Abstract
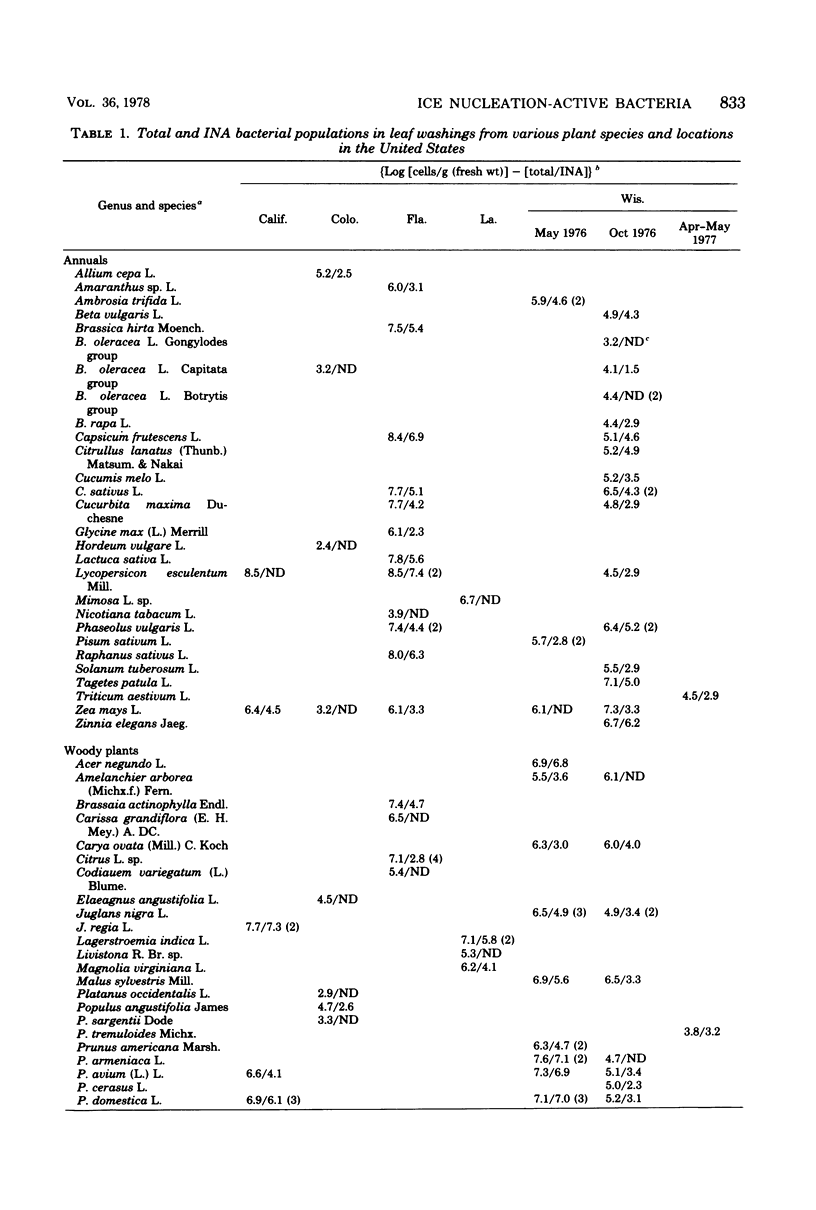
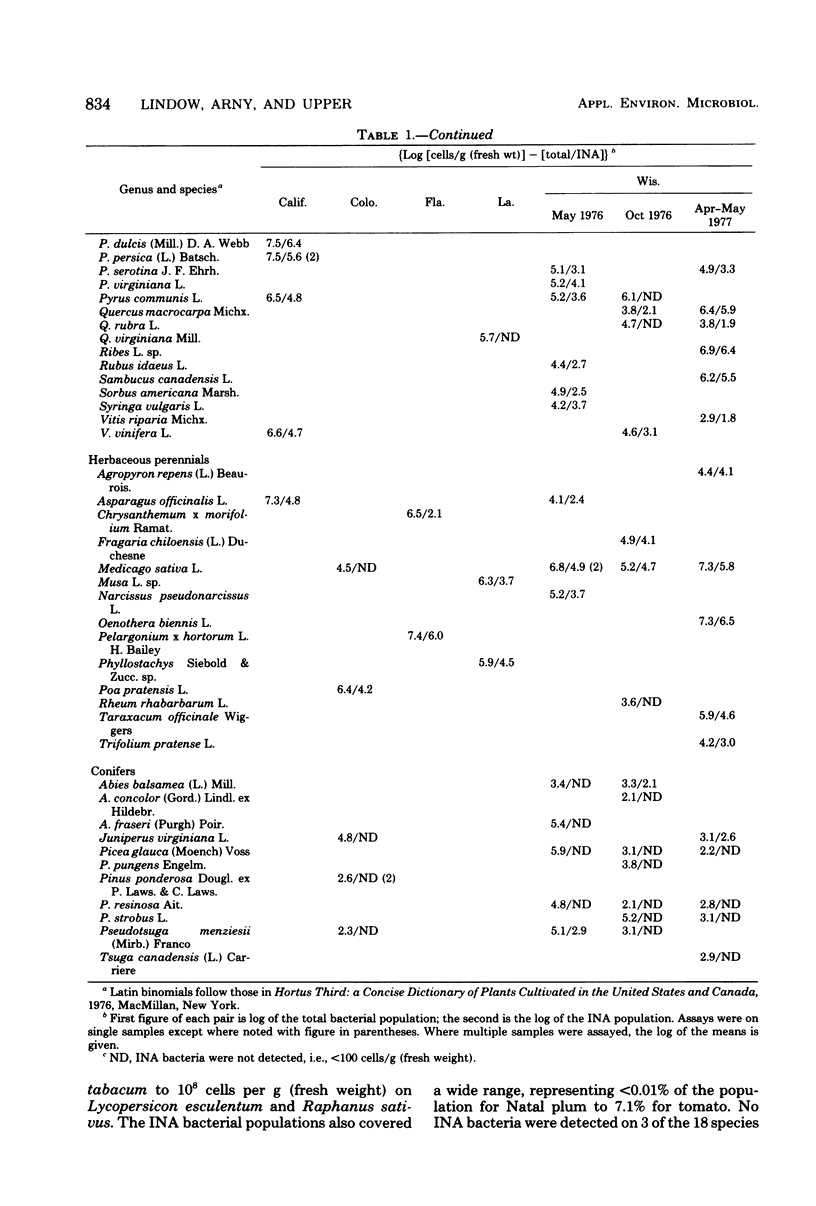
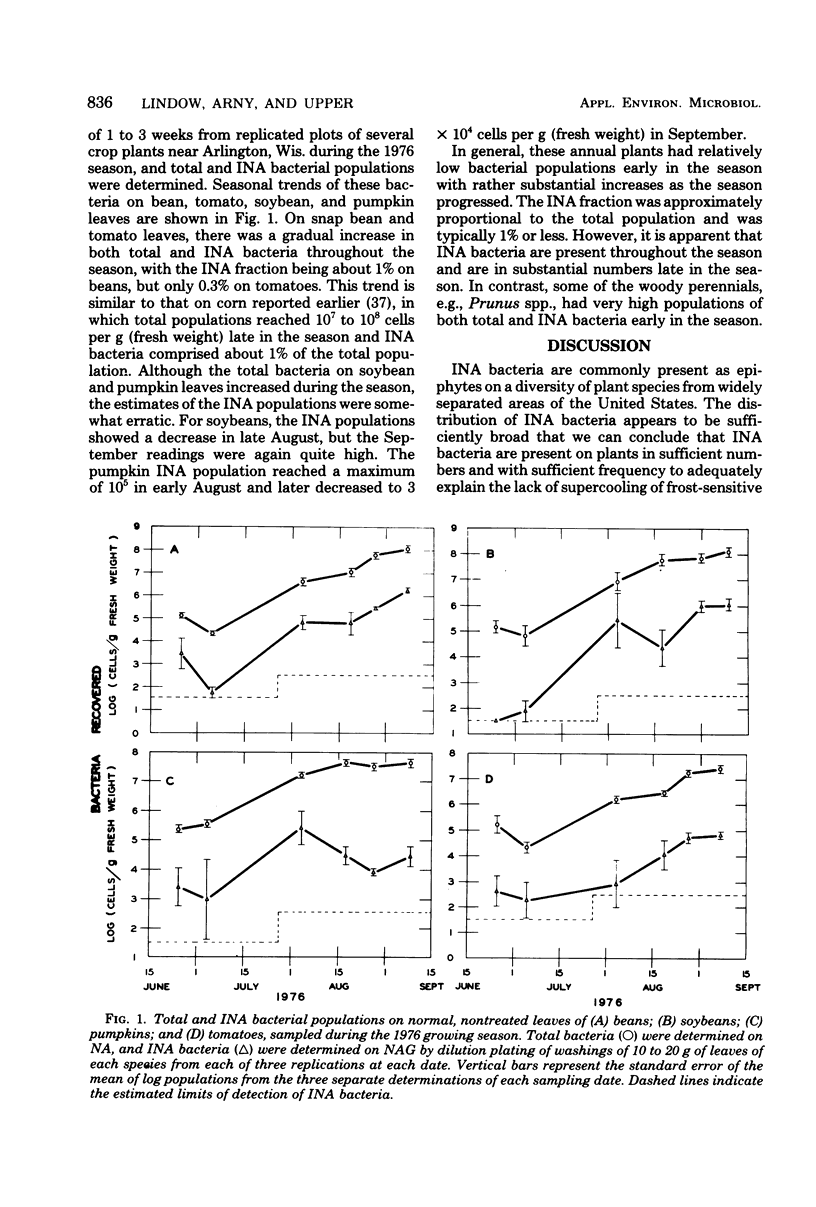
A replica plating method for rapid quantitation of ice nucleation-active (INA) bacteria was developed. Leaf washings of plant samples from California, Colorado, Florida, Louisiana, and Wisconsin were tested for the presence of INA bacteria. Of the 95 plant species sampled, 74 were found to harbor INA bacteria. Only the conifers were, as a group, unlikely to harbor INA bacteria. All of the INA bacteria isolated resembled either Pseudomonas syringae or Erwinia herbicola. Sufficient numbers of INA bacteria were present on the samples to account for the ice nuclei associated with leaves that are necessary for freezing injury to occur. Numbers of INA bacteria were large enough to suggest that plant surfaces may constitute a significant source of atmospheric ice nuclei.