Abstract
In modern cochlear implants, much of the information required for recognition of important sounds is conveyed by temporal modulation of the charge per phase in interleaved trains of electrical pulses. In this study, modulation detection thresholds (MDTs) were used to assess listeners’ abilities to detect sinusoidal modulation of charge per phase at each available stimulation site in their 22-electrode implants. Fourteen subjects were tested. MDTs were found to be highly variable across stimulation sites in most listeners. The across-site patterns of MDTs differed considerably from subject to subject. The subject-specific patterns of across-site variability of MDTs suggest that peripheral site-specific characteristics, such as electrode placement and the number and condition of surviving neurons, play a primary role in determining modulation sensitivity. Across-site patterns of detection thresholds (T levels), maximum comfortable loudness levels (C levels) and dynamic ranges (DRs) were not consistently correlated with across-site patterns of MDTs within subjects, indicating that the mechanisms underlying across-site variation in these measures differed from those underlying across-site variation in MDTs. MDTs sampled from multiple sites in a listener’s electrode array might be useful for diagnosing across-subject differences in speech recognition with cochlear implants and for guiding strategies to improve the individual’s perception.
I. INTRODUCTION
Most modern auditory prostheses use both spectral cues mapped to electrode place and temporal cues mapped to the envelope of the electrical signal. Typically, the acoustic signal is received by a microphone and divided into a number of channels using bandpass filters. The temporal envelopes of the filtered signals are then extracted and used to amplitude modulate the charge per phase of pulses in continuous-interleaved pulse trains. These trains of charge-modulated pulses are then sent to individual electrodes arranged along the tonotopic axis of the cochlea or a central auditory nucleus. Spectral resolution with current auditory prostheses is limited, so listeners are strongly dependent on the temporal-envelope information in the electrical signal. Listeners can typically achieve reasonable speech recognition with as few as four spectral channels as long as there is sufficient temporal-envelope information (Shannon et al., 1995; Xu et al., 2005).
Temporal modulation patterns are important for the perception of voicing and manner of consonants (Van Tasell et al., 1987), recognition of lexical tone (Xu et al., 2002), and for sound-source segregation (Bregman et al., 1990; Chatterjee et al., 2006). Thus, improvements in cochlear implant users’ abilities to detect temporal modulation might lead to improved perception of speech and other important auditory signals.
Modulation detection thresholds (MDTs) are a useful measure of the listener’s acuity for detection of temporal modulations. They can be used to assess the listener’s ability to detect relatively slow modulations in the charge per phase of electrical pulse trains, like those in the temporal-envelope encoding of speech information in an auditory prosthesis. MDTs have been found to correlate with speech recognition across listeners using cochlear and brainstem implants (Fu, 2002; Colletti and Shannon, 2005). These studies have demonstrated considerable across-listener variability in MDTs. In comparing MDTs in two populations of patients with auditory brainstem implants, Colletti and Shannon (2005) found that a population of patients with excised acoustic tumors had higher MDTs than patients who had no acoustic tumor. They concluded that the tumor patients had higher MDTs because neural pathways important for modulation detection were disrupted.
Although these studies examining modulation detection in listeners with auditory prostheses provided informative results pointing to possible causes of across-listener variability, they did not investigate within-listener variability of MDTs across stimulation sites in the electrode array. Cochlear implants typically have up to 22 stimulation sites positioned along the length of the scala tympani. Each of these sites could feasibly differ in sensitivity to modulation. Fu’s (2002) examination of modulation detection used only one stimulation site located near the center of the electrode array. In a recent report, we tested MDTs at three sites spaced evenly in the basal, middle, and apical parts of the electrode array and found that there was variation in MDTs across the three sites (Pfingst et al., 2007). In the current study, we explored this variation in greater detail by measuring MDTs at all available sites along the electrode array at two listening levels.
Examining across-site variability in MDTs is important because it could provide clues about what mechanisms underlie modulation detection in cochlear implant users and this in turn could guide rehabilitation strategies. First, it is important to know if the origins of differences in MDTs across various cochlear implant users are peripheral or central. That is, do MDTs reflect the general ability of the listener to detect modulation or does modulation detection depend on conditions near the individual stimulation sites? We hypothesize that a listener’s ability to detect modulation in the electrical signal depends on peripheral conditions in the implanted listener’s cochlea. Such conditions might include the nerve survival pattern near each electrode and/or the location of each electrode with respect to the modiolus. Conditions in the scala tympani, such as bone and tissue growth, might also affect the current path from the electrodes to the excitable neural elements. Each of these variables could affect the number of fibers activated and the sites of activation and thus they could affect the perception of the signal. These conditions are known to vary along the length of the implanted cochlea in a deaf ear, and the pattern of this variation differs from person to person (Hinojosa and Lindsay, 1980; Nadol, 1997; Saunders et al., 2002). We reason that if these conditions affect modulation detection, then we should find variation in MDTs across the individual stimulation sites within most listeners. Alternatively, if modulation detection simply relates to a listener’s general ability to perceive modulated signals, then we would expect a more uniform across-site pattern of MDTs within listeners. Further, it is important to know, assuming the conditions determining MDTs are peripheral in origin, the degree to which these conditions are localized with respect to the individual stimulation sites in the cochlear implant electrode array.
Previous studies conducted in our laboratory demonstrated across-site variability in detection thresholds (T levels), maximum comfortable loudness levels (C levels), and dynamic ranges (DRs) (Pfingst et al., 2004; Pfingst and Xu, 2004, 2005). Given this result, it is reasonable to expect that other psychophysical measures would also vary across stimulation sites in most cochlear implant users. If across-site variation in MDTs is found, it is important to know whether or not the across-site patterns of variation in MDTs match the across-site patterns of T levels, C levels, and DRs. Examining the relationship between T levels, C levels, and DRs could tell us whether or not the across-site patterns are due to common underlying mechanisms.
