SUMMARY
It is intuitive to speculate that nutrient availability may influence differentiation of mammalian cells. Nonetheless, a comprehensive complement of the molecular determinants involved in this process has not been elucidated yet. Here, we have investigated how nutrients (glucose) affect skeletal myogenesis. Glucose restriction (GR) impaired differentiation of skeletal myoblasts and was associated with activation of the AMP-activated protein kinase (AMPK). Activated AMPK was required to promote GR-induced transcription of the NAD+ biosynthetic enzyme Nampt. Indeed, GR augmented the Nampt activity, which consequently modified the intracellular [NAD+]/[NADH] ratio and nicotinamide levels, and mediated inhibition of skeletal myogenesis. Skeletal myoblasts derived from SIRT1+/− heterozygous mice were resistant to the effects of either GR or AMPK activation. These experiments reveal that AMPK, Nampt, and SIRT1 are the molecular components of a functional signaling pathway that allows skeletal muscle cells to sense and react to nutrient availability.
IINTRODUCTION
The execution of essential and specialized functions relies on the cell’s ability to sense and adapt to the environment, with nutrient availability being one of the most critical variables. The nematode C.elegans reacts to overcrowding and unfavorable nutrient conditions by entering the dauer diapause, a non-feeding, resistant to oxidative stress, and long-lived larval developmental stage (Cassada and Russell, 1975). Moreover, decreasing calorie intake is the sole intervention that successfully increases lifespan across species (Masoro, 2006). A mechanistic understanding of these phenomena requires the identification of the molecules that mediate the cellular response to nutrients.
While their role on lifespan is still a matter of intense debate (Chen and Guarente, 2007; Kaeberlein and Powers, 2007; Longo and Kennedy, 2006; Michan and Sinclair, 2007), it is well established that the sirtuins- a family of evolutionarily conserved deacetylases - play important roles in numerous physiological processes (Haigis and Guarente, 2006). By deacetylating cofactors such as PCAF (Fulco et al., 2003) (Brunet et al., 2004), p300 (Motta et al., 2004) (Brunet et al., 2004), PGC-1α (Nemoto et al., 2005; Rodgers et al., 2005), and numerous transcriptional activators, the NAD+-dependent deacetylase sirtuin SIRT1 controls critical functions of mammalian cell physiology including stress resistance (Vaziri et al., 2001) (Luo et al., 2001) (Cohen et al., 2004), replicative senescence (Chua et al., 2005), aging and differentiation (Blander and Guarente, 2004). Differentiation of skeletal muscle cells (Fulco et al., 2003) and adipocytes (Picard et al., 2004), angiogenesis (Potente et al., 2007), survival of neurons (Araki et al., 2004) and pancreatic β-cells (Kitamura et al., 2005), insulin secretion (Bordone et al., 2006) (Moynihan et al., 2005), lipid (Li et al., 2007) and liver metabolism (Rodgers et al., 2005), and increased physical activity during calorie restriction (Chen et al., 2005), are all regulated by SIRT1.
In contrast to class I-II histone deacetylases (HDACs), the enzymatic activity of SIRT1 is modulated by physiological cofactors and inhibitors. NAD+ is an obligate co-substrate (Imai et al., 2000), whereas NADH (Lin et al., 2004) and nicotinamide (NAM) are inhibitors of SIRT1 (Bitterman et al., 2002). The central role of the NAD+ salvage pathway in regulating the enzymatic activity of Sir2- the SIRT1 yeast ortholog- is illustrated by the observation that the nicotinamidase PNC1- the yeast functional equivalent of mammalian Nampt (also known as PBEF or visfatin)- is both necessary and sufficient for lifespan extension caused by calorie restriction and low-intensity stress in a Sir2-dependent fashion (Anderson et al., 2003). Moreover, Nampt retards senescence of cultured human cells (van der Veer et al., 2007). Overexpression of exogenous Nampt regulates the transcriptional activity of a transiently transfected Gal-SIRT1 fusion protein in mammalian cells (Revollo et al., 2004). Nampt was recently identified as a stress- and nutrient-responsive gene that increases mitochondrial NAD+ levels and promotes survival during genotoxic stress via the mitochondrial sirtuins SIRT3 and SIRT4 (Yang et al., 2007). Even though it remains unclear as to what the relative contribution of increased [NAD+]/[NADH] ratio versus reduced NAM is, overall, these findings are consistent with the suggestion that modifications in NAD+ and NAM levels are likely to be the most important regulators of sirtuins activity (Grubisha et al., 2005). Despite a wealth of information on the molecules and mechanisms that mediate the effects of SIRT1 on several biological processes (Michan and Sinclair, 2007), the identification and mechanistic elucidation of the signals that activate the NAD+ salvage pathway and, as a consequence, regulate the deacetylase activity of SIRT1 and of other sirtuins in response to nutrient availability and oxidative stress in mammalian cells remain to be fully understood. Within this context, an attractive candidate is the AMP-activated protein kinase (AMPK). AMPK activation is observed in fasting and calorie restricted-animals and it has been proposed as a one of the several mechanisms involved in regulating mammal longevity (McCarty, 2004). In agreement with this hypothesis -and similarly to Sir2-, extra copies of AMPK can extend lifespan in C. elegans (Apfeld et al., 2004) and mediate the effects of dietary restriction on longevity through the FOXO transcription factors (Greer et al., 2007a). Finally, the SIRT1 agonist resveratrol- shown to augment survival of mice on a high-calorie diet (Baur et al., 2006) and improve mitochondrial function (Lagouge et al., 2006) - induces phosphorylation and activation of AMPK (Baur et al., 2006) (Dasgupta and Milbrandt, 2007).
Skeletal muscle cell differentiation is accompanied by modifications of the [NAD+]/[NADH] ratio that exerts regulatory functions on SIRT1(Sartorelli and Caretti, 2005). A decrease of the [NAD+]/[NADH] ratio coincides with skeletal myogenesis, whereas its increase inhibits it (Fulco et al., 2003). The regulatory function of the [NAD+]/[NADH] ratio offers the opportunity of investigating whether a link exists between the mechanisms that preside to differentiation and those that mediate the response to nutrient availability in skeletal muscle cells. Here, we report that GR causes AMPK activation and prevents proper differentiation of mouse skeletal muscle cells. Activated AMPK is required to induce Nampt transcription, thus increasing intracellular [NAD+]/[NADH] ratio and lowering NAM levels. Blockade of either AMPK or Nampt counteracts the effects of GR and, conversely, activation of AMPK, in normocaloric conditions, augments intracellular [NAD+]/[NADH] ratio, reduces NAM levels, and mimics GR. Inhibition of cell differentiation induced by GR, AMPK activation, or Nampt is dependent on SIRT1, as skeletal myoblasts derived from SIRT1+/− heterozygous animals are less sensitive to either GR or AMPK activation and continue to differentiate in extremely low caloric conditions. These findings provide an initial description and mechanistic explanation of how mammalian skeletal muscle cells sense, decode, and respond to nutrient availability through a series of highly regulated enzymatic reactions leading to modifications of metabolic parameters consonant with promoting activation of SIRT1.
