Abstract
Replication-defective herpes simplex virus 2 (HSV-2), used as an immunization strategy, protects against HSV-2 challenge in animal models. The roles of replication-defective virus-induced T cell subsets in control of HSV-2 infection have not been established. Mice lacking B cells (μMT) were immunized, depleted of CD4 or CD8 T cells, and then challenged intravaginally with HSV-2 to elucidate T cell subset contributions in the absence of virus-specific antibody. Immunized, CD4-depleted μMT mice developed severe infection of the genital tract and nervous system. In contrast, depletion of CD8 T cells from μMT mice did not attenuate protection. Immunized wild-type mice depleted of CD4 T cells also developed more severe HSV-2 infection than mice from which CD8 T cells were depleted. Thus, immunization with replication-defective virus induces T cell responses that effectively control HSV-2 infection in the absence of HSV-immune antibody, and CD4 T cells play the predominant role in this protective effect.
Keywords: HSV-2, vaginal, B cell-deficient, CD4 T cells, immunization
Introduction
Herpes simplex virus 2 (HSV-2) is a sexually transmitted virus that causes most genital ulcerative disease. The virus currently infects 17% of adults in the United States and up to 75% of the adult population worldwide (Obasi, Mosha et al., 1999;Kamali, Nunn et al., 1999;Xu, Lee et al., 2007). Lesions caused by HSV-2 facilitate infection by HIV (Wald & Link, 2002;Freeman, Weiss et al., 2006), and HSV-2 shed in the genital tract of pregnant women can be transmitted to babies during birth, often with devastating consequences (Kimberlin & Whitley, 2005). No vaccine against HSV-2 has been licensed for use despite decades spent developing inactivated or glycoprotein-based vaccines. An adjuvanted glycoprotein vaccine provides limited protection, but only to seronegative women (Stanberry, Spruance et al., 2002). Development of alternative vaccination strategies is therefore warranted, and increased understanding of immune protective mechanisms that underlie their success in animal models may help further improve vaccine design.
Studies examining mechanisms of immune protection of the mouse vaginal tract and sacral sensory ganglia from HSV infection have often utilized intravaginal (i.vag.) inoculation of mice with attenuated HSV-2 as a method of generating immunity (McDermott, Smiley et al., 1984;Milligan & Bernstein, 1995b;Parr, Kepple et al., 1994;Harandi, Svennerholm et al., 2001). In these mice, genital secretions contain virus-specific secretory IgA and IgG (Milligan & Bernstein, 1995b;Parr, Bozzola et al., 1998), and circulating virus-specific IgG is also stimulated (McDermott, Brais et al., 1990;Parr & Parr, 1998a). Transfer of serum from HSV-immune mice to non-immune mice does not reduce HSV replication in the vaginal mucosa but does protect the nervous system and prevent a lethal outcome (McKendall, Klassen et al., 1979;Openshaw, Asher et al., 1979;McDermott, Brais, & Evelegh, 1990;Eis-Hubinger, Schmidt et al., 1993;Schneweis, Brado et al., 1988). B-cell-deficient (μMT) mice inoculated i.vag. with attenuated HSV-2 do not control challenge virus replication in the vaginal mucosa as readily as wild-type mice, but fare better than unimmunized mice (Dudley, Bourne et al., 2000;Parr & Parr, 2000;Harandi, Svennerholm et al., 2001). These observations indicate first, that the initial inoculation with attenuated HSV-2 stimulates T cell responses with some protective efficacy, and second, that HSV-specific antibody does influence virus replication in the mucosa. Interestingly, HSV-immune serum fully reconstitutes protection of the vaginal epithelium when transferred to immunized μMT mice (Dudley, Bourne, & Milligan, 2000), suggesting that early control of HSV-2 replication in the genital mucosa mediated by HSV-specific serum antibody requires the presence of immune T cells. Collectively, these studies indicate antibody generated by immunization with attenuated HSV-2 can ameliorate subsequent vaginal infection, but suggest a critical contribution of cell-mediated immunity to protection against HSV-2 challenge.