In addition, it would be clinically useful to know if the across-site patterns of MDTs and these other psychophysical measures are similar. T and C levels are routinely collected before programming a patient’s processor and DRs are derived from these measures. Thus, if T levels, C levels, or DRs show the same across-site pattern as MDTs, they could serve as a more clinically convenient and less time-consuming tool for identifying stimulation sites that are weak in modulation detection.
Knowledge about the causes of variation in modulation detection and the relationship of modulation detection to other psychophysical measures is also important for the design of clinical rehabilitation strategies. If the variation in MDTs is due to peripheral physiological and anatomical variables, then it might be appropriate to design rehabilitation strategies on an individual-electrode basis. Sites that show weaknesses in modulation detection might be ineffective or even distracting during speech perception tasks. Rehabilitation strategies could involve deactivating electrodes identified as having high MDTs or adjusting the stimulation parameters (e.g., pulse rate, electrode configuration, etc.) at a given stimulation site based on parameters that are most conducive to detecting modulation at that site. On the other hand, if poor modulation detection abilities are caused by general perceptual deficits, perceptual training might be a more effective method of lowering MDTs.
In summary, across-site patterns of MDTs might reflect the mechanisms that underlie modulation detection abilities in cochlear implant users and thus inform clinicians as to the best approaches for rehabilitation. Highly variable across-site MDTs would suggest that site-specific characteristics contribute to modulation detection and direct the focus to site-specific treatments. Alternatively, if listeners have poor MDTs that vary minimally across sites, a more general deficit in recognizing temporal modulations might be present, which would suggest using a training procedure to improve the use of available cues. Thus, the knowledge gained from this study might be useful in developing clinical rehabilitation strategies to help improve temporal-envelope processing, which could lead to more accurate consonant recognition and overall improvements in perception with cochlear implants.
In this study, a 40 Hz modulation frequency was used to modulate the phase duration of pulses in a constant-rate pulse train. This frequency was chosen as an intermediate value in the range of modulation frequencies important for cochlear implant users. For English phoneme recognition, the most important temporal envelope information occurs in the frequency region below 50 Hz (Drullman et al., 1994a, b; Shannon et al., 1995; Fu and Shannon, 2000; Xu et al., 2005), whereas higher-frequency cues (50–500 Hz) have been found to benefit lexical-tone recognition (Fu et al., 1998; Xu et al., 2002) and voice gender recognition (Fu et al., 2004). Most current cochlear prosthesis speech processors provide temporal envelope cues up to about 200–400 Hz (Wilson, 2004). However, modulation detection in cochlear implant users typically begins to decline above about 100 Hz modulation frequency and most subjects cannot detect modulation above 300 Hz (Shannon, 1992).
II. METHOD
A. Subjects
Fourteen postlingually deafened adults fitted with Nucleus cochlear implants participated in the study. Eleven of the subjects used the Nucleus CI24R (Contour) array, and three of the subjects used the CI24M (straight) array. All subjects were native speakers of American English and had at least two years (mean of 4.4 years) of experience with their device before beginning the experiment. Table I lists these and other demographic and clinical characteristics of the subjects. The use of human subjects for this research was approved by the University of Michigan Medical School Institutional Review Board.
TABLE I.
Demographic and clinical characteristics of the subjects used in the current study.
| ID | Gender | Age at onset of deafness (years) | Age at Testing (years) | Duration of deafness before implantation (years) | Duration of CI use | Implant | Processing strategy | Etiology of deafness | Sites tested |
|---|---|---|---|---|---|---|---|---|---|
| S45 | M | 44 | 50 | 1 | 5 | CI24R(CS) | ACE | Head injury | 3–22 |
| S53 | F | 26 | 58 | 26 | 6 | CI24R(CS) | ACE | Unknown | 3–22 |
| S54 | F | 32 | 71 | 35 | 4 | CI24R(CS) | ACE | Ototoxicity | 3, 5–22 |
| S57 | F | 60 | 68 | 3 | 5 | CI24M | SPEAK | Fever/Ménière’s | 3–21 |
| S58 | F | 42 | 57 | 6 | 9 | CI24M | SPEAK | Autoimmune | 3–22 |
| S60 | M | 62 | 67 | 2 | 3 | CI24R(CS) | ACE | Hereditary | 3–22 |
| S64 | F | 50 | 59 | 1 | 8 | CI24M | SPEAK | Unknown | 3–22 |
| S65 | F | 35 | 38 | 1 | 2 | CI24R(CS) | ACE | Hereditary | 2–13, 15–22 |
| S67 | M | 59 | 65 | < 1 | 6 | CI24R(CS) | ACE | Hereditary | 1–5, 7–21 |
| S71 | M | 57 | 60 | < 1 | 3 | CI24R(CS) | ACE | Unknown | 2–7, 9–22 |
| S72 | F | 16 | 66 | 47 | 3 | CI24R(CS) | ACE | Enlarged vestibular aqueduct | 5–13, 15–22 |
| S73 | M | 50 | 64 | 12 | 2 | CI24R(CS) | ACE | Unknown | 4–22 |
| S74 | F | 61 | 66 | 2 | 3 | CI24R(CS) | ACE | Unknown | 3–22 |
| S75 | M | 53 | 58 | 2 | 3 | CI24R(CS) | ACE | Noise induced | 2–21 |
B. Hardware and software for electrical stimulation
The listeners completed psychophysical tests wearing a laboratory-owned SPrint processor (Serial Number 408594, Cochlear Corporation, Englewood, CO) connected to a Processor Control Interface (Cochlear Corporation). The input to the processor was generated through the Nucleus Implant Communicator software libraries (version 3.27) and an IF5 ISA card. Listeners’ own implanted receiver/stimulators received radio frequency pulses generated by the processor, and these pulses were transmitted as electrical current to the appropriate sites in the implanted 22-electrode array. The calibration value of each listener’s receiver/stimulator was obtained from Cochlear Corporation and used to calculate the stimulation levels in peak microamperes. These levels were then converted to decibels of current using the formula
where x is the level in microamperes.
