxf) 1990 Apr;32(4):507–518. doi: 10.1111/j.1365-2265.1990.tb00892.x. [DOI] [PubMed] [Google Scholar]
- DeRose J., Singer F. R., Avramides A., Flores A., Dziadiw R., Baker R. K., Wallach S. Response of Paget's disease to porcine and salmon calcitonins: effects of long-term treatment. Am J Med. 1974 Jun;56(6):858–866. doi: 10.1016/0002-9343(74)90815-8. [DOI] [PubMed] [Google Scholar]
- Detheridge F. M., Guyer P. B., Barker D. J. European distribution of Paget's disease of bone. Br Med J (Clin Res Ed) 1982 Oct 9;285(6347):1005–1008. doi: 10.1136/bmj.285.6347.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch H. Diphosphonates: history and mechanisms of action. Metab Bone Dis Relat Res. 1981;3(4-5):279–287. doi: 10.1016/0221-8747(81)90044-8. [DOI] [PubMed] [Google Scholar]
- Gray R. E., Yates A. J., Preston C. J., Smith R., Russell R. G., Kanis J. A. Duration of effect of oral diphosphonate therapy in Paget's disease of bone. Q J Med. 1987 Sep;64(245):755–767. [PubMed] [Google Scholar]
- Grunstein H. S., Clifton-Bligh P., Posen S. Paget's disease of bone: experiences with 100 patients treated with salmon calcitonin. Med J Aust. 1981 Sep 19;2(6):278–280. doi: 10.5694/j.1326-5377.1981.tb128316.x. [DOI] [PubMed] [Google Scholar]
- Hamilton C. R., Jr Effects of synthetic salmon calcitonin in patients with Paget's disease of bone. Am J Med. 1974 Mar;56(3):315–322. doi: 10.1016/0002-9343(74)90613-5. [DOI] [PubMed] [Google Scholar]
- Huskisson E. C. Measurement of pain. Lancet. 1974 Nov 9;2(7889):1127–1131. doi: 10.1016/s0140-6736(74)90884-8. [DOI] [PubMed] [Google Scholar]
- MacIntyre I., Evans I. M., Hobitz H. H., Joplin G. F., Stevenson J. C. Chemistry, physiology, and therapeutic applications of calcitonin. Arthritis Rheum. 1980 Oct;23(10):1139–1147. doi: 10.1002/art.1780231011. [DOI] [PubMed] [Google Scholar]
- Recker R. R., Saville P. D. Intestinal absorption of disodium ethane-1-hydroxy-1,1-diphosphonate (disodium etidronate) using a deconvolution technique. Toxicol Appl Pharmacol. 1973 Apr;24(4):580–589. doi: 10.1016/0041-008x(73)90219-6. [DOI] [PubMed] [Google Scholar]
- Reginster J. Y., Colson F., Morlock G., Combe B., Ethgen D., Geusens P. Evaluation of the efficacy and safety of oral tiludronate in Paget's disease of bone. A double-blind, multiple-dosage, placebo-controlled study. Arthritis Rheum. 1992 Aug;35(8):967–974. doi: 10.1002/art.1780350819. [DOI] [PubMed] [Google Scholar]
- Reginster J. Y. Oral tiludronate: pharmacological properties and potential usefulness in Paget's disease of bone and osteoporosis. Bone. 1992;13(5):351–354. doi: 10.1016/8756-3282(92)90450-b. [DOI] [PubMed] [Google Scholar]
- Roux C., Gennari C., Farrerons J., Devogelaer J. P., Mulder H., Kruse H. P., Picot C., Titeux L., Reginster J. Y., Dougados M. Comparative prospective, double-blind, multicenter study of the efficacy of tiludronate and etidronate in the treatment of Paget's disease of bone. Arthritis Rheum. 1995 Jun;38(6):851–858. doi: 10.1002/art.1780380620. [DOI] [PubMed] [Google Scholar]
- Winfield J., Stamp T. C. Bone and joint symptoms in Paget's disease. Ann Rheum Dis. 1984 Dec;43(6):769–773. doi: 10.1136/ard.43.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]