Several additional lines of evidence directly support a prominent role for HSV-immune T cells in protecting against HSV re-infection. Treatment with cyclosporine A selectively depresses T cell function and results in more severe HSV infections and reactivations in humans and in animal models (Field & Gottsch, 1995;Schneweis, Brado et al., 1988). Transfer of HSV-immune T cells but not B cells to naïve mice reduces lethality after HSV-2 infection (McDermott, Goldsmith et al., 1989). In addition, depletion of T cells from mice previously inoculated i.vag. with attenuated HSV-2 completely abrogates resistance to vaginal HSV-2 challenge over the first 5 days post-infection (Dudley, Bourne, & Milligan, 2000;Parr & Parr, 1998b). The T cell subset principally responsible for protection remains at issue. Some studies using depletion of HSV-immune T cell subsets found that CD4 T cells play the more significant role in protection of the genital tract and prevent acute infection of the nervous system by HSV (Milligan, Bernstein et al., 1998;Schmidt, Eis-Hubinger et al., 1993;Milligan & Bernstein, 1997;Kuklin, Daheshia et al., 1998). In addition, depletion of CD4 T cells from wild-type mice immunized intranasally with vaccinia virus expressing HSV glycoprotein B, or from μMT mice immunized intranasally with attenuated HSV-1, reduces protective efficacy against HSV-1 replication after vaginal challenge more than does CD8 T cell depletion (Kuklin, Daheshia et al., 1998). Another study similarly using T cell subset depletion from mice previously immunized with attenuated HSV-2 concluded that CD8 T cells provide protection of the genital epithelium from subsequent HSV-2 infection (Parr & Parr, 1998b); however, in this study the extent of vaginal infection was evaluated by histology and quantification of virus protein instead of virus replication. Adoptive transfer of HSV-immune CTL has also suggested virus-immune CD8 T cells are protective against reinfection (McDermott, Goldsmith et al., 1989;Schneweis, Brado et al., 1988). Collectively, these studies indicate an important role for HSV-immune T cells in protection of the genital tract and nervous system, but the role of individual T cell subsets may depend on such variables as the immunizing agent or evaluation methods.
Although i.vag. administration of attenuated HSV-2 has been exceedingly useful in illuminating specific immune mechanisms protecting the genital tract against re-infection, this method of immunization causes extensive infection of vaginal tissue and sub-lethal disease even at low doses (McDermott, Smiley et al., 1984) and so is not tenable as a vaccination strategy. Replication-defective virus offers increased safety over attenuated, replication-competent virus, making it a potentially viable approach to vaccination. Furthermore, the broad spectrum of immunogenic viral proteins expressed by replication-defective virus may elicit immunity of greater magnitude and sophistication than a glycoprotein subunit vaccine. Replication-defective HSV-2, administered subcutaneously (s.c.) as an immunizing agent, greatly reduces the severity of subsequent genital HSV-2 infection in mice and guinea pigs (Da Costa, Morrison et al., 2001;Morrison, Da Costa et al., 1998;Da Costa, Bourne et al., 1997). We previously determined that HSV-specific antibody generated by s.c. immunization of WT mice with an ICP8−, replication-defective HSV-2 strain protects the nervous system against infection and signs of disease and also reduces infection of the vaginal mucosa if immune T cells are present (Morrison, Zhu, & Thebeau, 2001). However, the role of T cells independent of antibody, and the T cell subsets stimulated by replication-defective virus that mediate protection have not been elucidated. We therefore depleted T cell subsets from mice previously immunized with replication-defective HSV-2, and examined infection and disease of the genital mucosa and nervous system that occurs in their absence after HSV-2 challenge.
Results
The protective roles of replication-defective virus-immune, CD4 and CD8 T cell subsets would ideally be examined in a system that did not include the potentially confounding presence of immune serum antibody, such as in mice lacking B cells. Concerns linger, however, about the integrity of T cell responses in mice genetically deficient in B cells, with some studies noting differences (Dudley, Bourne, & Milligan, 2000;Homann, Tishon et al., 1998;Deshpande, Zheng et al., 2000) and others finding normal responses (Deshpande, Zheng et al., 2000;Topham, Tripp et al., 1996;Asano & Ahmed, 1996;Morrison, Zhu, & Thebeau, 2001). We therefore studied the contributions of immune CD4 and CD8 T cells to protection in B cell-deficient (μMT) mice, bred onto a BALB/c background, and then repeated the studies in BALB/c (WT) mice to compare and confirm results in an otherwise intact host.