C. Identification of testable sites
Prior to testing, each listener’s map was evaluated using CustomSound software (Cochlear Corporation) to determine which sites could be tested. Sites were excluded from testing if they were not used in the listener’s regular clinically programmed map. Stimulation sites are commonly turned off during mapping if their stimulation results in uncomfortable or non-auditory sensations or if there is an electrical short. Stimulation sites used for each listener are listed in Table I.
D. Psychophysical testing
Listeners completed psychophysical tests to determine thresholds (T levels), comfort levels (C levels), and MDTs at all available sites in the electrode array.
T and C levels were obtained using symmetric-biphasic pulses of 50 μs/phase with an 8 μs interphase gap and a pulse rate of 250 pulses/s (pps). The stimulus burst duration was 600 ms presented in an on/off duty cycle with a 600 ms interburst interval. Monopolar stimulation (MP1+2) was used in all cases. In this configuration current is passed between a single electrode in the scalatympani implant and two connected electrodes outside the cochlea.
Listeners used the method of adjustment to set T and C levels. Each trial started with the initiation of the on/off cycling of the stimulus. To record the T level, listeners were instructed to adjust the level of the signal up or down until it was “just barely audible.” Adjustments were made by using the mouse to click on large and small boxes on the computer screen representing 5 Cochlear Level Unit and 1 Cochlear Level Unit increases and decreases (where 1 Cochlear Level Unit equals 0.176 dB of current). Listeners recorded their T level by clicking on a button when they were satisfied with the level they reached. Once the T level was recorded, listeners began increasing the stimulus level until the C level was reached. Listeners were instructed to record a C level when they reached a level that was “the loudest they could listen to comfortably for an extended period of time.”
The stimuli used for the modulation detection task were symmetric-biphasic pulses with a mean pulse duration of 50 μs/phase and an interphase gap of 8 μs. The pulse rate was 250 pps and stimulus duration was 600 ms. The interstimulus duration was 600 ms. Monopolar stimulation (MP1+2) was used in all cases. The phase duration of the pulses was modulated by a 40 Hz sinusoid which started and ended at zero phase. The positive and negative phases of the pulses were modulated equally to maintain charge balance while the interphase gap was held constant.
The modulation index (m) was defined as:
where PDmax and PDmin are the maximum and minimum phase durations, respectively. We report modulation values in decibels (dB) re 100% modulation (i.e., 20 log m). In the results section, these values are plotted with the lowest values (most sensitive modulation thresholds) at the top of the ordinate and/or the right of the abscissa.
Phase duration modulation rather than amplitude modulation was used for these experiments because the limits of the implanted stimulators allowed finer control of charge per phase when phase duration was modulated compared to when amplitude was modulated. The smallest step size in phase duration available with these stimulators was 0.2 μs. Thus, with a mean phase duration of 50 μs the smallest achievable modulation was 0.4% or −47.96 dB re 100% modulation.
Listeners’ T and C levels were used to determine their DRs for each site and to set the levels of the stimuli for the modulation detection task. MDTs were measured at 30% and 70% of DR in decibels of current. Fu (2002) has suggested that measurement of MDTs at multiple levels is necessary to adequately characterize the listener’s modulation-detection ability. The decision to measure MDTs at only two levels was based on an analysis of data from a previous study (Pfingst et al., 2007). In that study, MDTs were measured in 12 cochlear implant users at five stimulus levels (10%, 30%, 50%, 70%, and 90% of DR in decibels of current) and three stimulation sites: one basal, one middle, and one apical site. MDTs were measured for two carrier rates: 250 pps and 4 kpps. For this analysis, we used the data for the 250 pps carrier to match the rate used in the current study. A bivariate, across-listeners, correlation of mean MDTs taken at two levels and mean MDTs taken at five levels was significant at all three sites (r = 0.99, p < 0.001 in each case). Based on this analysis, we concluded that MDTs could be adequately characterized by measuring MDTs at only two levels: 30% and 70% of DR.
MDTs were obtained using a two-interval, forced-choice paradigm with flanking cues. On each trial, listeners were presented with four sequential observation intervals marked by squares on the computer screen. These squares were illuminated in sequence as the electrical stimuli were presented to the implant. The interstimulus interval was 600 ms. The first and fourth interval contained identical unmodulated pulse trains which served as flanking cues. One of the other intervals (interval 2 or interval 3, chosen at random on each trial) also contained this unmodulated signal. The modulated pulse train occurred in the remaining interval. Listeners were instructed to choose the interval (interval 2 or interval 3) containing the stimulus that sounded different from the other three. Selections were made by using the computer mouse to click on the desired square.
A two-down, one-up adaptive procedure (Levitt, 1971) was used, starting with a modulation depth of 50% and ending when 14 reversals were recorded. Modulation depth was increased or decreased in steps of 6 dB to the first reversal, 2 dB for the next two reversals, and 1 dB for the next 10 reversals. The MDT was defined as the mean of the levels at the last 8 reversal points. MDTs were measured in each listener at all available stimulation sites and at both stimulus levels (30% and 70% of DR) two times in random order. If the difference between the two estimates obtained for a condition exceeded 7 dB, a third estimate of MDT was made for that condition and the outlier was discarded (see Pfingst et al., 2007). A third measurement was required for only 5.9% of the total measurements.