RESULTS
Glucose Restriction-Mediated Activation of AMPK Prevents Differentiation of Skeletal Muscle Cells
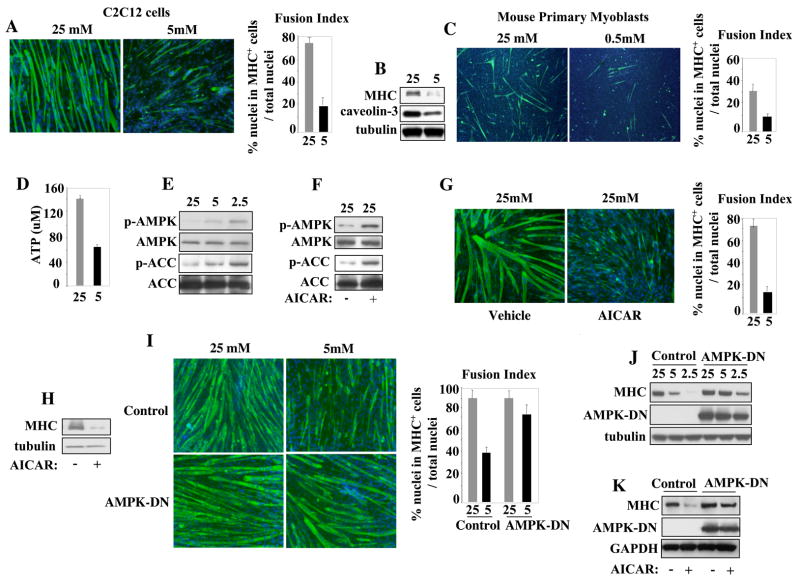
We investigated the effect of reducing the levels of glucose -the major source of calories in the culture medium - on the differentiation process of either C2C12 skeletal muscle cell line or mouse primary skeletal myoblasts. Cells cultured in low glucose - 5mM or lower concentrations- failed to appropriately differentiate as indicated by reduced expression of the sarcomeric myosin heavy chain (MHC), caveolin-3, and impaired formation of multinucleated myotubes (Figure 1A,B, C and Fig. 1SA). Primary skeletal myoblasts differentiated in 5mM glucose (data not shown), showing defective differentiation only at a lower glucose concentration (0.5mM) (Fig. 1C). Within the time frame of our experiments (48 hours in differentiation medium, DM), GR did not induce apoptosis and, once normocaloric conditions were re-established, cells resumed differentiation (Fig. S1B, C). We evaluated whether fatty acids –which are effectively utilized by the mitochondrial metabolism- could overcome the effects of low glucose by exposing C2C12 cells to 0.1mM of oleic acid. Oleic acid promoted differentiation (Hurley et al., 2006) but was ineffective in counteracting the differentiation defects exerted by low glucose (Fig. S1D), indicating that increased β-oxidation fueled by lipids is insufficient to compensate for glucose reduction. As expected, cells cultured with low glucose had decreased intracellular ATP levels (Fig. 1D). In response to ATP depletion, the AMP-activated protein kinase (AMPK) is phosphorylated and activated (Hardie et al., 2006). Accordingly, progressive reduction of glucose induced phosphorylation of AMPK and of its substrate acetyl-CoA-carboxylase (ACC) in C2C12 cells (Fig. 1E). To evaluate whether AMPK activation is sufficient to recapitulate the effects of GR, we employed the AMP mimetic 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside (AICAR) (Kemp et al., 1999). AMPK is required for AICAR-stimulated glucose uptake in skeletal muscle, indicating that this AMP mimetic is a specific activator of AMPK in this tissue (Mu et al., 2001). AICAR promoted AMPK and ACC phosphorylation in normocaloric (NC) conditions (Fig. 1F) and cells exposed to AICAR in NC conditions failed to appropriately differentiate (Fig. 1 G, H). In addition to AICAR, two other AMPK activators - the furancarboxylic acid derivative D942 (Kosaka et al., 2005) and the hypoglycemic drug metformin (Zhou et al., 2001)- also inhibited cell differentiation in a dose-dependent manner (Fig. S1E,F). To test whether AMPK activation is necessary to mediated GR, an AMPK dominant negative (AMPK-DN) construct -bearing the K45R mutation in the α-2 catalytic subunit of rat AMPK (Mu et al., 2001) - was retrovirally-transduced in myoblasts. Cells that received the AMPK-DN efficiently differentiated despite the GR conditions (Fig. 1I, J) and were refractory to AICAR-induced block of differentiation (Fig. 1K). Moreover, compound C, an AMPK inhibitor (Zhou et al., 2001), also rescued the GR-induced differentiation defects of both C2C12 cells and primary skeletal myoblasts (Fig. S1G,H). Thus, AMPK activation is required to mediate the effects of GR on skeletal muscle differentiation.
Figure 1.
Glucose Restriction Inhibits Differentiation of Skeletal Myoblasts and Activates AMPK. (A) C2C12 cells were differentiated in a medium (differentiation medium, DM) supplemented with either 25mM or 5mM glucose for 48 hours. Immunofluorescence (IF) was performed with a MHC antibody. DAPI marks the cell nuclei. The fusion index reported throughout this study was determined as described in Experimental Procedure (p<0.05). (B) Immunoblot for MHC, caveolin-3, and tubulin proteins from cell extracts derived from C2C12 cells cultured as in (A). (C) Mouse primary myoblasts were cultured in DM with either 25mM or 0.5mM glucose for 48 hours. IF was performed as in (A). (D) The ATP levels were determined in extracts of C2C12 cells cultured in DM with either 25mM or 5mM glucose. (E) Phosphorylation of AMPK (pT172) and of the AMPK substrate ACC (pS79) in C2C12 cells cultured in DM with 25mM, 5mM, or 2.5mM glucose determined by immunoblot with phospho-specific AMPK and ACC antibodies. The total levels of AMPK and ACC were determined with AMPK and ACC antibodies. (F) C2C12 cells were exposed to AICAR (0.5mM) and AMPK and ACC phosphorylation evaluated as in (E). (G) C2C12 cells were cultured in DM with 25mM glucose and exposed to either vehicle (DMSO) or AICAR (0.5mM) for 36 hours. IF was performed with an MHC antibody. (H) MHC and tubulin immunoblot of extracts of the cells described in (G). (I) C2C12 cells transduced with a retrovirus expressing a dominant negative form of AMPK (myc-tagged AMPK-DN) were cultured in DM supplemented with either 25mM or 5mM glucose. IF was performed with a MHC antibody. (J) Immunoblot of extracts derived from the cells described in (I) with the MHC, myc, and tubulin antibodies. (K) MHC, myc, and GAPDH immunoblot of extracts derived from the cells expressing myc-AMPK-DN exposed to AICAR (0.5mM).
Glucose Restriction and AMPK Require SIRT1
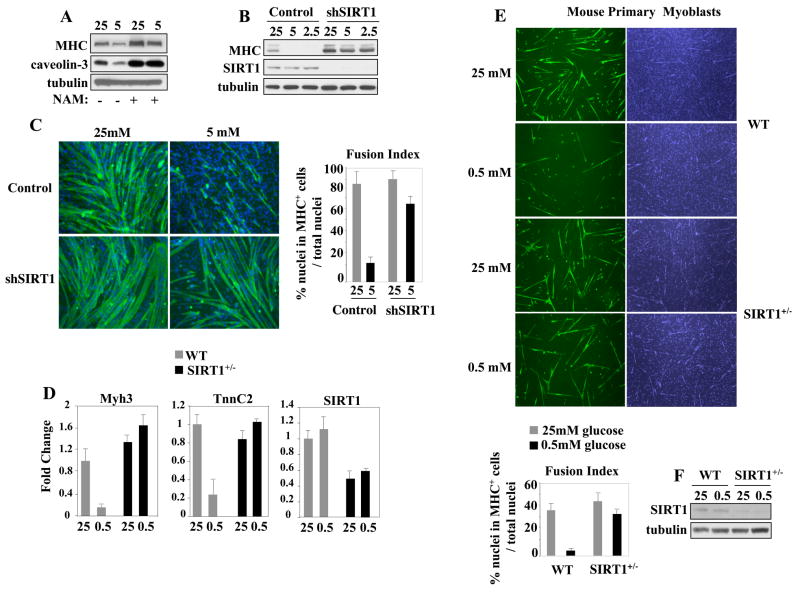
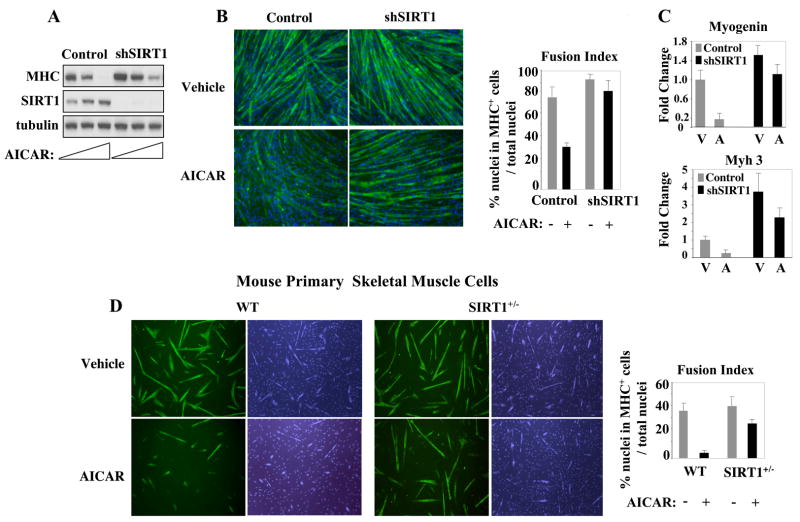