μMT or WT mice were sham-immunized or immunized s.c. with replication-defective HSV-2 to generate vaccine-specific immunity. Prior to i.vag. challenge with virulent HSV-2, CD4 or CD8 T cells were depleted in vivo using rat monoclonal antibodies specific for either subset. Control depletions consisted of injecting normal rat IgG. Depletions were continued every 4 days through day 7 post-challenge. μMT mice immunized with replication-defective HSV-2 and then control-depleted were able to restrict challenge virus replication in the genital mucosa (Fig. 1A). Titers were not reduced until 3 days post-challenge, however, consistent with previous observations (Morrison, Zhu, & Thebeau, 2001). Immunized μMT mice depleted of CD8 T cells also readily controlled replication in the genital mucosa by 3 days post-challenge (Fig. 1A). Titers were not significantly different than those observed in immunized control-depleted animals. In contrast, immunized CD4-depleted mice showed prolonged replication in the genital mucosa, with elevated titers at 3 to 5 days post-challenge that were indistinguishable from those of unimmunized mice (Fig. 1A). In WT mice, CD8 depletion had only a modest effect on the capacity of the immune response to limit virus infection. Slightly higher titers in CD8-depleted than control-depleted mice were observed only on days 2 and 3 post-challenge (Fig 1B). Overall, CD8- and control-depleted WT mice curtailed replication more effectively at early times post-challenge than their μMT counterparts. In contrast, CD4-depleted WT mice did not control replication of challenge virus in the genital mucosa (Fig. 1B), and virus titers resembled those seen in CD4-depleted μMT mice. Thus, replication-defective virus-immune, CD4 T cells have the principal role in limiting replication in the genital tract.
Figure 1. Replication of HSV-2 in the genital mucosa of immunized depleted of CD4+ or CD8+ T cells.
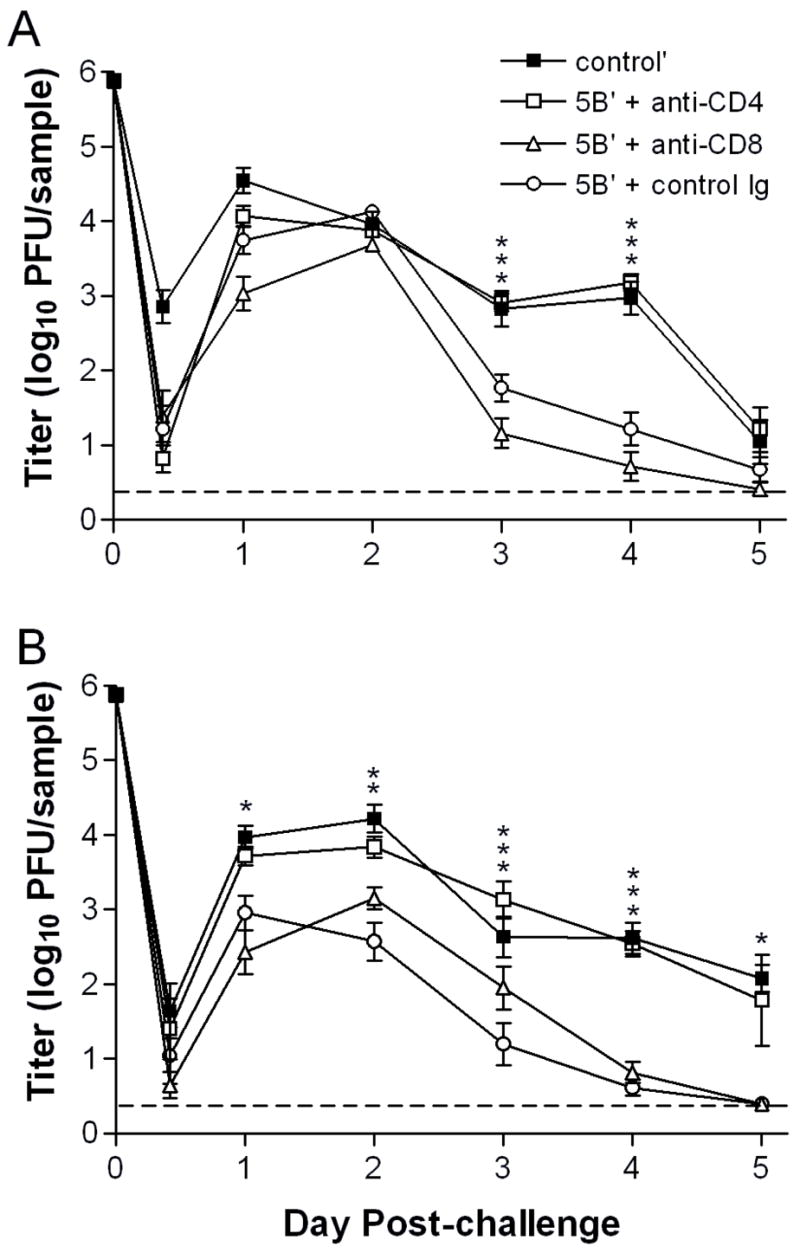
Groups of A) μMT or B) WT BALB/c mice were immunized s.c. with 2x106 pfu of HSV-2 5BlacZ and rested for 4 wk, or were left unimmunized. Immunized mice were then depleted of CD4+ or CD8+ T cells by injection of CD4 or CD8-specific monoclonal antibody, and challenged by i.vag. inoculation of 7.5x105 pfu HSV-2 G-6. A third group was injected with control antibody before challenge. Vaginal swab samples were collected at the indicated times post-challenge