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean P. G., Everton J. R. Observations on the taxonomy of chromogenic bacteria isolated from cannery environments. J Appl Bacteriol. 1969 Mar;32(1):51–59. doi: 10.1111/j.1365-2672.1969.tb02188.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee A. K., Gibbins L. N., Carpenter J. A. Some observations on the physiology of Erwinia herbicola and its possible implication as a factor antagonistic to Erwinia amylovora in the "fire-blight" syndrome. Can J Microbiol. 1969 Jun;15(6):640–642. [PubMed] [Google Scholar]
- DUNCAN C. L., COLMER A. R. COLIFORMS ASSOCIATED WITH SUGARCANE PLANTS AND JUICES. Appl Microbiol. 1964 Mar;12:173–177. doi: 10.1128/am.12.2.173-177.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GELDREICH E. E., KENNER B. A., KABLER P. W. OCCURRENCE OF COLIFORMS, FECAL COLIFORMS, AND STREPTOCOCCI ON VEGETATION AND INSECTS. Appl Microbiol. 1964 Jan;12:63–69. doi: 10.1128/am.12.1.63-69.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON T., STIRLING A. C., KEDDIE R. M., ROSENBERGER R. F. Bacteriological changes in silage made at controlled temperatures. J Gen Microbiol. 1958 Aug;19(1):112–129. doi: 10.1099/00221287-19-1-112. [DOI] [PubMed] [Google Scholar]
- JAMES N. Yellow chromogenic bacteria on wheat. II. Determinative studies. Can J Microbiol. 1955 Aug;1(7):479–485. doi: 10.1139/m55-061. [DOI] [PubMed] [Google Scholar]
- KING E. O., WARD M. K., RANEY D. E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954 Aug;44(2):301–307. [PubMed] [Google Scholar]
- Kaku S. Analysis of freezing temperature distribution in plants. Cryobiology. 1975 Apr;12(2):154–159. doi: 10.1016/s0011-2240(75)80007-1. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]