Since the SIRT1 ortholog Sir2 mediates the effects of calorie restriction in yeast (Lin et al., 2000) and counteracts skeletal myogenesis in mammalian cells (Fulco et al., 2003), we evaluated the potential involvement of SIRT1 in mediating the effects of GR on skeletal muscle differentiation. NAM – a sirtuin inhibitor (Bitterman et al., 2002) - rescued differentiation of GR cells (Fig. 2A), suggesting that the deacetylase activity of sirtuins is relevant in mediating the effects of CR. NAM inhibits the deacetylase activities of several sirtuins. Therefore, we assessed the specific role of SIRT1 in GR by reducing its levels with a retrovirus expressing a short hairpin RNA predicted to target solely the SIRT1 mRNA (shSIRT1). Under these conditions, the differentiation ability was efficiently rescued, even when the cells were cultured in very low glucose conditions (2.5mM glucose) (Fig. 2B,C). In contrast, siRNA-mediated knockdown of two other mitochondrial sirtuins - SIRT3 and SIRT4- was ineffective in preventing GR-mediated inhibition of cell differentiation (Fig. S2). To unequivocally test for SIRT1 involvement, we isolated skeletal myoblasts derived from mice with germline mutation of the SIRT1 gene. Since SIRT1−/− homozygous mice are perinatal lethal (McBurney et al., 2003), we compared the response to GR of primary myoblasts isolated from 4 weeks-old wild-type and SIRT1+/− heterozygous mice. Despite the extreme GR regimen (0.5mM glucose), SIRT1+/− primary myoblasts efficiently activated muscle gene expression and differentiated, whereas the cells derived from wild-type littermates were impaired in these processes (Fig. 2D, E). Neither the SIRT1 transcripts, nor the protein levels were affected by GR in C2C12 cells, WT, or SIRT1+/− myoblasts (Figs. 2B,D,F). Thus, myoblasts cultured in low glucose are impaired in their differentiation process and SIRT1 is required to mediate this phenomenon. We then asked whether AMPK also requires SIRT1. When SIRT1 levels were reduced, the cells became partially refractory to AICAR (Fig. 3A,B,C). Similarly, myoblasts from SIRT1+/− animals differentiated despite the presence of AICAR in the culture medium (Fig. 3D). The residual inhibitory effect of AICAR on cell differentiation (on either shSIRT1 C2C12 cells or SIRT1+/− myoblasts) is likely due to the remaining SIRT1. SIRT1 was also required for the effects of the AMPK activator D942 (Fig. S1E). Overall, the results of the experiments reported in this paragraph indicate that the effects of either GR or AMPK on skeletal myogenesis require SIRT1.
Figure 2. SIRT1 Mediates the Effects of Glucose Restriction on Skeletal Myoblasts.
(A) Extracts of C2C12 cells cultured in DM with either 25mM or 5mM glucose and without or with NAM (5mM) were immunoblotted with the MHC, caveolin-3, and tubulin antibodies. (B) C2C12 cells were transduced with a retrovirus expressing a RNA hairpin directed against SIRT1 (shSIRT1) and cultured in DM with 25mM, 5mM, or 2.5mM glucose. Immunoblot was performed with antibodies against MHC, SIRT1, and tubulin. (C) MHC IF of cells expressing control or hSIRT1 cultured in DM with 25mM or 5mM glucose. (D) RT-qPCR of transcripts (embryonic myosin heavy chain Myh3, troponin C2, and SIRT1) derived from freshly isolated mouse primary skeletal myoblasts of 4 weeks-old wild-type (WT) or SIRT1+/− heterozygous mice cultured in DM with 25mM or 0.5mM glucose. Three independently obtained RNA samples were evaluated. The values of each transcript were corrected for those of GAPDH transcripts. Error bars represent standard deviations (p< 0.05). (E) MHC IF of WT and SIRT1+/− skeletal myoblasts cultured in DM with 25mM or 0.5mM glucose. (F) SIRT1 and tubulin immunoblot of extracts from mouse primary myoblasts obtained from either WT or SIRT1+/− heterozygous animals cultured in either 25mM or 0.5mM glucose.
Figure 3. AMPK Activation Recapitulates the Effects of Glucose Restriction and is SIRT1-Dependent.
(A) MHC, SIRT1, and tubulin immunoblot of extracts of C2C12 cells expressing shSIRT1 cultured in 25mM glucose and exposed to increasing (0.25–0.5mM) concentrations of AICAR for 36 hours. (B, C) MHC IF (B) and myogenin and Myh3 transcripts (C) of cells described in (A). V, DMSO; A, AICAR. (D) MHC IF of mouse primary skeletal myoblasts from WT or SIRT1+/− heterozygous mice cultured in DM with DMSO (vehicle) or AICAR (0.5mM) for 36–48 hours.
Modulation of Gene Expression by GR
To identify genes transcriptionally modulated by glucose, we performed whole-genome microarray analysis of C2C12 cells grown in either 25mM or 5mM glucose. In addition to transcripts for structural and regulatory muscle proteins, numerous others involved in glucose and lipid metabolism, xenobiotic detoxification, mitochondrial energy production and respiration were modulated by GR (GEO Database at http://www.ncbi.nlm.nih.gov/geo/ and Table S1). Expression of several transcripts was verified by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) (data not shown and Fig. S3). Downregulation of glycolysis is a hallmark of calorie restriction and it has been suggested as one of the mechanisms that mediates its effects (Ingram et al., 2006) (Hipkiss, 2006). Accordingly, the transcripts of the glycolytic genes phosphoglycerate mutase (PGAM), phosphofructokinase and beta enolase were reduced (Table S1 and Fig. S3A,B). Expression of the phosphoglycerate mutase, beta enolase, and phosphofructokinase was also reduced in skeletal muscles of mice subjected to a 48 hr fasting (Fig. S3B). Conversely, the UDP-glucuronosyltransferase 1, epoxide hydrolase 1(Epx1) and glutathione S transferase (GST) (xenobiotic detoxification), and Gadd45 gamma (stress-induced) transcripts were increased in GR cells (Table S1 and Fig. S3C). As observed in C.elegans subjected to dietary glucose restriction (Schulz et al., 2007), several transcripts encoding for proteins involved in lipid metabolism were increased, whereas a number of transcripts encoding collagen or collagen-like proteins were decreased in GR cells (Table S1, extracellular matrix). We next asked whether the modifications on gene expression induced by GR were dependent on SIRT1 by either exposing the GR cells to NAM or by overexpressing SIRT1 in NC conditions. The results of these experiments indicate that NAM reversed the effects of GR on gene expression and, conversely, SIRT1 mimicked them under NC (Fig. S3A, C). The transcripts for the PGAM, GST and Epx1genes were also evaluated in myoblasts from either wild type or SIRT1+/− mice. While GR affected their expression in control myoblasts, it had no effect on SIRT1+/− cells. (Fig. S4). Overall, the results of these experiments indicate that GR induces specific modifications on the gene expression profile and that this gene modulation involves SIRT1.