III. RESULTS
A. Across-site patterns of MDTs
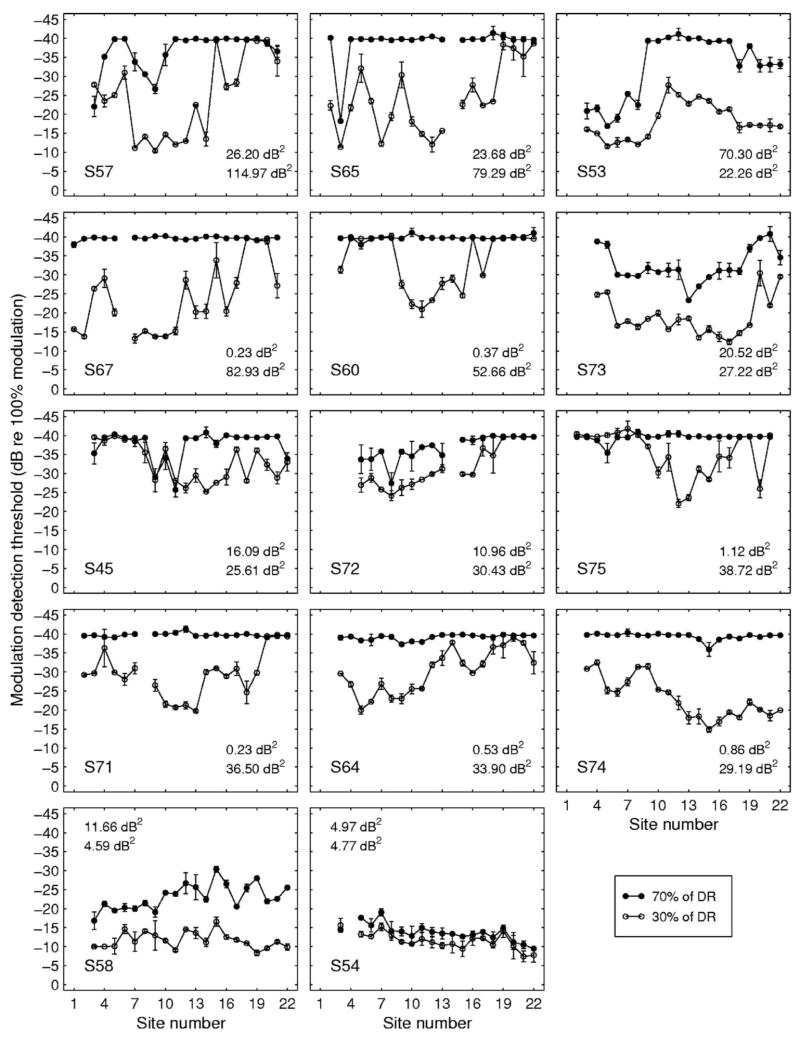
There was considerable across-subject and across-site (within-subject) variability in MDTs. Further, the pattern of variation across sites differed markedly from subject to subject. Figure 1 shows the across-site patterns for the 14 subjects. The across-site variances of each listener’s MDTs for each of the two levels tested are shown in the lower right-hand or upper left-hand corner of each panel. The top number is the across-site variance in square decibels for MDTs at 70% of DR and the number below is the across-site variance for MDTs at 30% of DR. The subjects demonstrated a wide range of variability in MDTs measured at 30% of DR (σ = 4.59–114.97 dB2) and 70% of DR (σ = 0.23–70.30 dB2). Many listeners had near minimal MDTs at the majority of sites when the stimulus level was 70% of DR (M = −34.93 dB re 100% modulation, SD = 7.62) and thus the across-site variance was lower at this level. MDTs were higher (M = −24.91 dB, SD = 7.73) and more variable at 30% of DR.
FIG. 1.
Across-site patterns of MDTs for 14 subjects. Each panel shows data for one subject. MDTs measures at 70% of DR (filled symbols) and 30% of DR (open symbols) are plotted as a function of stimulation site. Error bars show the two MDTs obtained for each level at each site and the open and filled circles show the means of these two values. Stimulation sites are numbered in order from the most basal electrode in the 22-electrode array (Site 1) to the most apical electrode (Site 22). Subject numbers are given in the lower left corner of each panel. The variances shown in each panel (dB2) are the across-site variance for MDTs measured at 70% of DR (top number) and the across-site variance for MDTs measured at 30% of DR (bottom number). The mean of these two variances was used to order the panels with the data for the subject with the highest mean variance shown in the upper left-hand panel and the subject with the lowest mean variance shown in the lower right-hand panel.
Across-site variation in MDTs could be due to localized variation in physiological and biophysical variables that result from subject-specific patterns of pathology along the length of the cochlea. However, systematic normal physiological differences along the length of the cochlea, systematic differences in susceptibility to pathology along the cochlear length, or systematic differences in medial-lateral electrode location as a function of cochlear length could also contribute to across-site variation in MDTs. To determine if there were systematic differences in MDTs along the cochlear length, the MDT data were divided into three groups based on the segment of the implant where the stimulation occurred: Basal (Sites 1–8), middle (Sites 9–15), and apical (Sites 16–22). Across-subject mean MDTs for each segment are shown in Fig. 2. A repeated-measures analysis of variation (ANOVA) indicated that there was an effect of segment on MDTs when the MDTs were measured at 30% of DR [F(2,41) = 4.695, p = 0.0182]. A post-hoc Tukey test indicated that the mean of the MDTs from the apical segment (M = −27.76 dB re 100% modulation, SD = 9.84 dB) was lower than the mean of the MDTs from the middle segment (M = −22.12 dB, SD = 6.13 dB). The mean of the MDTs from the basal segment (M = −24.92 dB, SD = 9.48 dB) was not significantly different from those from the two other segments. There was no statistically significant effect of segment on MDTs when measurements were made at 70% of DR [F(2,41) = 2.345, p = 0.1158].
FIG. 2.
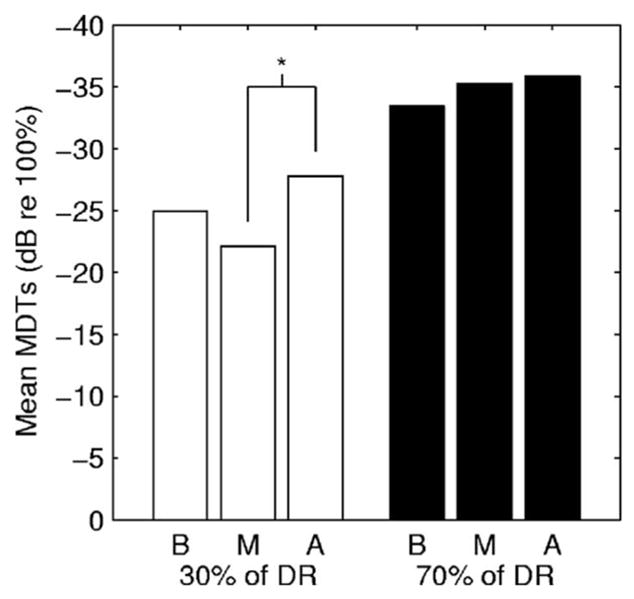
Across-subject mean MDTs for each of three segments of the implant. Basal segment (B) = Sites 1–8; middle segment (M) = Sites 9–15; apical segment (A) = Sites 16–22. MDTs for all tested sites within each segment were averaged. Mean MDTs measures at 30% of DR and 70% of DR are shown. The asterisk (*) indicates a significant difference between the mean MDTs for the apical and middle segments at 30% of DR in a post-hoc Tukey test.