and titered by standard plaque assay. Data represent the geometric mean titers for 7 to 8 mice per group ± SEM. The experiment was repeated once. Data point at time 0 indicates the inoculum dose. *, P = 0.0138–0.0478; **, P = 0.001; ***, P = <0.0001–0.0003 for CD4-depleted compared with control Ig-depleted mice. (For CD4-depleted compared with CD8-depleted mice: Fig. 1A, P = 0.0478 on day 2, and P = <0.0001–0.0002 on days 3 and 4; Fig. 1B, P = 0.0016–0.0092 on days 1 through 3, and P <0.0001 on day 4.)
Signs of genital inflammation in μMT mice depleted of CD4 T cells were as severe as unimmunized mice and were markedly worse than CD8-depleted or control-depleted μMT mice (Fig. 2A). Correspondingly, immunized μMT mice depleted of CD4 T cells prior to challenge lost significant weight, whereas the overall health of CD8-depleted mice was less severely affected by the challenge virus infection (data not shown). In contrast, WT mice showed a clear difference between CD4-depleted and unimmunized groups with only about half of the former developing lesions (Fig. 2B). WT mice depleted of CD8 T cells, like their μMT counterparts, showed only mild signs of genital inflammation. Control-depleted WT mice remained completely protected (Fig. 2B). HSV-2 causes a more severe infection in the mouse model than in humans, with signs of illness extending to the nervous system in non-immune mice. Consequently, hind-limb paralysis developed in 90% of CD4-depleted or unimmunized μMT mice but in only 30% of the CD8-depleted and in none of the control-depleted mice (Table 1). Hind-limb paralysis developed in fewer CD4-depleted WT mice than control-depleted mice, and those paralyzed developed paralysis approximately 1 day later (Table 1). Not surprisingly, the CD4-depleted μMT mice died as rapidly as unimmunized controls, whereas immunized, CD8-depleted μMT mice rarely succumbed to infection (Fig. 3A). Although not all CD4-depleted WT mice developed genital lesions and paralysis, nearly all of the mice eventually succumbed to infection (Fig. 3B). The lethality of the infection in CD4-depleted mice precluded study of latency. Together, these results reveal a major contribution of virus-immune CD4 T cells to protection of the genital tract and nervous system from HSV-2-induced disease, but scant evidence of a CD8 T cell contribution.
Figure 2. Genital disease in immunized mice depleted of CD4+ or CD8+ T cells.