The Nicotinamide Phosphoribosyltransferase (Nampt) of the NAD+ Salvage Pathway Mediates The Effects of GR or AMPK on Cell Differentiation in a SIRT1-Dependent Manner
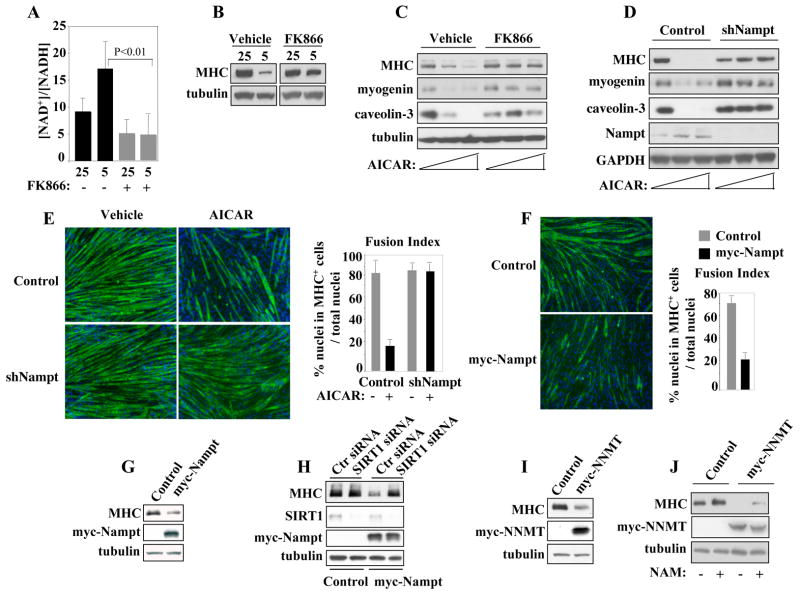
Since the SIRT1 levels were not increased by GR, we considered the possibility that its enzymatic activity may be modulated. Indeed, extracts derived from GR cells sustained an increased SIRT1 activity (Fig. 4A). SIRT1 activity is stimulated by an increased [NAD+]/[NADH] ratio and/or reduced NAM levels. Given that either GR or AMPK requires the presence of SIRT1 and its activity is increased in GR cells, we asked whether the [NAD+]/[NADH] ratio and NAM levels were influenced by GR or AMPK activation. Extracts derived from GR cells displayed a significantly increased [NAD+]/[NADH] ratio and decreased NAM (Fig. 4 B, C and Fig. S5A). Similarly, AICAR increased SIRT1 activity, the [NAD+]/[NADH] ratio and decreased the NAM levels (Fig. 4D–F and Fig. S5B). AICAR also stimulated SIRT1 activity in wild type mouse primary myoblasts and -consistent with the residual inhibitory effect of AICAR on cell differentiation (Fig. 3D)- in SIRT1+/− myoblasts (Fig. S4B). The increased intracellular [NAD+]/[NADH] ratio and reduced NAM levels observed in GR and AICAR-treated cells are consistent with activation of the NAD+ salvage pathway. In a highly regulated deacetylation reaction, SIRT1 cleaves NAD+, yielding NAM, 2′-3′-O-acetyl ADP ribose and the deacetylated lysine (Tanny and Moazed, 2001) (Sauve et al., 2006). NAM is then employed as a precursor of NAD+ synthesis through the NAD+ salvage pathway. In mammalian cells, the NAM phosphoribosyltransferase (Nampt) catalyzes the conversion of NAM and phosphoribosyl pyrophosphate (PRPP) into nicotinamide mononucleotide (NMN) (Magni et al., 1999). NMN is further converted into NAD+ by the nicotinamide/nicotinic acid mononucleotide adenylyltransferase (Emanuelli et al., 2001) (Fig. 4G). Since Nampt is the first and rate-limiting enzyme of this pathway, we tested for its involvement in GR- and AMPK-mediated effects on skeletal myogenesis. To evaluate the Nampt enzymatic activity, cell extracts derived from skeletal muscle cells cultured in either NC or GR conditions were incubated with 14C-labeled NAM (the Nampt substrate) and formation of 14C-labeled NMN (the final product of the Nampt reaction) measured. Cell extracts from either GR or AICAR-treated cells sustained an increased production of 14C-NMN, when compared to extracts of NC cells (Fig. 4 H, I). The function of Nampt was directly addressed by lowering its levels with a retrovirus expressing a short hairpin specific RNA (shNampt) (Fig. 4J). Cells with reduced Nampt did not increase the intracellular [NAD+]/[NADH] ratio and efficiently differentiated in GR conditions (Fig. 4K, L and Fig. S5C). We then probed the role of the enzymatic activity of Nampt with FK866, a highly specific inhibitor (Hasmann and Schemainda, 2003) (Khan et al., 2006). FK866 prevented the increase of the intracellular [NAD+]/[NADH] ratio caused by GR and allowed differentiation of myoblasts cultured in GR conditions (Fig. 5A,B). To further substantiate these findings, cells were transduced with a Nampt mutant (A244M) that retains the phosphoribosyltransferase activity but is FK866-insensitive (Khan et al., 2006). Since the Nampt A244M protein escapes FK866 inhibition, these cells had an increased intracellular [NAD+]/[NADH] ratio and failed to appropriately differentiate, despite exposure to FK866 (Fig. S6A, B, middle panels). The enzymatic activity of Nampt was inactivated by introducing a mutation (S314A) within its active domain (Wang et al., 2006). Cell transduced with Nampt (A244M/S314A) and exposed to FK866 failed to upregulate the [NAD+]/[NADH] ratio and properly differentiated (Fig. S6A, B, right panels). Overall, these results indicate that the enzymatic activity of Nampt is responsible for modulating the [NAD+]/[NADH] ratio and is associated with lack of cell differentiation observed during GR. In parallel experiments, we employed AICAR to ask whether Nampt was also required to mediate the effects of AMPK. As shown in Fig. 5 (C–E) and Fig. S7, inhibiting Nampt activity with FK866 or reducing the Nampt levels rendered the cells refractory to AICAR. Lastly, we investigated whether Nampt requires SIRT1. To this end, skeletal myoblasts were transduced with a retrovirus encoding Nampt. Under NC conditions, cells overexpressing Nampt were impaired in their differentiation process (Fig. 5F,G). Reducing SIRT1 levels, resumed differentiation of Nampt-overexpressing cells, as indicated by increased MHC expression (Fig. 5H, compare Ctr and SIRT1 siRNA lanes). In an attempt to distinguish the contribution of the two possible effects mediated by Nampt (NAM depletion vs. increased NAD+ synthesis), we transduced C2C12 cells with a retrovirus expressing the NAM N-methyltransferase (NNMT). NNMT is an enzyme outside of the NAD+ salvage pathway that removes NAM by converting it to N-methyl-NAM without boosting NAD+ biosynthesis (Aksoy et al., 1994) (Anderson et al., 2003). Cells expressing NNMT were impaired in their differentiation despite being cultured with high glucose (Fig. 5I). Consistent with a role of NAM in this process, its addition partially reverted the NNMT-mediated inhibition on differentiation (Fig. 5J). Altogether, the results presented in this paragraph indicate that: 1) GR and AMPK activate Nampt; 2) Nampt is required for the effects exerted by GR and AMPK; 3) the effects of Nampt on cell differentiation require SIRT1; 4) expression of NNMT- which reduces NAM levels (Aksoy et al., 1994)- is sufficient to mimic GR.
Figure 4. Effects of Glucose Restriction, AMPK Activation, or Nampt on [NAD+]/[NADH] Ratio, SIRT1 Activity, and Nicotinamide (NAM) Levels of Skeletal Muscle Cells.
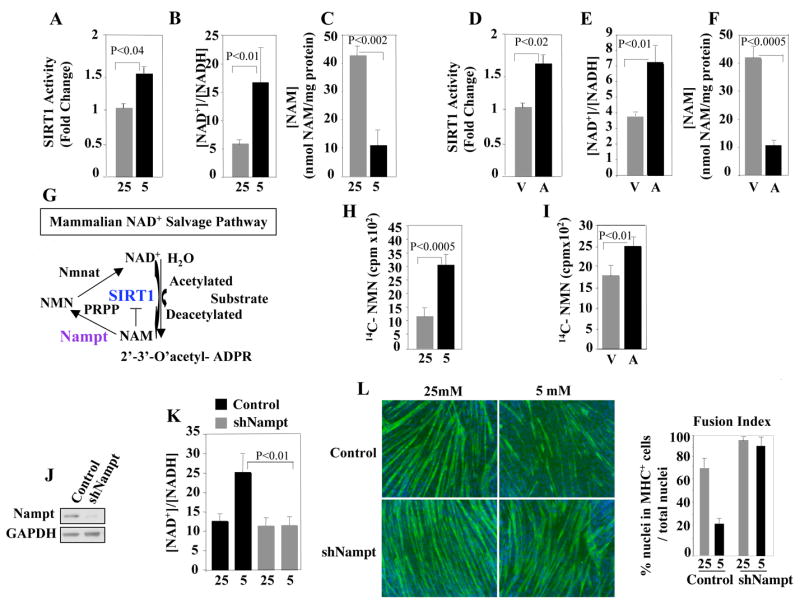
(A) SIRT1 activity was evaluated in extracts derived from C2C12 cells cultured in DM with 25mM or 5mM glucose, respectively, for 24 hr. (B) [NAD+]/[NADH] ratio and (C) NAM levels were determined in extracts of C2C12 cells cultured in DM with 25mM or 5mM glucose. (D) SIRT1 activity was evaluated in extracts derived from C2C12 cells exposed to vehicle or AICAR (0.5mM) for 24 hours. (E) [NAD+]/[NADH] ratio and (F) NAM levels were evaluated in C2C12 cells cultured in DM with 25mM glucose in the absence (vehicle, V) or presence of AICAR (A). (G) Schematic representation of the SIRT1-mediated deacetylation reaction and NAD+ salvage pathway. (H) Nampt enzymatic activity in extracts of C2C12 cells cultured in DM with 25mM or 5mM glucose. (I) Nampt enzymatic activity in extracts of C2C12 cells cultured in DM with 25mM glucose in the absence (V) or presence of AICAR (A). (J) Nampt and GAPDH immunoblot of C2C12 cells expressing a RNA hairpin against Nampt (shNampt). (K) [NAD+]/[NADH] ratio in cells expressing shNampt cultured in DM with 25mM or 5mM glucose. (L) MHC IF of control or shNampt-expressing C2C12 cells cultured in DM with 25mM or 5mM glucose. All the enzymatic measurements reported in this figure were repeated with three independent samples. Error bars represent standard deviations.