Although statistically significant regional differences in MDTs were seen in the data averaged across all listeners at 30% of DR, these differences were small and they were not found in all individuals. Notable exceptions to the pattern shown in Fig. 2 are the data for subjects S53, S74, and S54 shown in Fig. 1.
B. Characterizing the listeners
Although across-site patterns of modulation detection are useful for many purposes, it might also be useful in some cases to have a single number to characterize a listener’s modulation-detection acuity relative to that of other listeners. This can be done by averaging the MDTs across all sites, averaging across a subsample of sites within the electrode array or by determining the MDT for only a single site (as was done by Fu, 2002). Presumably, averaging MDTs across all sites would result in the most accurate assessment of a listener’s overall modulation-detection acuity. However, this approach is very time consuming. If MDTs from a small sample of sites or a single site are closely related to, and accurately reflect the across-site mean of, MDTs at all sites, then the subjects’ overall modulation detection skills could be more efficiently assessed by testing fewer sites. To determine the relationship between these three methods of acquiring a single value to quantify listeners’ modulation-detection acuity, we calculated correlations between MDTs at each individual site (each available site from 3 to 22) and the mean MDTs across all sites. In most cases, the correlations calculated at both 30% and 70% of DR were large and highly significant after the Bonferroni method of correction was used to adjust the criterion p-value (0.05/20 = 0.0025). At 70% of DR, the r values of all correlations ranged from 0.74 to 0.98, and the p-values for all but one of these correlations were 0.001 or less. At 30% of DR, r values ranged from 0.63 to 0.92. All but five of these correlations resulted in significant p-values. One example of the relationship between MDTs for a single site (Site 11) and the means for all sites is shown in the left-hand panel of Fig. 3.
FIG. 3.
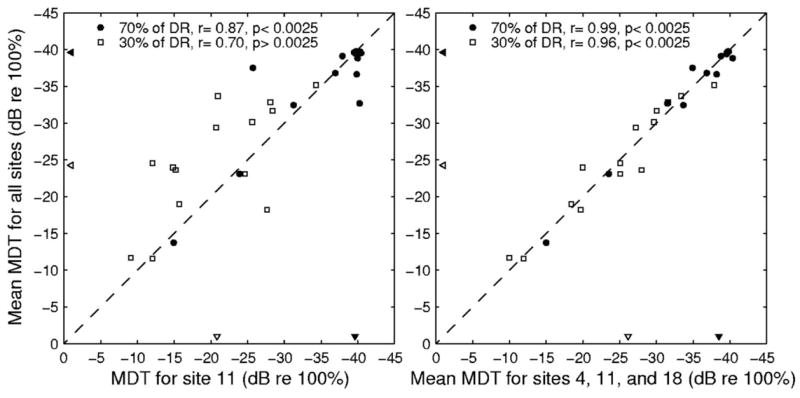
Scatterplots illustrating the correlations between mean MDTs for all sites and MDTs at Site 11 (left-hand panel) or the mean MDT for Sites 4, 11 and 18 (right-hand panel) at 70% of DR (filled circles) and 30% of DR (open squares). The filled and open triangles pointing to the abscissa represent the medians of the MDTs across all 14 subjects at Site 11 (left-hand panel) and at Sites 4, 11, and 18 (right-hand panel) for 70% and 30% of DR, respectively. The filled and open triangles pointing to the ordinate represent the medians of the MDTs across all 14 subjects at all sites for 70% and 30% of DR, respectively.
This analysis indicates that single-site measures of MDTs are highly related to the across-site mean MDTs. Listeners with relatively high MDTs at single sites also had high MDTs when MDTs from all sites were averaged together. Thus, measuring MDTs at only one site seems to provide a reasonable estimate of a listener’s modulation-detection acuity relative to that of other listeners. However, simple correlations between single-site measures of MDTs and across-site means of MDTs do not indicate if measuring at a single site will result in comparable quantitative values or if measuring at a single site could significantly under- or overestimate listeners’ modulation-detection acuity. For example, although MDTs measured at Site 11 were significantly correlated with mean MDTs across all sites, the two measures were not equivalent for many of the listeners. At 30% of DR, the MDTs measured at Site 11 tended to underestimate the mean modulation-detection acuity for all sites (points above the diagonal in the left-hand panel of Fig. 3). In some instances, it might be desirable to have more precise estimates of listeners’ MDTs rather than just an estimated rank order of their modulation-detection acuity. To determine if MDTs measured at single sites were comparable to the across-site means for all sites, we calculated paired-samples t-tests between MDTs measured at single sites (each available site from 3 to 22) and the across-site mean MDTs. Because many listeners performed at ceiling when 70% of DR was used, these comparisons were only done for the data collected at 30% of DR. The results of these comparisons indicate that, in this group of listeners, MDTs measured at 6 of the 20 sites were significantly different from the across-site mean MDTs. MDTs measured in the middle of the array at Site 10 [t(13) = 2.79, p < 0.05], Site 11 [t(13) = 2.67, p < 0.05], and Site 13 [t(13) = 2.32, p < 0.05] significantly underestimated listeners’ overall modulation-detection acuity. MDTs measured at the apical end of the array at Site 19 [t(13) = −2.86, p < 0.05], Site 20 [t(13) = −2.43, p < 0.05], and Site 21 [t(13) = −2.39, p < 0.05] significantly overestimated listeners’ overall modulation-detection acuity.