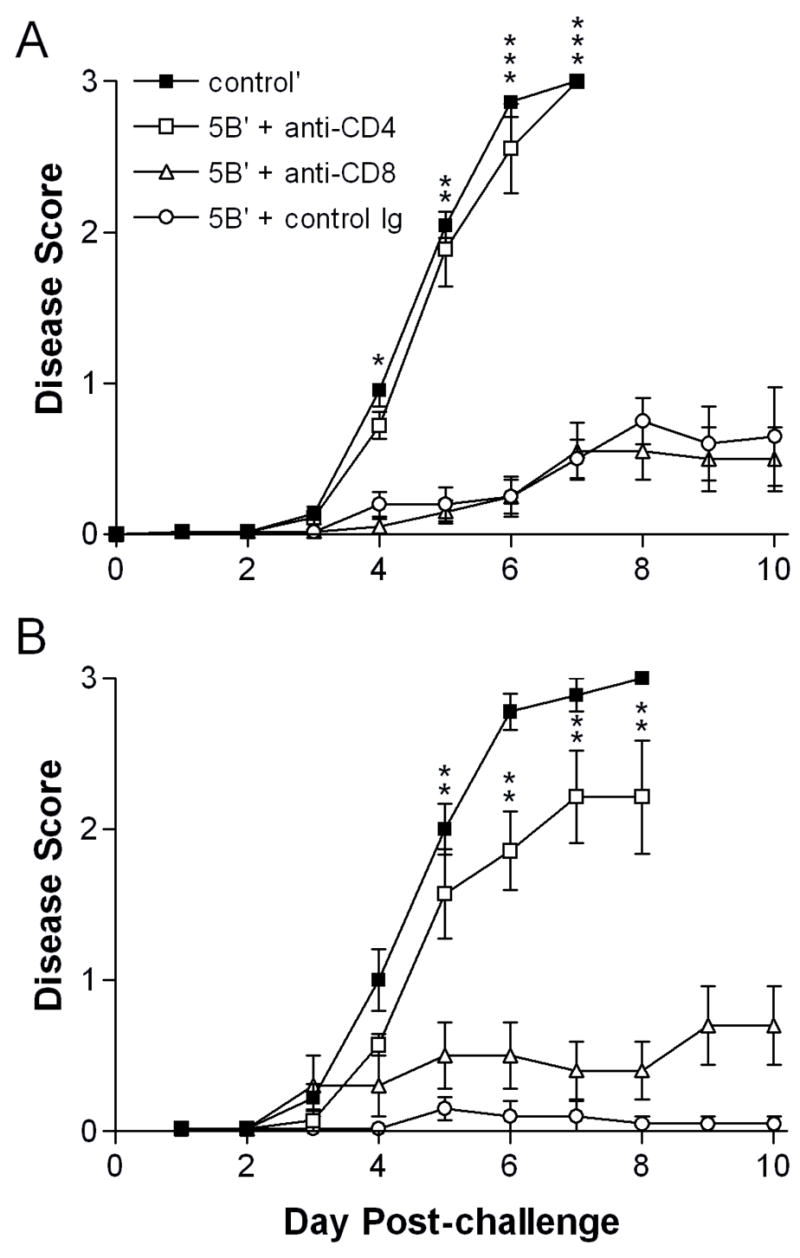
Groups of A) μMT (n=9 to 11) or B) WT (n=7 to 10) mice treated as in Fig. 1 were examined over time post-challenge for signs of genital and neurologic disease. 0, no signs; 1, mild erythema and edema of the external genitalia; 2, moderate erythema and edema; 3, severe erythema and edema accompanied by lesions. Data were pooled from two independent experiments and represent the arithmetic mean ± SEM of all mice per group. *, P = 0.021; **, P = 0.002–0.006; ***, P <0.001 for CD4-depleted compared with control Ig-depleted mice. (For CD4-depleted compared with CD8-depleted mice: Fig. 2A, P = 0.021 on day 4, P = 0.002 on day 5, and P <0.001 on days 6 and 7; Fig. 2B, P = 0.015–0.026 for days 6 through 8.)
Table 1.
Percentage of mice developing hind-limb paralysis
| Mouse type | Depletion group | Percent developing paralysis | Mean day post-challenge ± SD |
|---|---|---|---|
| μMT | Control’ | 90.9 | 7.6±0.7 |
| 5B′ + anti-CD4 | 88.9a | 7.6±1.3 | |
| 5B′ + anti-CD8 | 30.0 | 9.0±1.7 | |
| 5B′ + control Ig | 0.0 | -- | |
| BALB/c (WT) | Control’ | 85.7 | 7.5±0.8 |
| 5B′ + anti-CD4 | 57.1b | 8.3±1.3 | |
| 5B′ + anti-CD8 | 0.0 | -- | |
| 5B′ + control Ig | 0.0 | -- |
P = 0.0001 for anti-CD4 v. control Ig, and P = 0.0198 for anti-CD4 v. anti-CD8
P = 0.0147 for anti-CD4 v. control Ig or anti-CD8
Figure 3. Survival of immunized mice depleted of CD4+ or CD8+ T cells.