Figure 5. The Outcomes of AMPK Activation on Cell Differentiation Depend on Nampt and SIRT1.
(A) [NAD+]/[NADH] ratio in C2C12 cells exposed to the Nampt-inhibitor FK866 (10nM) cultured in DM with either 25mM or 5mM glucose. (B) MHC and tubulin immunoblot of extracts from C2C12 cells exposed to FK866 (10nM). (C) MHC, myogenin, caveolin-3, and tubulin immunoblot of extracts of C2C12 cells exposed to increasing concentrations (0.25–0.5mM) of AICAR and FK866 (10nM) in DM with 25mM glucose. (D) MHC, myogenin, caveolin-3, Nampt and GAPDH immunoblot of extracts from C2C12 cells (control or shNampt) exposed to AICAR in DM with 25mM glucose. (E) MHC IF of control or shNampt-expressing C2C12 cells cultured in DM with 25mM glucose and 0.5mM AICAR or vehicle. (F) MHC IF of controls or myc-Nampt-expressing C2C12 cells cultured in DM with 25mM glucose. (G) MHC, myc, and tubulin immunoblot of extracts from the C2C12 cells described in (F). (H) MHC, SIRT1, myc, and tubulin immunoblot of extracts from C2C12 cells expressing myc-Nampt and transfected with either control (scrambled) or SIRT1 siRNA. Cells were cultured in DM with 25mM glucose. (I) MHC, myc, and tubulin immunoblot of extracts from C2C12 cells expressing myc-NNMT and cultured in DM with 25mM glucose. (J) Immunoblot were as described in (I) with extracts from control or NNMT-expressing C2C12 cells cultured in the absence or presence of NAM (5mM).
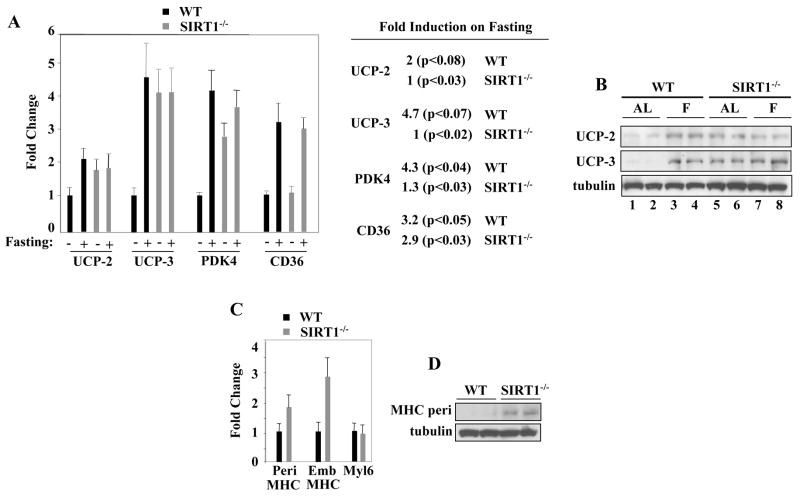
Fasting Induces Expression of Some AMPK Target Genes in a SIRT1-Dependent Manner
To evaluate the role of AMPK-SIRT1 in regulating the response to nutrient availability in vivo, wild type CD1 or SIRT1 −/− homozygous animals outbred on the CD1 strain were fed AL or fasted for 48 hours. We chose to analyze SIRT1−/− homozygous/CD1 mice since these animals have no detectable SIRT1 and, in contrast to inbred SIRT1−/−/129/Sv, survive to adulthood (McBurney et al., 2003). Uncoupling protein 2 (UCP-2) expression is induced by AICAR (Jager et al., 2007) and is repressed by SIRT1 in basal conditions (Bordone et al., 2007) (Moynihan et al., 2005). Compared to fed AL wild type (Fig. 6A and B, lanes 1–2), UCP-2 was increased in muscles of fed AL SIRT1−/− mice (Fig. 6A and B, lanes 5–6). While fasting induced UCP-2 expression in wild type (Fig. 6A and B, lanes 3–4), it failed to further increase the already elevated levels of UCP-2 in SIRT1−/− animals (Fig. 6A and B, lanes 7–8). A similar behavior was noted for the uncoupling protein 3 (UCP-3) – another gene whose expression is induced by AICAR (Jager et al., 2007) and repressed by SIRT1 (Amat et al., 2007) (Fig. 6A and B) and for the pyruvate kinase-4 (PDK4) (Fig. 6A). In contrast, expression of the fatty acid translocase CD36 – a gene activated by AICAR (Chabowski et al., 2006)- was stimulated by fasting, irrespective of SIRT1 (Fig. 6A). Consistent with a repressive role of SIRT1 on muscle gene expression (Fulco et al., 2003), skeletal muscles of SIRT1−/− had elevated levels of perinatal and embryonic myosins- two isoforms that are normally repressed in the adult muscle (Weydert et al., 1987). On the contrary, the non-muscle myosin Myl6 –whose expression is not developmentally regulated - was comparably expressed in wild type and SIRT1−/− mice (Fig. 6 C, D). Since the levels of Pax7, Myf5, cyclins D1 and E, and PCNA - all markers of quiescent or activated and proliferating satellite cells (Holterman and Rudnicki, 2005)- were not increased (Fig. S7 C,D), we are incline to exclude the possibility that elevated expression of perinatal and embryonic myosins is a reflection of muscle damage and regeneration occurring in SIRT1−/− animals. Overall, the results presented in this paragraph indicate that SIRT1 negatively regulates expression of some AMPK targets in normocaloric conditions in vivo and is required to mediate their induction by fasting. They further indicate that skeletal muscles of adult SIRT1−/− mice retain expression of embryonic and neonatal differentiation markers without displaying signs of satellite cell activation.
Figure 6. In Vivo Role of SIRT1 during Fasting.