We also examined if MDTs averaged over three sites would be correlated to, and quantitatively equal to, the mean MDT calculated across all sites. For these analyses, the mean MDTs for a basal site (Site 4), a middle site (Site 11), and an apical site (Site 18) were averaged and compared to the mean MDTs across all sites. Figure 3 (right panel) illustrates that MDTs averaged across the three sites along the electrode array were significantly correlated to MDTs averaged across all sites at both 30% (r = 0.99, p < 0.001) and 70% (r = .96, p < 0.001) of the dynamic range. A paired-samples t-test was conducted to compare quantitatively the mean MDTs of three sites to the mean MDTs of all sites. There was no significant difference found between the mean MDT for Sites 4, 11, and 18 and the mean MDT of all sites at either 30% of DR [t(13) = 0.02, p = 0.99] or 70% of DR [t(13) = 0.60, p = 0.56]. Thus, sampling at three sites (Fig. 3, right-hand panel) clearly gave a better estimate of the mean MDT for all sites than just sampling at the middle site (Fig. 3, left-hand panel) or several other single sites along the electrode array.
C. Relation to across-site patterns of other measures
Correlational analysis was used to determine if across-site patterns of MDTs could be predicted from measures of T and C levels or DRs. In each listener, bivariate correlations were conducted between MDTs at the individual sites and T levels, C levels, and DRs at the individual sites. This analysis showed only sporadic relationships between the psychophysical measures within listeners. The sign of the correlation was also variable across listeners. For some listeners, values of T levels, C levels, and DRs increased as a function of MDT. For other listeners, these values decreased. Table II lists the r-values obtained for each listener for correlation of the MDT at each site with the C level, T level, and DR at each site. Significance levels were determined using the Bonferroni correction (p = 0.05/42 = 0.001). Only 6 of the 42 correlations were significant by this criterion and those did not all have the same sign: Three were positive and three were negative. Thus, it was not possible to consistently predict MDTs from T levels, C levels, or DRs.
TABLE II.
Bivariate correlations between individual subjects’ modulation detection thresholds (MDTs) and detection threshold levels (T levels; column 2), maximum comfortable loudness levels (C levels; column 3), and dynamic ranges (DRs; column 4) across all available stimulation sites. Asterisks (*) indicate significance at the p < 0.001 level.
| Subject | MDT vs. T level | MDT vs. C level | MDT vs. DR |
|---|---|---|---|
| 45 | 0.14 | 0.54 | 0.25 |
| 53 | 0.00 | −0.72* | −0.76* |
| 54 | 0.35 | 0.11 | −0.18 |
| 57 | −0.20 | −0.42 | −0.55 |
| 58 | −0.17 | 0.18 | 0.35 |
| 60 | −0.02 | 0.21 | 0.23 |
| 64 | −0.20 | 0.28 | 0.53 |
| 65 | −0.52 | −0.34 | −0.38 |
| 67 | −0.37 | 0.06 | 0.23 |
| 71 | −0.23 | 0.38 | 0.39 |
| 72 | 0.85* | −0.24 | −0.85* |
| 73 | 0.01 | 0.44 | 0.67* |
| 74 | 0.20 | 0.66* | 0.34 |
| 75 | 0.10 | 0.42 | 0.38 |
IV. DISCUSSION
This study demonstrated that MDTs are highly variable across stimulation sites of cochlear-implant electrode arrays, consistent with the hypothesis that the MDTs are affected by local variation in the number of neurons activated by each site and/or the condition of those neurons. Most of the listeners in our sample had MDTs that were highly variable across sites at a stimulus level that was 30% of DR.
These results suggest that strategies to improve a listener’s modulation detection performance might best be directed at individual stimulation sites in the electrode array. Such strategies might include adjusting stimulation parameters at individual sites to optimize modulation detection for each site or simply removing weaker sites from the processor map in order to improve the overall mean modulation detection scores and/or reduce any negative effects of the weak sites or the high across-site variability. One caution regarding such an approach is that strategies to improve modulation detection could potentially degrade other important features of perception. Before proceeding with such strategies, we need to know more about the relationship between across-site patterns of MDTs and across-site patterns of other perceptual features of electrical stimulation and to better understand the importance of these across-site patterns for speech recognition and other aspects of perception with auditory prostheses.
Potential rehabilitation strategies based on modulation-detection acuity rely on the assumption that modulation-detection acuity is closely related to speech recognition with auditory prostheses. There is some information about these relationships in the literature (Cazals et al., 1994; Fu, 2002; Colletti and Shannon, 2005), but additional studies are needed to determine how the patterns of MDTs across the whole implant electrode array relate to speech recognition. It is also important to determine how these across-site patterns are affected by the presence of interleaved stimulation at other stimulation sites. Modulation detection interference is known to occur with multichannel stimulation (Richardson et al., 1998; Chatterjee and Oba, 2004), and this could have a large effect on the across-site pattern of modulation detection and its relationship to speech recognition with auditory prostheses.
The MDTs in the experiments reported here were measured using monopolar stimulation, which is the electrode configuration used in most contemporary auditory prostheses. Monopolar stimulation has been shown to activate a broad spatial extent of neurons at levels a few decibels above the neural threshold (Merzenich and White, 1980; Hartmann et al., 1984; Bierer and Middlebrooks, 2002). This suggests that there is considerable overlap in the neural populations activated by stimulation of sites in the implant that are close together. However, the large variation seen in MDTs across adjacent or nearby stimulation sites in the current study suggests that the neural responses for stimulation of nearby sites are not identical in most cases. It is possible that stimulation using electrode configurations that produce more restricted activation patterns, such as tripolar stimulation, would result in even greater across-site variation in MDTs. However, given that these configurations are not currently used in most speech processors, the practical significance of using these configurations for evaluation of a subject’s temporal acuity is limited.
