Abstract
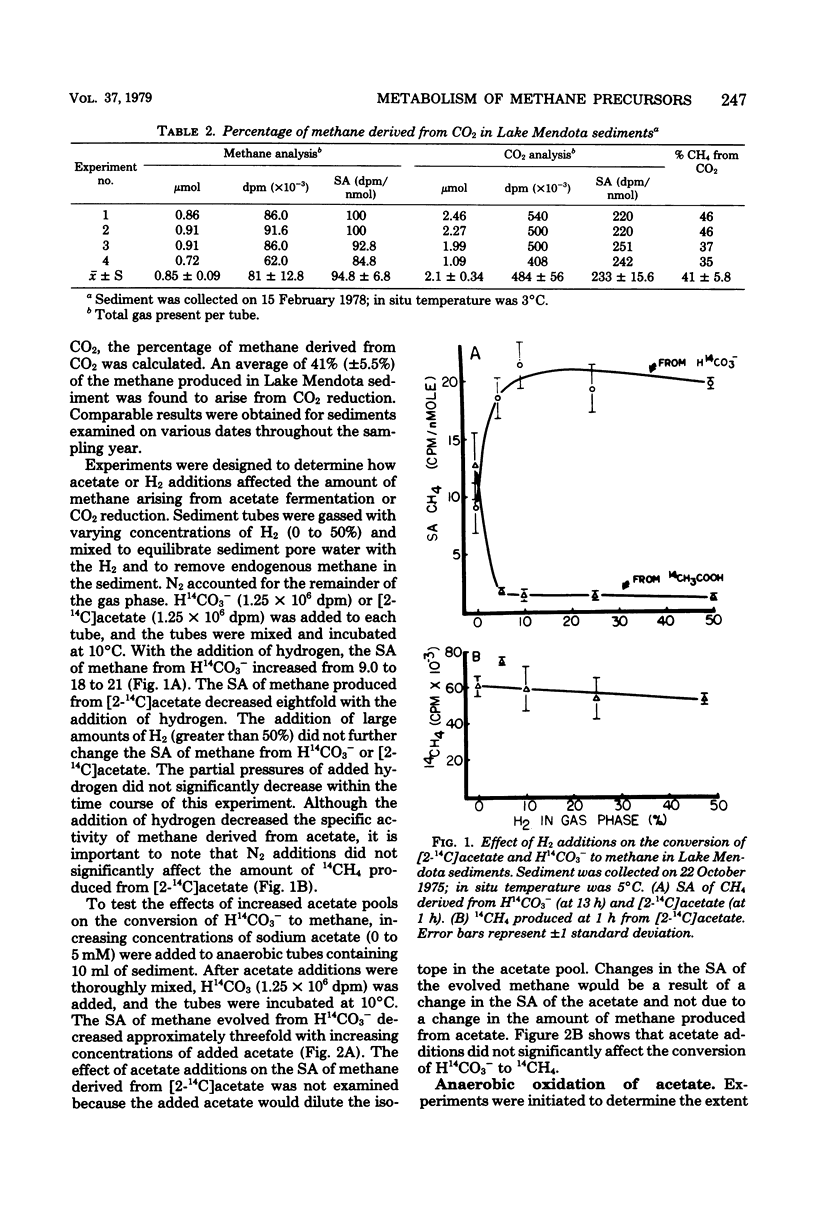
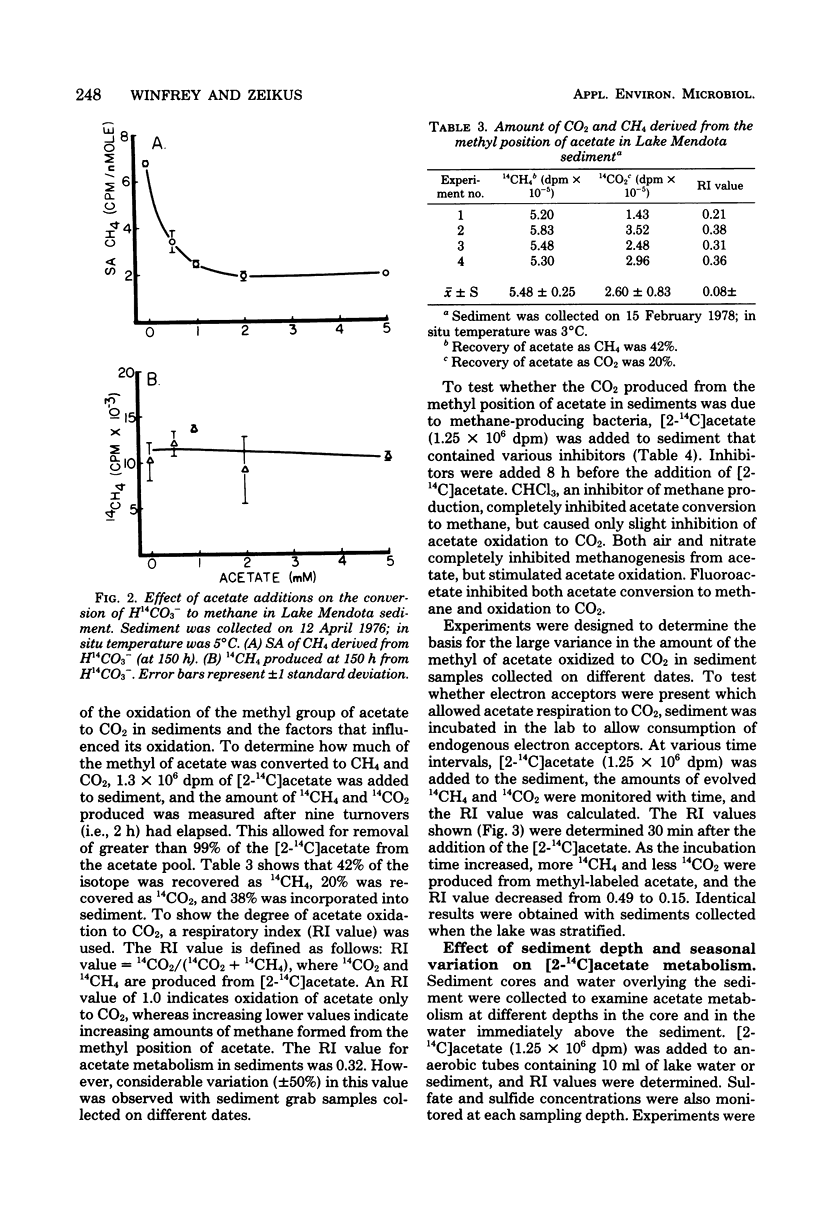
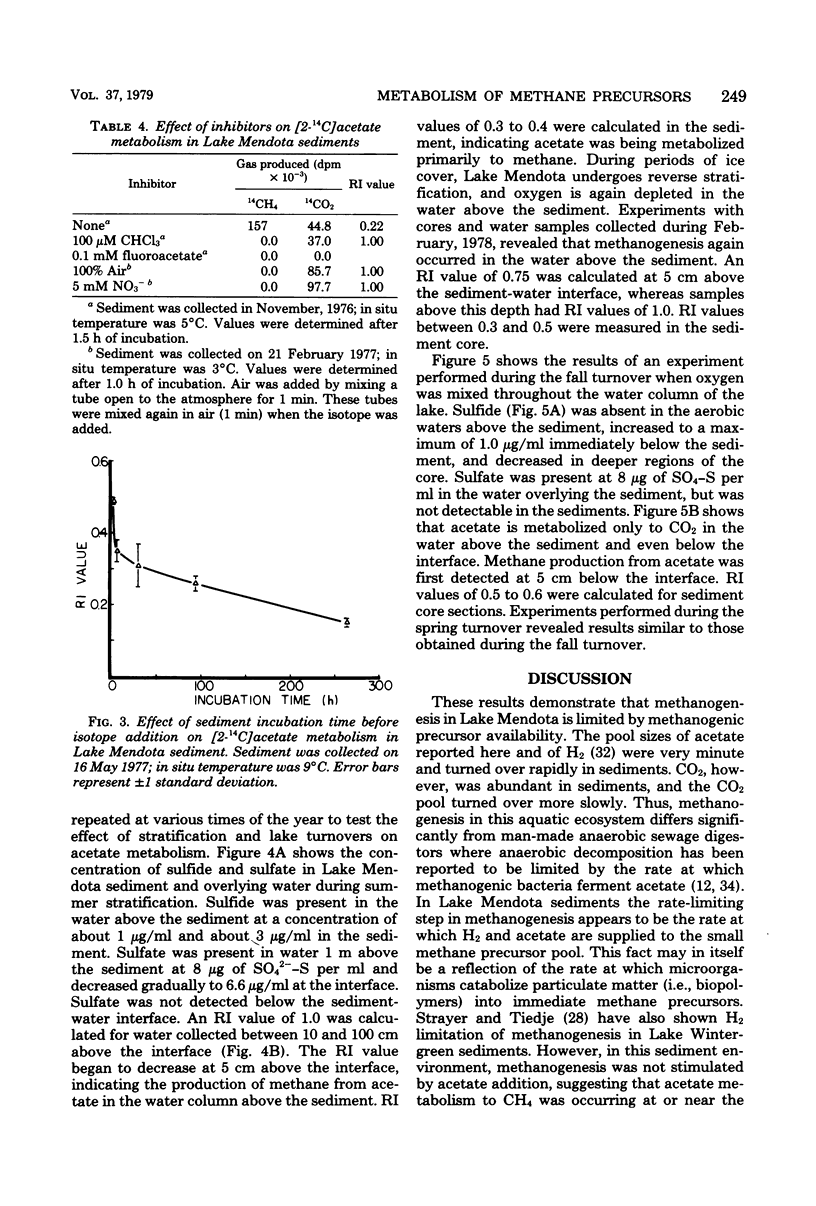
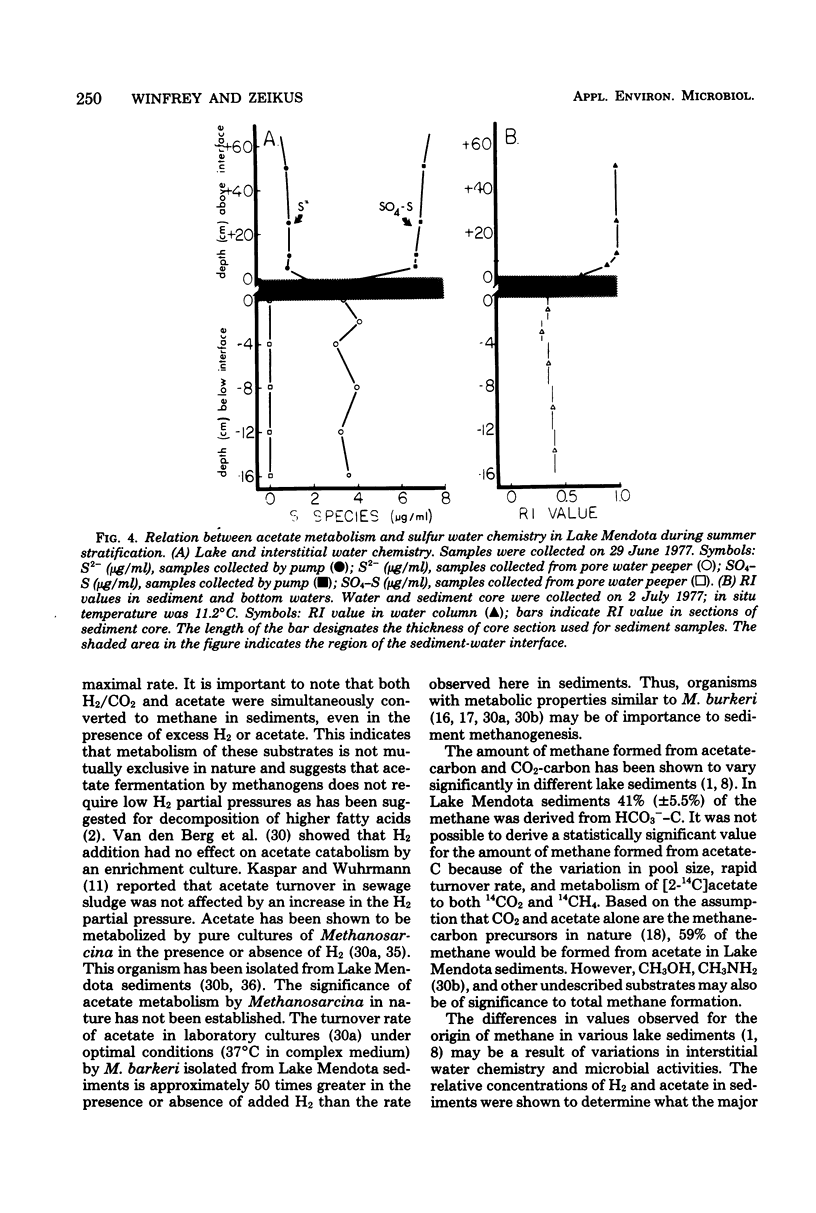
Lake Mendota sediments and the immediate overlying water column were studied to better understand the metabolism of the methanogenic precursors H2/CO2 and acetate in nature. The pool size of acetate (3.5 microns M) was very small, and the acetate turnover time (0.22h) was very rapid. The dissolved inorganic carbon pool was shown to be large (6.4 to 8.3 mM), and the turnover time was slow (111 H.). CO2 was shown to account for 41 +/- 5.5% of the methane produced in sediment. Acetate and H2/CO2 were simultaneously converted to CH4. The addition of H2 to sediments resulted in an increase specific activity of CH4 from H(14)CO3- and a decrease in specific activity of CH4 from [2-14C]acetate. Acetate addition resulted in a decrease in specific activity of CH4 from H(14)CO3-. The metabolism of H(14)CO3- or [2-14C]acetate to 14CH4 was not inhibited by addition of acetate or H2. After greater than 99% of added [2-14C]acetate had been turned over, 42% of the label was recovered as 14CH4 20% was recovered as 14CO2 and 38% was incorporated