Abstract
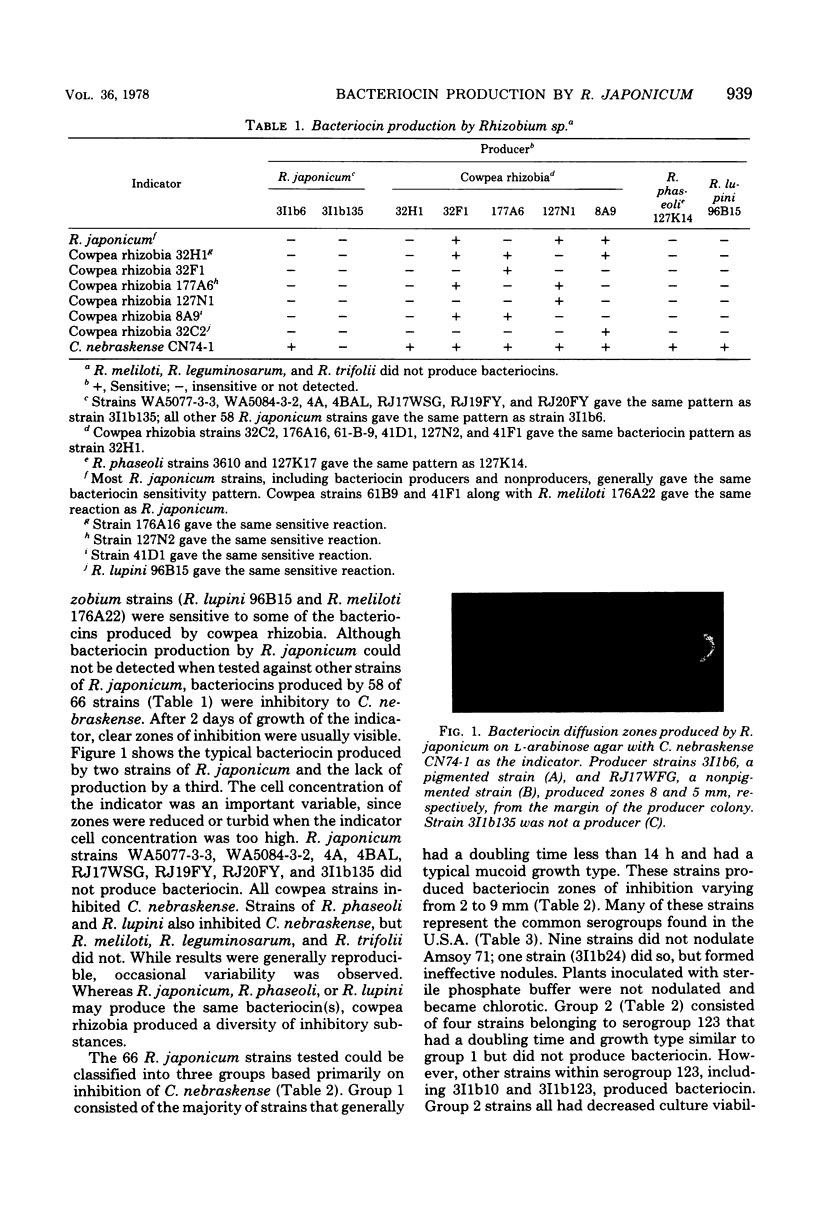
Bacteriocin-like substances were commonly produced by slow-growing Rhizobium japonicum and cowpea rhizobia on an L-arabinose medium. Antagonism between strains of R. japonicum was not detected in vitro; however, such strains were often sensitive to some bacteriocins produced by cowpea rhizobia. Inhibitory zones (2 to 8 mm from colony margins), produced by 58 of 66 R. japonicum test strains, were reproducibly detected with Corynebacterium nebraskense as an indicator. Quantitative production was not related to symbiotic properties of effective strains, since nine noninfective strains and one ineffective strain produced bacteriocin. Eight R. japonicum strains that did not produce bacteriocin nevertheless formed effective nodules on soybeans. R. japonicum strains that produced bacteriocin in vitro had no antagonistic effect on nonproducer strains during soybean nodulation. Under controlled conditions, a nonproducer (3I1b135) predominated over a bacteriocin producer (3I1b6) when inoculated at 1:1 and 1:9 ratios. Depending on the particular ratio, up to 38% of the total nodules formed were infected with mixed combinations. The bacteriocin(s) had a restricted host range and antibiotic-like properties which included the ability to be dialyzed and resistance to heat (75 to 80°C, 30 min), Pronase, proteinase K, trypsin, ribonuclease, and deoxyribonuclease. R. japonicum strains representing genetic, serological, cultural, and geographic diversity were differentiated into three groups on the basis of bacteriocin production.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Johnston A. W., Beringer J. E. Identification of the rhizobium strains in pea root nodules using genetic markers. J Gen Microbiol. 1975 Apr;87(2):343–350. doi: 10.1099/00221287-87-2-343. [DOI] [PubMed] [Google Scholar]
- Johnston A. W., Beringer J. E. Mixed inoculations with effective and ineffective strains of Rhizobium leguminosarum. J Appl Bacteriol. 1976 Jun;40(3):375–380. doi: 10.1111/j.1365-2672.1976.tb04186.x. [DOI] [PubMed] [Google Scholar]