This study, as well as previous studies, showed that MDTs improve as a function of stimulus level in almost all cases. However, the shape of the MDT versus level function varies considerably from listener to listener (Fu, 2002; Galvin and Fu, 2005; Pfingst et al., 2007). In some listeners, these functions have steep slopes at low levels but reach a ceiling and plateau at higher levels. Other listeners show steady improvements in MDTs throughout the dynamic range. These variations are also seen across stimulation sites within listeners (e.g., see Fig. 4 in Pfingst et al., 2007). In many cases the differences across sites are reduced at higher levels in the dynamic range because the MDTs have reached ceiling performance levels. However, in some cases, the across-site differences are maintained over a large extent of the dynamic range. For example, see the data for S53 in Fig. 1 from the current study where MDTs at Sites 2–5 at 70% of DR are poorer than those at Sites 10–14 at 30% of DR. Because of the differences in growth of MDT versus level functions, it would be possible in some cases to reduce across-site variation in MDTs by making small site-specific adjustments in stimulus level, i.e., changing the input–output functions for each channel. On the other hand, some across-site differences persist over such a large range of levels that this approach would not be practical.
The large variability in MDTs across stimulation sites suggests that measurements made at a single site might not provide an adequate assessment of cochlear implant users’ modulation-detection acuity. For example, although MDTs obtained at a single site in the middle of the electrode array (Site 11) at 30% of DR were significantly correlated with the mean MDTs obtained at all sites, substantial differences were found between the MDTs at Site 11 and the mean MDTs for all sites in several individual listeners. Thus, measuring MDTs at a single site could result in over- or underestimations of temporal processing acuity in many cochlear implant users. Correspondence to the mean MDT for all sites was much closer when the mean of MDTs measured at three sites spaced throughout the electrode array (Sites 4, 11, and 18) was used. Thus, measuring MDTs at more than one site is advantageous for obtaining accurate estimates of cochlear implant users’ modulation-detection acuity. Such an estimate of a listener’s mean modulation-detection acuity might be useful for predicting cochlear implant performance (e.g., Fu, 2002) and for identifying whether or not the weaknesses in speech recognition or other complex perceptual abilities are due to deficits in temporal-envelope perception (e.g., Colletti and Shannon, 2005). However, assuming that the source of a listener’s perceptual weakness is related to temporal-envelope perception, a more detailed analysis of MDTs across the whole electrode array would be needed to identify the specific stimulation sites where changes are required.
In this study, we also found a weak dependence of MDTs on position of the stimulation sites along the apical-basal dimension of the scala tympani. MDTs were significantly lower at apical sites than at sites located in the middle of the array. A number of variables could contribute to this regional difference including (1) more hair cells present in the cochlear apex (Kiefer et al., 2004) that contribute to spontaneous activity in the neurons and alter sensitivity to temporal modulations; (2) systematic differences in temporal response properties of neurons from base to apex in the cochlea (Adamson et al., 2002; Liu and Davis, 2006); (3) better nerve survival in the apex of the cochlea (Nadol, 1997); and (4) systematic variation in the positions of the electrodes with respect to the modiolus along the cochlear-implant electrode array (Saunders et al., 2002). However, although regional differences in MDTs were seen in the average data across all listeners at 30% of DR, they were not found in all individuals. The more prominent characteristic of these data was the listener-specific variation in the across-site patterns of MDTs. This finding suggests that localized listener-specific patterns of the pathology of deafness and/or electrode placement play the dominant role in determining the across-site patterns of modulation detection.
If across-site patterns of MDTs are determined by the pattern of variation in physiological and biophysical variables along the length of the electrode array, then we might expect that other measures of implant function would show similar patterns of across-site variation. It is known that T levels, C levels, and DRs do vary as a function of stimulation site along the electrode array (Pfingst and Xu, 2004, 2005). However, in this study we found that, for most listeners, the across-site patterns of these psychophysical measures were not correlated with across-site patterns of MDTs. This suggests that the specific mechanisms underlying the across-site patterns of variation differ across these various psychophysical measures. The weak and inconsistent correlations between MDTs and T levels, C levels, and DRs make it improbable that these clinical measures would be successful predictors of modulation-detection acuity.
Acknowledgments
The authors express appreciation to their research subjects for their cheerful participation in these studies, to Ian Hsu for assistance with data analysis and presentation, to Thyag Sadasiwan for his computer programming work, and to the associate editor and reviewers of JASA for insightful comments and helpful suggestions on earlier drafts of this article. This work was supported by NIH NIDCD Grant Nos. R01 DC04312, T32 DC00011, and P30 DC05188.
Footnotes
Initial reports of these data were presented at the Fourth Joint Meeting of the Acoustical Society of America and the Acoustical Society of Japan, Honolulu, HI.
Contributor Information
Bryan E. Pfingst, Kresge Hearing Research Institute, Department of Otolaryngology, University of Michigan, Ann Arbor, Michigan 48109-5616.
Rose A. Burkholder-Juhasz, Kresge Hearing Research Institute, Department of Otolaryngology, University of Michigan, Ann Arbor, Michigan 48109-5616
Li Xu, School of Hearing, Speech and Language Sciences, Ohio University, Athens, Ohio 45701 and Kresge Hearing Research Institute, Department of Otolaryngology, University of Michigan, Ann Arbor, Michigan 48109-5616.