(A) RT-qPCR of UCP-2, UCP-3, PDK4, and CD36 transcripts from hindlimb muscles of WT and SIRT1−/− mice fed ad libitum (AL) or fasted for 48 hours. The values of each transcript were corrected for those of GAPDH transcripts. Error bars represent standard deviations. The RNAs obtained from three (n=3) animals per experimental group were analyzed. Fold induction and p-values are indicated in the right panel. (B) UCP-2, UCP-3, and tubulin immunoblot of extracts derived from hindlimb muscles of WT mice fed AL (lanes1–2) or fasted (F) for 48 hours (lanes 3–4) and SIRT1−/− mice fed AL (lanes 5–6) or fasted (F) for 48 hours (lanes 7–8). Muscles derived from two WT and two SIRT1−/− animals for each experimental condition were analyzed. (C) RT-qPCR of embryonic Myh3 and perinatal Myh8, and non-muscle Myl6 myosin transcripts in WT and SIRT1−/− mice. The values of each transcript were corrected for those of GAPDH transcripts. Error bars represent standard deviations. The RNAs obtained from three (n=3) animals per experimental group were analyzed. (D) MHC perinatal and tubulin immunoblot of extracts derived from hindlimb muscles of WT and SIRT1−/− mice. Two WT and two SIRT1−/− animals were analyzed
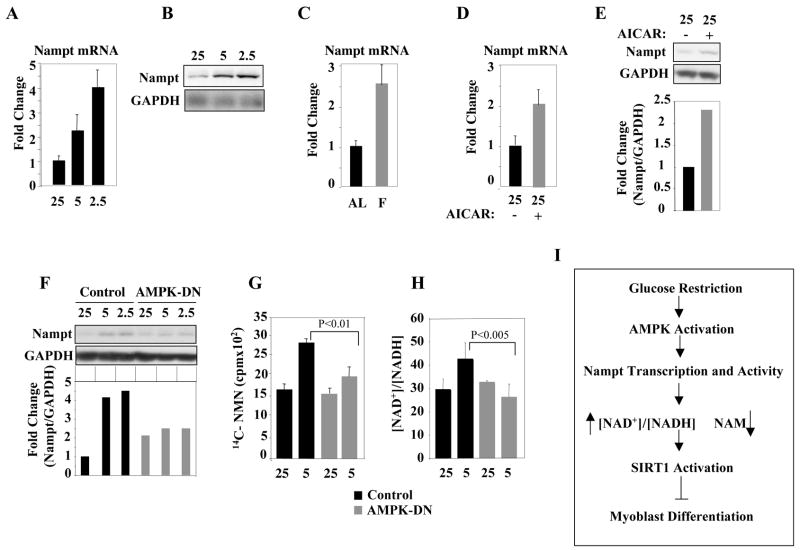
Glucose Restriction Induces Nampt Transcription via AMPK
Extracts derived from either GR or AICAR-treated cells have an elevated Nampt enzymatic activity (Fig. 4H,I). Therefore, we asked whether these two interventions might influence Nampt transcription. Following GR of skeletal muscle cells or animal fasting, the Nampt mRNA and protein levels were increased (Fig. 7A–C). Cells exposed to AICAR had also increased Nampt mRNA and protein levels (Fig. 7 D, E). Such increase in Nampt was blunted in cells cultured in GR conditions and expressing an AMPK-dominant negative (AMPK-DN) form (Fig. 7F), indicating that AMPK regulates GR-induced expression of Nampt. Consonant with a lack of Nampt induction, neither the Nampt activity nor the [NAD+]/[NADH] ratio was increased in AMPK-DN cells exposed to GR (Fig. 7G, H). Overall, these experiments indicate that activated AMPK is required to mediate transcriptional induction of Nampt occurring during GR.
Figure 7. AMPK Mediates the Glucose Restriction-Induced Nampt Transcription.
(A) RT-qPCR of Nampt RNA transcripts from C2C12 cells cultured in DM with 25mM, 5mM, or 2.5mM glucose. In all the experiments reported in this figure, the Nampt values were corrected for those of GAPDH transcripts. Error bars represent standard deviations. (B) Nampt and GAPDH immunoblot of extracts from C2C12 cells cultured as described in (A). (C) RT-qPCR of Nampt RNA transcripts from hindlimb muscles of mice (n=3) subjected to a 48 hr fast (F) or fed AL. (D) Nampt transcripts from C2C12 cells exposed to AICAR in DM with 25mM glucose. (E) Nampt immunoblot of extracts from C2C12 cells exposed to AICAR in DM with 25mM glucose. Quantification was performed by scanning the bands corresponding to the Nampt signal and correcting the values for those obtained from the GAPDH signal (Nampt/GAPDH ratio). (F) Nampt and GAPDH immunoblot of extracts from C2C12 cells expressing AMPK-DN in DM with 25mM, 5mM, or 2.5mM glucose. (G) Nampt activity in extracts from control or AMPK-DN-expressing C2C12 cells cultured in DM with 25mM or 5mM glucose (H) [NAD+]/[NADH] ratio in control or AMPK-DN-expressing C2C12 cells cultured in DM with 25mM or 5mM glucose. (I) Schematic illustration of the CR-AMPK-Nampt-SIRT1 pathway.
DISCUSSION
SIRT1 regulates skeletal muscle differentiation in a tissue culture system (Fulco et al., 2003) but how signals emanating from the microenvironment control this process is not understood. More specifically, whether and how modifications of the microenvironment may impact on the intracellular NAD+ biosynthesis and/or the nicotinamide levels -and therefore modulate SIRT1 activity- in skeletal muscle cells, remains to be determined. The results of this study indicate that skeletal myoblasts cultured in low glucose media are impaired in their differentiation through activation of a pathway that targets the enzymatic activity of SIRT1. The lack of proper differentiation when nutrients are scarce may be simplistically interpreted as the result of passive adaptation of cells incapable of sustaining energy-demanding processes such as those associated with modification of gene expression, sarcomere assembly, and reorganization of the Golgi apparatus, that accompany differentiation. However, the results of our experiments suggest an alternative interpretation of the phenomenon and rather indicate the existence of a defined pathway that actively controls cell behavior in response to low nutrients. AMPK, Nampt, and SIRT1 are the molecular components of this pathway and each of them is required for the cell to respond to GR. Pharmacological inhibition, RNA interference, or hemizigosity of the single molecular components render skeletal muscle cells oblivious to the calorie-poor microenvironment, allowing them to differentiate in an otherwise non-permissive microenvironment. As such, the AMPK-Nampt-SIRT1 pathway can be viewed as a checkpoint that allows the cell to sense and respond to a scarcity of nutrients in the immediate surroundings (Fig. 7I). Experiments conducted in fasting mice revealed that SIRT1 mediates response of some AMPK target genes to nutrient availability. Since the contribution of satellite cells to physiological muscle fiber growth and maintenance in the adult animal is small (Spalding et al., 2005), it is likely that AMPK and SIRT1 may be differently activated and mediate distinct outcomes in cultured muscle cells exposed to low glucose and in muscles of adult fasting animal. Central to the regulation of SIRT1 in cultured cells and to the functional consequences of GR on cell differentiation is the modulation of the intracellular [NAD+]/[NADH] ratio and the NAM levels engendered by GR. These two metabolic parameters are regulated by the Nampt enzyme of the NAD+ salvage pathway and are essential to convey cellular response induced by GR. The likelihood that free cellular NAD+ is regulatory for SIRT1 activity is suggested by the estimated concentration of free nuclear NAD+, which approximates Km values of several sirtuins (Bedalov et al., 2003). Nonetheless, the elevated ratio of cytoplasmic free [NAD+]/[NADH] ratio (Williamson et al., 1967) makes it unlikely that the enzymatic activity of SIRT1 is modulated by a generalized increase of the NAD+. Compartmentalization of the NAD+ biosynthesis (Yang et al., 2006) may create local gradients - in defined nuclear chromatin domains or organelles (Yang et al., 2007) - of NAD+, [NAD+]/[NADH] ratio and NAM levels that could effectively regulate SIRT1 and other sirtuins. Indeed, reduction of the NAM levels obtained via expression of the NNMT in skeletal myoblasts is sufficient to recapitulate the effects of GR on cell differentiation. While these findings do not exclude the possibility that the [NAD+]/[NADH] ratio may be functionally relevant, they further underscore the important role that NAM exerts in regulating phenomena controlled by SIRT1 (Anderson et al., 2003). Nampt expression is increased following several stress stimuli (Yang et al., 2007). Our experiments provide evidence that AMPK is involved in this regulation. AMPK activation – induced by GR or AICAR- causes the Nampt transcripts to increase in normocaloric conditions, while blockade of AMPK obtained using an AMPK dominant negative form prevents GR-mediated increased expression of Nampt. Intriguingly, a genomic region directing Nampt expression encompasses several putative binding sites for the forkhead FOXO transcription factors (M.F. and V.S., unpublished results), which are phosphorylated and activated by AMPK (Greer et al., 2007b). In addition to intracellular Nampt, a secreted form of Nampt has been described that reportedly exhibits robust NAD+ biosynthetic activity. Importantly, Nampt+/− heterozygous mice show impaired glucose tolerance and reduced glucose-stimulated insulin secretion (Revollo et al., 2007). Modification of the intracellular [NAD+]/[NADH] ratio and of NAM levels are necessary but not sufficient to mediate the effects of GR and require the enzymatic activity of SIRT1. Increased expression (Cohen et al., 2004) (Nemoto et al., 2004) coupled to increased SIRT1 activity -as we report here in GR skeletal muscle cells- may be part of a functionally coherent strategy developed by the cell to cope with reduced nutrient availability. It seems that increased Nampt expression controls different cellular outcomes depending on the location of the target sirtuins. In the case of genotoxic stress- mediated cells death, the protective role of Nampt is exerted through the mitochondrial SIRT3 and SIRT4 (Yang et al., 2006), whereas GR-induced Nampt expression mediates its effects on the differentiation process of skeletal muscle cells through the nuclear SIRT1. Whether AMPK has a role also in the SIRT3-SIRT4 regulated response to genotoxic stress awaits further investigation.