- Lotz W., Mayer F. Isolation and characterization of a bacteriophage tail-like bacteriocin from a strain of Rhizobium. J Virol. 1972 Jan;9(1):160–173. doi: 10.1128/jvi.9.1.160-173.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCardell B. A., Pootjes C. F. Chemical nature of agrocin 84 and its effect on a virulent strain of Agrobacterium tumefaciens. Antimicrob Agents Chemother. 1976 Sep;10(3):498–502. doi: 10.1128/aac.10.3.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W. L., Chakrabarty K., Klucas R. V., Vidaver A. K. Nitrogen fixation (acetylene reduction) associated with roots of winter wheat and sorghum in Nebraska. Appl Environ Microbiol. 1978 Jan;35(1):129–135. doi: 10.1128/aem.35.1.129-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister H., Lodderstaedt G. Adsorption of a phage tail-like bacteriocin to isolated lipopolysaccharide of Rhizobium. J Gen Virol. 1977 Nov;37(2):337–347. doi: 10.1099/0022-1317-37-2-337. [DOI] [PubMed] [Google Scholar]
- Roberts W. P., Tate M. E., Kerr A. Agrocin 84 is a 6-N-phosphoramidate of an adenine nucleotide analogue. Nature. 1977 Jan 27;265(5592):379–381. doi: 10.1038/265379a0. [DOI] [PubMed] [Google Scholar]
- Roslycky E. B. Bacteriocin production in the rhizobia bacteria. Can J Microbiol. 1967 Apr;13(4):431–432. doi: 10.1139/m67-057. [DOI] [PubMed] [Google Scholar]
- Schwinghamer E. A., Pankhurst C. E., Whitfeld P. R. A phage-like bacteriocin of Rhizobium trifolii. Can J Microbiol. 1973 Mar;19(3):359–368. doi: 10.1139/m73-059. [DOI] [PubMed] [Google Scholar]
- Schwinghamer E. A. Properties of some bacteriocins produced by Rhizobium trifolii. J Gen Microbiol. 1975 Dec;91(2):403–413. doi: 10.1099/00221287-91-2-403. [DOI] [PubMed] [Google Scholar]
- Skrdleta V., Karimová J. Competition between two somatic serotypes of Rhizobium japonicum used as double-strain inocula in varying proportions. Arch Mikrobiol. 1969;66(1):25–28. doi: 10.1007/BF00414659. [DOI] [PubMed] [Google Scholar]
- Veldkamp H. Saprophytic coryneform bacteria. Annu Rev Microbiol. 1970;24:209–240. doi: 10.1146/annurev.mi.24.100170.001233. [DOI] [PubMed] [Google Scholar]
- Vidaver A. K., Mathys M. L., Thomas M. E., Schuster M. L. Bacteriocins of the phytopathogens Pseudomonas syringae, P. glycinea, and P. phaseolicola. Can J Microbiol. 1972 Jun;18(6):705–713. doi: 10.1139/m72-113. [DOI] [PubMed] [Google Scholar]
- Watson B., Currier T. C., Gordon M. P., Chilton M. D., Nester E. W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975 Jul;123(1):255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]