into sediment. Inhibitor studies of [2-14C]acetate metabolism in sediments demonstrated that CHCl3 completely inhibited CH4 formation, but not CO2 production. Air and nitrate addition inhibited CH4 formation and stimulated CO2 production, whereas fluoroacetate addition totally inhibited acetate metabolism. The oxidation of [2-14C]acetate to 14CO2 was shown to decrease with time when sediment was incubated before the addition of label, suggesting depletion of low levels of an endogenous sediment electron acceptor. Acetate metabolism varied seasonally and was related to the concentration of sulfate in the lake and interstitial water. Methanogenesis occurred in the sediment and in the water immediately overlying the sediment during period of lake stratification and several centimeters below the sediment-water interface during lake turnovers. These data indicate that methanogenesis in Lake Mendota sediments was limited by "immediate" methane precursor availability (i.e., acetate and H2), by competition for these substrates by nonmethanogens, and by seasonal variations which altered sediment and water chemistry.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell D. E., Tiedje J. M. The structure of anaerobic bacterial communities in the hypolimnia of several Michigan lakes. Can J Microbiol. 1975 Mar;21(3):377–385. doi: 10.1139/m75-052. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. II. Inhibition experiments. Antonie Van Leeuwenhoek. 1974;40(2):297–306. doi: 10.1007/BF00394388. [DOI] [PubMed] [Google Scholar]
- Cappenberg T. E., Prins R. A. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. 3. Experiments with 14C-labeled substrates. Antonie Van Leeuwenhoek. 1974;40(3):457–469. doi: 10.1007/BF00399358. [DOI] [PubMed] [Google Scholar]
- Gerasimenko L. M., Goriunova S. V. Nekotorye tsitomorfologicheskie osobennosti Hydrodictyon reticulatum Lagerch. Mikrobiologiia. 1975 Mar-Apr;44(2):272–276. [PubMed] [Google Scholar]
- Kirsch E. J., Sykes R. M. Anaerobic digestion in biological waste treatment. Prog Ind Microbiol. 1971;9:155–237. [PubMed] [Google Scholar]
- Mah R. A., Smith M. R., Baresi L. Studies on an acetate-fermenting strain of Methanosarcina. Appl Environ Microbiol. 1978 Jun;35(6):1174–1184. doi: 10.1128/aem.35.6.1174-1184.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah R. A., Ward D. M., Baresi L., Glass T. L. Biogenesis of methane. Annu Rev Microbiol. 