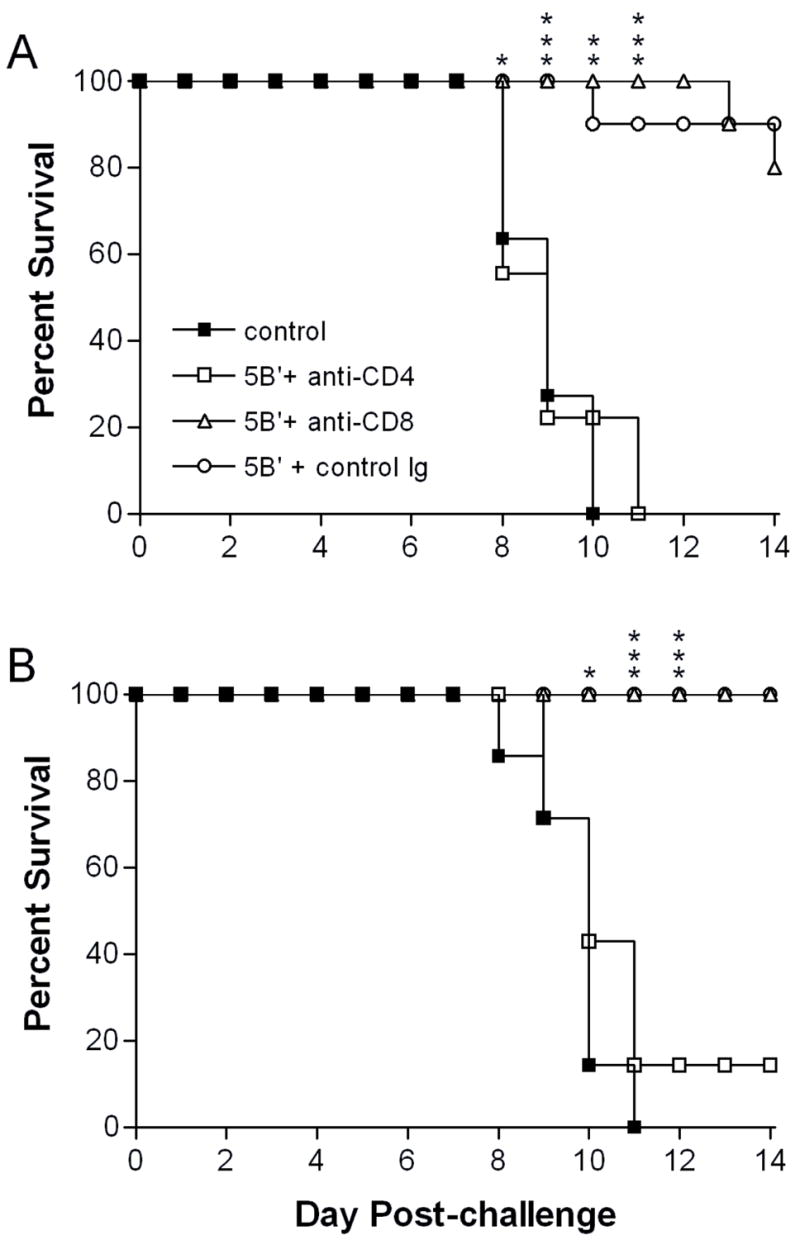
Groups of A) μMT (n=9 to 11) or B) WT (n=7 to 10) mice treated as in Fig. 1 were monitored over time post-challenge and sacrificed when moribund. *, P = 0.0147–0.0325; **, P =0.0055; ***, P = <0.0001–0.0007 for CD4-depleted compared with control Ig-depleted mice. (For CD4-depleted compared with CD8-depleted mice: Fig. 3A, P = 0.0325 on day 8, and P = <0.0001–0.0007 on days 9 through 14; Fig. 3B, P = 0.0047 for day 11 through day 14.)
