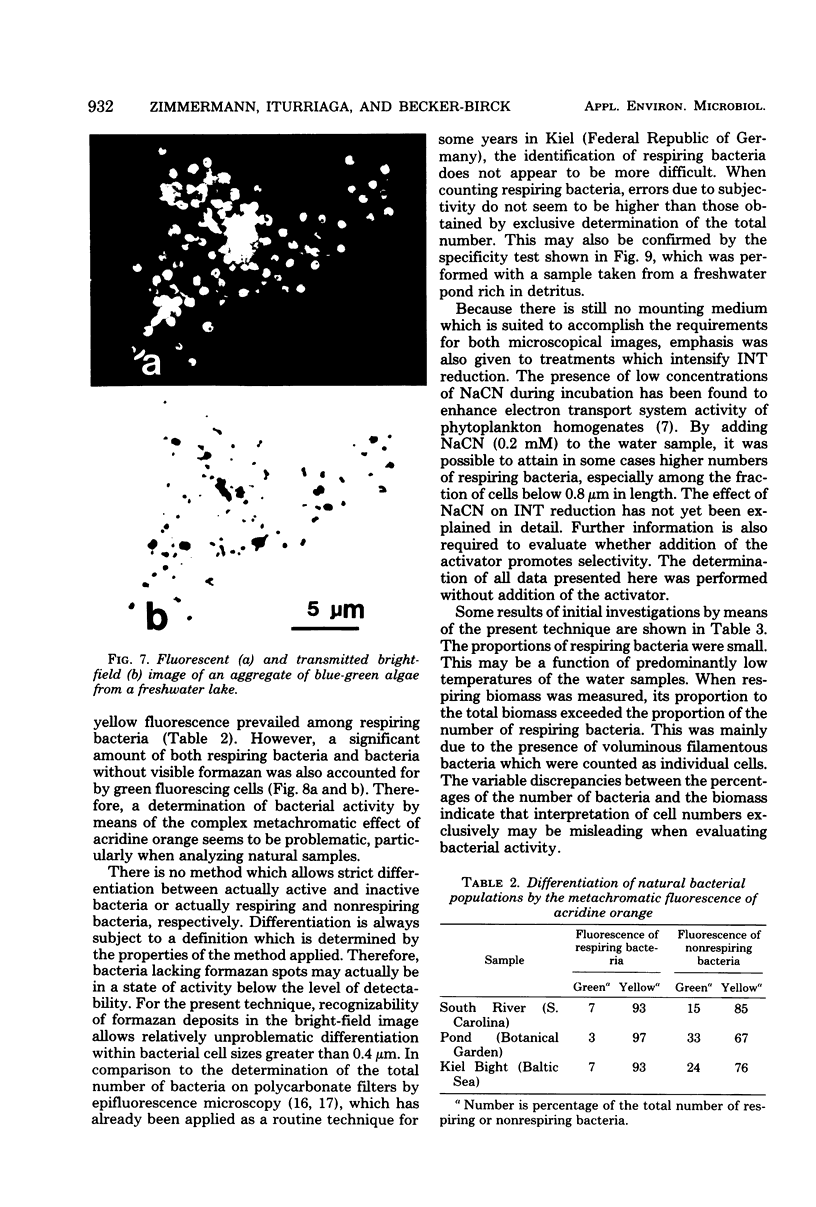
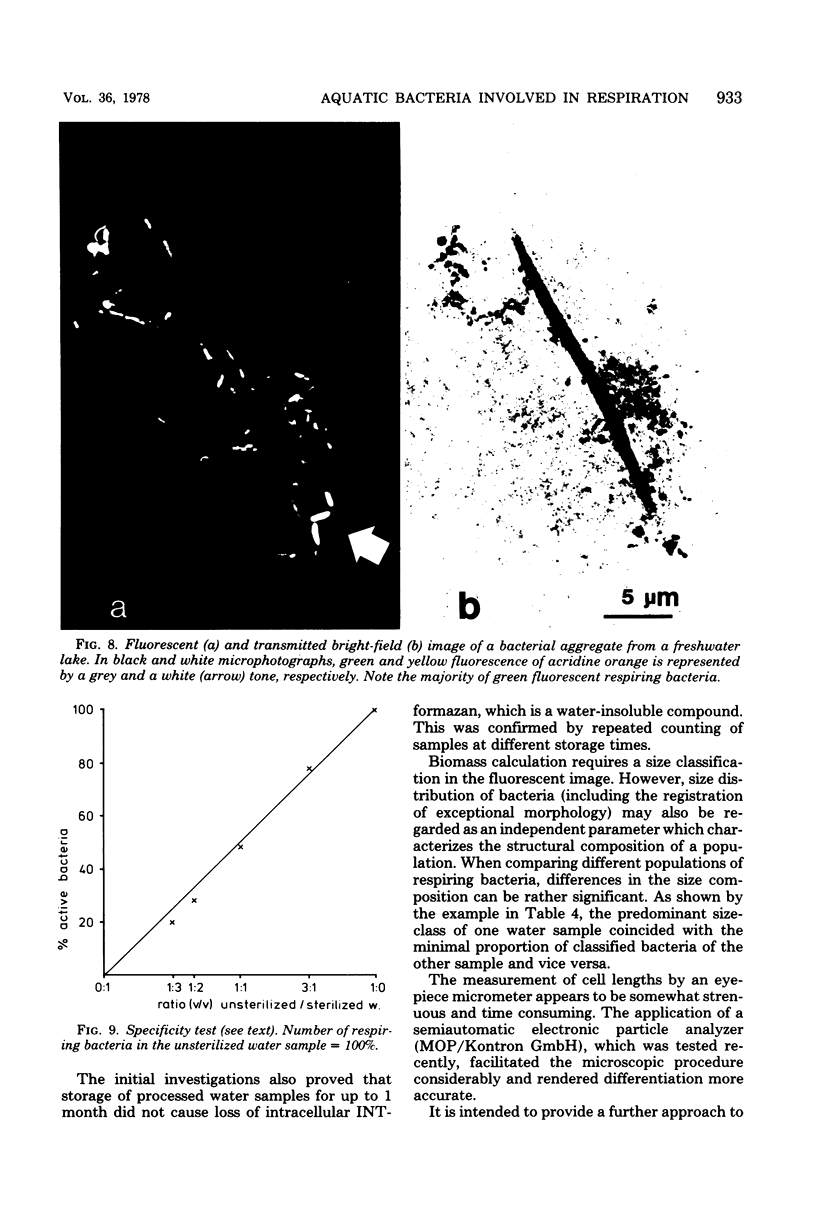
Abstract
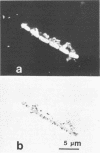
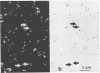
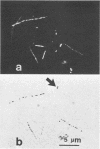
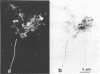
The electron transport system of respiring organisms reduces 2-(p-iodophenyl)-3-(p-nitrophenyl)-5-phenyl tetrazolium chloride (INT) to INT-formazan. Respiring bacteria deposit accumulated INT-formazan intracellularly as dark red spots. Corresponding to electron transport system activity, these deposits attain a size and a degree of optical density which allows them to be examined by light microscopy. If polycarbonate filters and epifluorescence microscopy are applied to analyze an INT-treated water sample, it is possible to differentiate between respiring and apparently nonrespiring bacteria. This differentiation, which permits determinations of the total number of bacteria and the proportion thereof involved in respiration, is realized directly within one and the same microscopic image. Initial applications of the present method for hydrobiological purposes showed that the proportion of respiring aquatic bacteria ranged between 6 to 12% (samples taken from coastal areas of the Baltic Sea) and 