1977;31:309–341. doi: 10.1146/annurev.mi.31.100177.001521. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Zeikus J. G. Rapid method for the radioisotopic analysis of gaseous end products of anaerobic metabolism. Appl Microbiol. 1974 Aug;28(2):258–261. doi: 10.1128/am.28.2.258-261.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S. Methane production in shallow-water, tropical marine sediments. Appl Microbiol. 1975 Oct;30(4):602–608. doi: 10.1128/am.30.4.602-608.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Powell M. R., Doebbler G. F., Hamilton R. W., Jr Serum enzyme level changes in pigs following decompression trauma. Aerosp Med. 1974 May;45(5):519–524. [PubMed] [Google Scholar]
- Smith P. H., Mah R. A. Kinetics of acetate metabolism during sludge digestion. Appl Microbiol. 1966 May;14(3):368–371. doi: 10.1128/am.14.3.368-371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strayer R. F., Tiedje J. M. Kinetic parameters of the conversion of methane precursors to methane in a hypereutrophic lake sediment. Appl Environ Microbiol. 1978 Aug;36(2):330–340. doi: 10.1128/aem.36.2.330-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. Acetate metabolism in Methanosarcina barkeri. Arch Microbiol. 1978 Nov 13;119(2):175–182. doi: 10.1007/BF00964270. [DOI] [PubMed] [Google Scholar]