There is little doubt that calorie restriction has several beneficial outcomes in adult organisms, especially in humans with a typical Western diet (Hursting et al., 2003). For instance, reducing calorie intake improves numerous functional indexes, and reduces metabolic risks associated with type II diabetes and metabolic syndrome. Intriguingly, alternate-day fasting (ADF)- a regimen involving one day of ad libitum diet followed by one day of food withholding or reduction- has also been shown, though not conclusively, to improve insulin sensitivity, reduce blood pressure, and increase muscle fat oxidation (Varady and Hellerstein, 2007). Our study raises the possibility that AMPK-Nampt-SIRT1 may be the molecular pathway activated by calorie restriction/ADF regimens and by the hypoglycemic agent metformin, a drug commonly used to treat type II diabetes (Krentz and Bailey, 2005). Less investigated are the effects of reduced caloric intake during embryonic and fetal development, while it is well known that undernourishment is detrimental for fetal development with lasting consequences in adulthood. Earlier studies have shown that the skeletal muscle fiber numbers in young mammals (Bedi et al., 1982)- including humans (Montgomery, 1962)- are reduced following undernutrition of the mother during gestation and lactation. Interestingly, the mononucleated cells lying within the basal lamina (satellite cells) were not reduced but the secondary myotubes were, indicating that the rate of myoblast differentiation (or proliferation) was impaired in the muscles of the undernourished animals (Wilson et al., 1988). Restoration of normal dietary intake could correct –within a given time frame- the muscle fibers defect of undernourished animals (Wilson et al., 1988). We speculate that, functioning as a cellular checkpoint, the AMPK-Nampt-SIRT1 pathway may be activated by reduced nutrient availability to prevent the undertaking of energy-demanding processes -such as cell differentiation- during calorie-unfavorable conditions and be inactivated, once nutrients become available, to allow resumption of physiological development.
EXPERIMENTAL PROCEDURES
Cell Culture, Small Molecules, and Retroviral Infection
C2C12 cells were obtained from ATCC and grown in complete DMEM medium supplemented with 20% fetal bovine serum (FBS) (growth medium, GM) and 100 μg/ml penicillin/streptomycin. When C2C12 were nearly confluent (60–70% confluency), they were induced to differentiate in DMEM supplemented with 2% horse serum, insulin, transferrin, and selenium (1X) (Invitrogen) (differentiation medium, DM) either in normocaloric (25mM glucose) or reduced glucose conditions, as indicated in the single experiments. The fusion index was determined by counting the number of nuclei present in MHC+ cells/number of total nuclei and expressed as percentage as described in (Iezzi et al., 2004). Nuclei present in at least 5 microscopic fields were counted. NAM and metformin were obtained from Sigma. AICAR and D942, compound C, and the oleic acid were obtained from Calbiochem. The Nampt specific inhibitor FK866 was kindly synthesized by NIMH Chemical Synthesis and Drug Supply Program at the NIH. Retroviruses were generated by transient transfection of retroviral constructs in Phoenix cells. The viral supernatants were employed to transduce C2C12 cells. Cells were selected with 2μg/ml puromycin.
Antibodies, Immunoblotting and Immunofluorescence
The methods and antibodies employed are reported in Supplemental Data.
Plasmids, siRNAs and Retroviral Constructs
The constructs and siRNAs source are described in Supplemental Data.
Expression Profiling and Data Analysis
The methods are described in Supplemental Data. A comprehensive annotation of the transcripts identified by genome-wide expression profiling has been deposited in the GEO database. The accession number is reported in Supplemental Data.
RT-qPCR
The methods and the oligonucleotide sequences are reported in Supplemental Data
Mouse Primary Myoblasts
Skeletal primary myoblasts from four weeks-old wilt type or SIRT1 +/−129/Sv mice were isolated and cultured as described in (Caretti et al., 2004). To assay for the effects of GR on muscle differentiation, cells were plated on gelatin-coated tissue culture dishes and induced to differentiate in DM supplemented with either 25mM or 0.5mM glucose.
Nampt Enzymatic Activity, NAD+/NADH Assays, and HPLC/MALDI/MS Determination of NAM
Detailed protocols for determining Nampt activity, NAD+ and NADH levels, and HPLC/MALDI/MS evaluation of nicotinamide are reported in Supplemental Data.
SIRT1 Deacetylase Activity
SIRT activity was measured using the SIRT1 Fluorimetric Drug Discovery Kit (Biomol) according to the manufacturer’s instructions with minor modifications as described in Supplemental Data.
ATP Measurements
ATP levels were determined as described in the Supplemental Data.
Animal Studies
Six weeks-old Balb/c mice were obtained from the Jackson Laboratory and housed at the animal vivarium of the NIAMS at the NIH. The control group (n=3) was fed ad libitum (AL) with a standard diet (Harland Tekland extruded diet 2018, 57.26% carbohydrate) while the experimental group (n=3) was fasted for 48 hours before being euthanized. Five months-old wild type/CD1 or SIRT1−/− homozygous/CD1 mice (McBurney et al., 2003) (n=3 for each group) were fed AL or fasted as described for the Balb/c mice. Water was available to both the control and fasted animals at all times. The hindlimb muscles from both control and experimental animals were isolated and immediately frozen in liquid nitrogen and stored at −80°C until RNA or protein extraction. Four weeks-old wild type or SIRT1+/− heterozygous/129/Sv mice (McBurney et al., 2003) were euthanized and the hindlimb muscles isolated and immediately employed to obtain skeletal myoblast cultures. All the animal studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Supplementary Material
Acknowledgments
We thank R.G. Jones and C.B. Thompson (University of Pennsylvania, Philadelphia), R.M Weinshilboum (Mayo Clinic, Rochester), and F.Andris (Universite’ Libre de Bruxelles, Gosselies, Belgium) for the generous gift of AMPK, NNMT, and Nampt (PBEF) plasmids, respectively, and the members of the NIMH Chemical Synthesis and Drug Supply Program at the NIH for chemical synthesis of FK866. We are indebted to M.T.King and R.L.Veech (Laboratory of Membrane Biochemistry and Biophysics, NIAAA, NIH) for measuring the ATP levels. The help of Kambiz Mousavi with the isolation and culture of mouse primary myoblasts and the advice of Aster H. Juan in animal handling is kindly acknowledged. A.A.S. is consultant to Sirtris Pharmaceuticals, a company aiming to treat diseases by modulating sirtuins. This work was supported in part by the Intramural Research Program of the National Institute of Arthritis, Musculoskeletal, and Skin Diseases of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksoy S, Szumlanski CL, Weinshilboum RM. Human liver nicotinamide N-methyltransferase. cDNA cloning, expression, and biochemical characterization. J Biol Chem. 1994;269:14835–14840. [PubMed] [Google Scholar]
- Amat R, Solanes G, Giralt M, Villarroya F. SIRT1 is involved in glucocorticoid-mediated control of uncoupling protein-3 gene transcription. J Biol Chem. 2007;282:34066–34076. doi: 10.1074/jbc.M707114200. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfeld J, O’Connor G, McDonagh T, DiStefano PS, Curtis R. The AMP-activated protein kinase AAK-2 links energy levels and insulin-like signals to lifespan in C. elegans. Genes Dev. 2004;18:3004–3009. doi: 10.1101/gad.1255404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedalov A, Hirao M, Posakony J, Nelson M, Simon JA. NAD+-dependent deacetylase Hst1p controls biosynthesis and cellular NAD+ levels in Saccharomyces cerevisiae. Mol Cell Biol. 2003;23:7044–7054. doi: 10.1128/MCB.23.19.7044-7054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedi KS, Birzgalis AR, Mahon M, Smart JL, Wareham AC. Early life undernutrition in rats. 1. Quantitative histology of skeletal muscles from underfed young and refed adult animals. Br J Nutr. 1982;47:417–431. doi: 10.1079/bjn19820053. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 2002;277:45099–45107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Blander G, Guarente L. The Sir2 family of protein deacetylases. Annu Rev Biochem. 2004;73:417–435. doi: 10.1146/annurev.biochem.73.011303.073651. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007 doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, et al. Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 2006;4:e31. doi: 10.1371/journal.pbio.0040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassada RC, Russell RL. The dauerlarva, a post-embryonic developmental variant of the nematode Caenorhabditis elegans. Dev Biol. 1975;46:326–342. doi: 10.1016/0012-1606(75)90109-8. [DOI] [PubMed] [Google Scholar]