5 to 36% (samples taken from freshwater lakes and ponds). Cells of 1.6 to 2.4 micrometer (freshwater) and 0.4 micrometer (Baltic Sea) account for the highest proportion of respiring bacteria.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. G., Simon B. M. An investigation of errors in direct counts of aquatic bacteria by epifluorescence microscopy, with reference to a new method for dyeing membrane filters. J Appl Bacteriol. 1975 Dec;39(3):317–329. doi: 10.1111/j.1365-2672.1975.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Sedar A. W., Burde R. M. The demonstration of the succinic dehydrogenase system in Bacillus subtilis using tetranitro--blue tetrazolium combined with techniques of electron microscopy. J Cell Biol. 1965 Oct;27(1):53–66. doi: 10.1083/jcb.27.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLHAUSSER K. H. Vitalfärbung von Bakterien. Wirkungsmechanismus der Antibiotica-Zellteilungsvorgänge (Vitalfärbung). Zentralbl Bakteriol Parasitenkd Infektionskr Hyg. 1952 Jun;158(3-5):358–362. [PubMed] [Google Scholar]
- WEIBULL C. Observations on the staining of bacillus megaterium with triphenyltetrazolium. J Bacteriol. 1953 Aug;66(2):137–139. doi: 10.1128/jb.66.2.137-139.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]