- Weimer P. J., Zeikus J. G. One carbon metabolism in methanogenic bacteria. Cellular characterization and growth of Methanosarcina barkeri. Arch Microbiol. 1978 Oct 4;119(1):49–57. doi: 10.1007/BF00407927. [DOI] [PubMed] [Google Scholar]
- Widdel F., Pfennig N. A new anaerobic, sporing, acetate-oxidizing, sulfate-reducing bacterium, Desulfotomaculum (emend.) acetoxidans. Arch Microbiol. 1977 Feb 4;112(1):119–122. doi: 10.1007/BF00446665. [DOI] [PubMed] [Google Scholar]
- Winfrey M. R., Nelson D. R., Klevickis S. C., Zeikus J. G. Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl Environ Microbiol. 1977 Feb;33(2):312–318. doi: 10.1128/aem.33.2.312-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G. The biology of methanogenic bacteria. Bacteriol Rev. 1977 Jun;41(2):514–541. doi: 10.1128/br.41.2.514-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg L., Patel G. B., Clark D. S., Lentz C. P. Factors affecting rate of methane formation from acetic acid by enriched methanogenic cultures. Can J Microbiol. 1976 Sep;22(9):1312–1319. doi: 10.1139/m76-194. [DOI] [PubMed] [Google Scholar]