- Chabowski A, Momken I, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A. Prolonged AMPK activation increases the expression of fatty acid transporters in cardiac myocytes and perfused hearts. Mol Cell Biochem. 2006;288:201–212. doi: 10.1007/s11010-006-9140-8. [DOI] [PubMed] [Google Scholar]
- Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med. 2007;13:64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Chua KF, Mostoslavsky R, Lombard DB, Pang WW, Saito S, Franco S, Kaushal D, Cheng HL, Fischer MR, Stokes N, et al. Mammalian SIRT1 limits replicative life span in response to chronic genotoxic stress. Cell Metab. 2005;2:67–76. doi: 10.1016/j.cmet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelli M, Carnevali F, Saccucci F, Pierella F, Amici A, Raffaelli N, Magni G. Molecular cloning, chromosomal localization, tissue mRNA levels, bacterial expression, and enzymatic properties of human NMN adenylyltransferase. J Biol Chem. 2001;276:406–412. doi: 10.1074/jbc.M008700200. [DOI] [PubMed] [Google Scholar]
- Fulco M, Schiltz RL, Iezzi S, King MT, Zhao P, Kashiwaya Y, Hoffman E, Veech RL, Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO Pathway Mediates Longevity Induced by a Novel Method of Dietary Restriction in C. elegans. Curr Biol. 2007a doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem. 2007b;282:30107–30119. doi: 10.1074/jbc.M705325200. [DOI] [PubMed] [Google Scholar]
- Grubisha O, Smith BC, Denu JM. Small molecule regulation of Sir2 protein deacetylases. Febs J. 2005;272:4607–4616. doi: 10.1111/j.1742-4658.2005.04862.x. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins--emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase--development of the energy sensor concept. J Physiol. 2006;574:7–15. doi: 10.1113/jphysiol.2006.108944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasmann M, Schemainda I. FK866, a highly specific noncompetitive inhibitor of nicotinamide phosphoribosyltransferase, represents a novel mechanism for induction of tumor cell apoptosis. Cancer Res. 2003;63:7436–7442. [PubMed] [Google Scholar]
- Hipkiss AR. Does chronic glycolysis accelerate aging? Could this explain how dietary restriction works? Ann N Y Acad Sci. 2006;1067:361–368. doi: 10.1196/annals.1354.051. [DOI] [PubMed] [Google Scholar]
- Holterman CE, Rudnicki MA. Molecular regulation of satellite cell function. Semin Cell Dev Biol. 2005;16:575–584. doi: 10.1016/j.semcdb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Hurley MS, Flux C, Salter AM, Brameld JM. Effects of fatty acids on skeletal muscle cell differentiation in vitro. Br J Nutr. 2006;95:623–630. doi: 10.1079/bjn20051711. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Lavigne JA, Berrigan D, Perkins SN, Barrett JC. Calorie restriction, aging, and cancer prevention: mechanisms of action and applicability to humans. Annu Rev Med. 2003;54:131–152. doi: 10.1146/annurev.med.54.101601.152156. [DOI] [PubMed] [Google Scholar]
- Iezzi S, Di Padova M, Serra C, Caretti G, Simone C, Maklan E, Minetti G, Zhao P, Hoffman EP, Puri PL, Sartorelli V. Deacetylase inhibitors increase muscle cell size by promoting myoblast recruitment and fusion through induction of follistatin. Dev Cell. 2004;6:673–684. doi: 10.1016/s1534-5807(04)00107-8. [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403:795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW., 3rd Sir2 and calorie restriction in yeast: a skeptical perspective. Ageing Res Rev. 2007;6:128–140. doi: 10.1016/j.arr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Mitchelhill KI, Stapleton D, Michell BJ, Chen ZP, Witters LA. Dealing with energy demand: the AMP-activated protein kinase. Trends Biochem Sci. 1999;24:22–25. doi: 10.1016/s0968-0004(98)01340-1. [DOI] [PubMed] [Google Scholar]
- Khan JA, Tao X, Tong L. Molecular basis for the inhibition of human NMPRTase, a novel target for anticancer agents. Nat Struct Mol Biol. 2006;13:582–588. doi: 10.1038/nsmb1105. [DOI] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Okuyama R, Sun W, Ogata T, Harada J, Araki K, Izumi M, Yoshida T, Okuno A, Fujiwara T, et al. Identification of molecular target of AMP-activated protein kinase activator by affinity purification and mass spectrometry. Anal Chem. 2005;77:2050–2055. doi: 10.1021/ac0484631. [DOI] [PubMed] [Google Scholar]
- Krentz AJ, Bailey CJ. Oral antidiabetic agents: current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang S, Blander G, Tse JG, Krieger M, Guarente L. SIRT1 Deacetylates and Positively Regulates the Nuclear Receptor LXR. Mol Cell. 2007;28:91–106. doi: 10.1016/j.molcel.2007.07.032. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. [DOI] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Raffaelli N, Ruggieri S. Enzymology of NAD+ synthesis. Adv Enzymol Relat Areas Mol Biol. 1999;73:135–182. xi. doi: 10.1002/9780470123195.ch5. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Dietary restriction-induced life extension: a broadly based biological phenomenon. Biogerontology. 2006;7:153–155. doi: 10.1007/s10522-006-9015-0. [DOI] [PubMed] [Google Scholar]
- McBurney MW, Yang X, Jardine K, Hixon M, Boekelheide K, Webb JR, Lansdorp PM, Lemieux M. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF. Chronic activation of AMP-activated kinase as a strategy for slowing aging. Med Hypotheses. 2004;63:334–339. doi: 10.1016/j.mehy.2004.01.043. [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery RD. Muscle morphology in infantile protein malnutrition. J Clin Pathol. 1962;15:511–521. doi: 10.1136/jcp.15.6.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–563. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Meneur C, Permutt MA, Imai S. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005;2:105–117. doi: 10.1016/j.cmet.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. Nutrient availability regulates SIRT1 through a forkhead-dependent pathway. Science. 2004;306:2105–2108. doi: 10.1126/science.1101731. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, Haendeler J, Mione M, Dejana E, Alt FW, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007;21:2644–2658. doi: 10.1101/gad.435107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 2004;279:50754–50763. doi: 10.1074/jbc.M408388200. [DOI] [PubMed] [Google Scholar]
- Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, Dasgupta B, Sasaki Y, Wolberger C, Townsend RR, et al. Nampt/PBEF/Visfatin Regulates Insulin Secretion in beta Cells as a Systemic NAD Biosynthetic Enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Caretti G. Mechanisms underlying the transcriptional regulation of skeletal myogenesis. Curr Opin Genet Dev. 2005;15:528–535. doi: 10.1016/j.gde.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauve AA, Wolberger C, Schramm VL, Boeke JD. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- Schulz TJ, Zarse K, Voigt A, Urban N, Birringer M, Ristow M. Glucose Restriction Extends Caenorhabditis elegans Life Span by Inducing Mitochondrial Respiration and Increasing Oxidative Stress. Cell Metab. 2007;6:280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Spalding KL, Bhardwaj RD, Buchholz BA, Druid H, Frisen J. Retrospective birth dating of cells in humans. Cell. 2005;122:133–143. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Tanny JC, Moazed D. Coupling of histone deacetylation to NAD breakdown by the yeast silencing protein Sir2: Evidence for acetyl transfer from substrate to an NAD breakdown product. Proc Natl Acad Sci U S A. 2001;98:415–420. doi: 10.1073/pnas.031563798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veer E, Ho C, O’Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J Biol Chem. 2007;282:10841–10845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang X, Bheda P, Revollo JR, Imai S, Wolberger C. Structure of Nampt/PBEF/visfatin, a mammalian NAD+ biosynthetic enzyme. Nat Struct Mol Biol. 2006;13:661–662. doi: 10.1038/nsmb1114. [DOI] [PubMed] [Google Scholar]
- Weydert A, Barton P, Harris AJ, Pinset C, Buckingham M. Developmental pattern of mouse skeletal myosin heavy chain gene transcripts in vivo and in vitro. Cell. 1987;49:121–129. doi: 10.1016/0092-8674(87)90762-8. [DOI] [PubMed] [Google Scholar]
- Williamson DH, Lund P, Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Ross JJ, Harris AJ. A critical period for formation of secondary myotubes defined by prenatal undernourishment in rats. Development. 1988;102:815–821. doi: 10.1242/dev.102.4.815. [DOI] [PubMed] [Google Scholar]
- Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, Perez E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-Sensitive Mitochondrial NAD(+) Levels Dictate Cell Survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.