Catherine S. Thompson, Kresge Hearing Research Institute, Department of Otolaryngology, University of Michigan, Ann Arbor, Michigan 48109-5616
References
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002;447:331–350. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: Dependence on electrode configuration. J Neurophysiol. 2002;87:478–492. doi: 10.1152/jn.00212.2001. [DOI] [PubMed] [Google Scholar]
- Bregman AS, Levitan R, Liao C. Fusion of auditory components: Effects of the frequency of amplitude modulation. Percept Psychophys. 1990;47:68–73. doi: 10.3758/bf03208166. [DOI] [PubMed] [Google Scholar]
- Cazals Y, Pelizzone M, Saudan O, Boex C. Low-pass filtering in amplitude modulation detection associated with vowel and consonant identification in subjects with cochlear implants. J Acoust Soc Am. 1994;96:2048–2054. doi: 10.1121/1.410146. [DOI] [PubMed] [Google Scholar]
- Chatterjee M, Oba SI. Across- and within-channel envelope interactions in cochlear implant listeners. J Assoc Res Otolaryngol. 2004;5:360–375. doi: 10.1007/s10162-004-4050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Sarampalis A, Oba SI. Auditory stream segregation with cochlear implants: A preliminary report. Hear Res. 2006;222:100–107. doi: 10.1016/j.heares.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colletti V, Shannon RV. Open set speech perception with auditory brainstem implant. Laryngoscope. 2005;115:1974–1978. doi: 10.1097/01.mlg.0000178327.42926.ec. [DOI] [PubMed] [Google Scholar]
- Drullman R, Festen JM, Plomp R. Effect of temporal envelope smearing on speech perception. J Acoust Soc Am. 1994a;95:1053–1064. doi: 10.1121/1.408467. [DOI] [PubMed] [Google Scholar]
- Drullman R, Festen JM, Plomp R. Effect of reducing slow temporal modulations on speech reception. J Acoust Soc Am. 1994b;95:2670–2680. doi: 10.1121/1.409836. [DOI] [PubMed] [Google Scholar]
- Fu Q-J. Temporal processing and speech recognition in cochlear implant users. NeuroReport. 2002;13:1635–1639. doi: 10.1097/00001756-200209160-00013. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Chinchilla S, Galvin JJ. The role of spectral and temporal cues in voice gender discrimination by normal-hearing listeners and cochlear implant users. J Assoc Res Otolaryngol. 2004;5:253–260. doi: 10.1007/s10162-004-4046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV. Effect of stimulation rate on phoneme recognition by Nucleus-22 cochlear implant listeners. J Acoust Soc Am. 2000;107:589–597. doi: 10.1121/1.428325. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Zeng FG, Shannon RV, Soli SD. Importance of tonal envelope cues in Chinese speech recognition. J Acoust Soc Am. 1998;104:505–510. doi: 10.1121/1.423251. [DOI] [PubMed] [Google Scholar]
- Galvin JJ, Fu QJ. Effects of stimulation rate, mode and level on modulation detection by cochlear implant users. J Assoc Res Otolaryngol. 2005;6:269–279. doi: 10.1007/s10162-005-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear Res. 1984;13:47–62. doi: 10.1016/0378-5955(84)90094-7. [DOI] [PubMed] [Google Scholar]
- Hinojosa R, Lindsay JR. Profound deafness. Associated sensory and neural degeneration. Arch Otolaryngol. 1980;106:193–209. doi: 10.1001/archotol.1980.00790280001001. [DOI] [PubMed] [Google Scholar]
- Kiefer J, Gstoettner W, Baumgartner W, Pok SM, Tillein J, Ye Q, von Ilberg C. Conservation of low-frequency hearing in cochlear implantation. Acta OtoLaryngol. 2004;124:272–280. doi: 10.1080/00016480310000755a. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J Acoust Soc Am. 1971;49:467–477. [PubMed] [Google Scholar]
- Liu Q, Davis RL. From apex to base: How endogenous neuronal membrane properties are distributed in the spiral ganglion. Assoc Res Otolaryngol Abstr. 2006;29:305. [Google Scholar]
- Merzenich MM, White M. Coding considerations in design of cochlear prostheses. Ann Otol Rhinol Laryngol Suppl. 1980;89:84–87. doi: 10.1177/00034894800890s523. [DOI] [PubMed] [Google Scholar]
- Nadol J. Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngol-Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Pfingst BE, Xu L. Across-site variation in detection thresholds and maximum comfortable loudness levels for cochlear implants. J Assoc Res Otolaryngol. 2004;5:11–24. doi: 10.1007/s10162-003-3051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L. Psychophysical metrics and speech recognition in cochlear implant users. Audiol Neuro-Otol. 2005;10:331–341. doi: 10.1159/000087350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CS. Across-site threshold variation in cochlear implants: Relation to speech recognition. Audiol Neuro-Otol. 2004;9:341–352. doi: 10.1159/000081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingst BE, Xu L, Thompson CA. Effects of carrier pulse rate and stimulation site on modulation detection by subjects with cochlear implants. J Acoust Soc Am. 2007;121:2236–2246. doi: 10.1121/1.2537501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson LM, Busby PA, Clark GM. Modulation detection interference in cochlear implant subjects. J Acoust Soc Am. 1998;104:442–452. doi: 10.1121/1.423248. [DOI] [PubMed] [Google Scholar]
- Saunders E, Cohen L, Aschendorff A, Shapiro W, Knight M, Stecker M, Richter B, Waltzman S, Tykocinski M, Roland T, Laszig R, Cowan R. Threshold, comfortable level and impedance changes as a function of electrode-modiolar distance. Ear Hear. 2002;23:28S–40S. doi: 10.1097/00003446-200202001-00004. [DOI] [PubMed] [Google Scholar]
- Shannon RV. Temporal modulation transfer functions in patients with cochlear implants. J Acoust Soc Am. 1992;91:2156–2164. doi: 10.1121/1.403807. [DOI] [PubMed] [Google Scholar]
- Shannon RV, Zeng F-G, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Van Tasell DJ, Soli SD, Kirby VM, Widin GP. Speech waveform envelope cues for consonant recognition. J Acoust Soc Am. 1987;82:1152–1161. doi: 10.1121/1.395251. [DOI] [PubMed] [Google Scholar]
- Wilson BS. Engineering design of cochlear implants. In: Zeng F-G, Popper AN, Fay RR, editors. Cochlear Implants: Auditory Prostheses and Electrical Hearing. Springer; New York: 2004. pp. 14–52. [Google Scholar]
- Xu L, Thompson C, Pfingst BE. Relative contributions of spectral and temporal cues for phoneme recognition. J Acoust Soc Am. 2005;117:3255–3267. doi: 10.1121/1.1886405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Tsai Y, Pfingst BE. Features of stimulation affecting tonal-speech perception: Implications for cochlear prostheses. J Acoust Soc Am. 2002;112:247–258. doi: 10.1121/1.1487843. [DOI] [PMC free article] [PubMed] [Google Scholar]