The uniform paralysis observed in CD4-depleted μMT mice likely resulted from direct infection of the spinal cord and associated ganglia, but inflammation in the spinal cord could also result in CNS dysfunction (Bishop & Hill, 1991). To distinguish between these possibilities, the peripheral and central nervous systems of a cohort of immunized, T cell-depleted μMT mice were dissected at 7 days post-challenge, when signs of paralysis were developing. CD4-depleted and unimmunized cohort animals showed high titers of virus in the spinal cord, brainstem and brain (Fig. 4). In contrast, immunized mice that were CD8-depleted or given control Ig had low titers of virus (Fig. 4). Thus, immune control of acute HSV-2 infection of the nervous system also largely depends on the presence of CD4 T cells, and paralysis in these mice is likely due to vigorous replication of challenge virus in neurons. Together, these results strongly support a critical role for CD4 T cells induced by replication-defective virus in protecting the genital tract and nervous system from the deleterious effects of challenge virus infection. Possible supporting roles for HSV-immune antibody and CD8 T cells are discussed.
Figure 4. Replication of HSV-2 in the nervous system of immunized and T cell-depletedμMT mice.
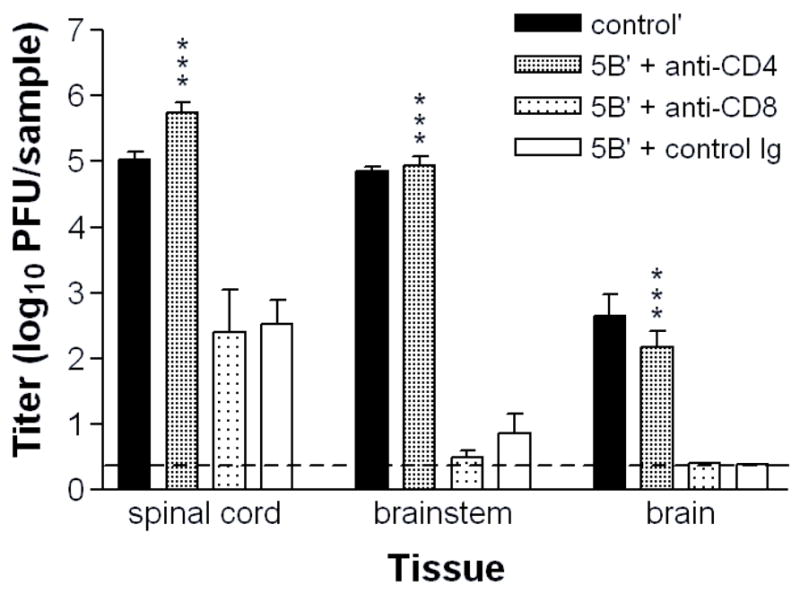
Groups of 6 to 8 5BlacZ-immunized μMT mice were depleted of CD4+ or CD8+ T cells prior to challenge as described in Figure 1. Mice were euthanized 7 days post-challenge. Neural tissues were dissected, homogenized and virus titer contained in them was determined by standard plaque assay. Values represent the geometric mean titers ± SEM. The experiment was repeated once. ***, P <0.0001 for CD4-depleted compared with control Ig-depleted mice. (For CD4-depleted compared with CD8-depleted mice: P = 0.0006 for spinal cord and P <0.0001 for brainstem and brain.)
Discussion
Depletion of T cell subsets from WT or μMT mice vaccinated with replication-defective HSV-2 has revealed a decisive role for CD4 T cells in reducing HSV-2 replication in the genital tract, signs of genital and neurologic disease, and acute infection of the nervous system. Furthermore, the critical effector function of CD4 T cells is not the provision of help for generating immune serum antibody because the protective effect of CD4 T cells is still observed in mice lacking B cells. Because immunization of μMT mice with attenuated HSV (Milligan, Bernstein, & Bourne, 1998;Kuklin, Daheshia et al., 1998), vector-expressed HSV glycoprotein (Kuklin, Daheshia et al., 1998), or replication-defective HSV-2 (this study) have yielded similar results, we can surmise as a general phenomenon that CD4 T cells mediate significant protection of the mouse genital mucosa and nervous system independently of HSV-immune antibody. Nonetheless, CD8 T cells play an important role elsewhere in the body, curtailing HSV-1 infection or reactivation after cutaneous or ocular inoculation (van Lint, Ayers et al., 2004;Banerjee, Biswas et al., 2005;Liu et al., 2000).
T cell subset depletion from μMT mice previously immunized with replication-defective virus allowed us to discern T cell roles in protection in the absence of contributions from HSV-specific antibody. We also performed the same depletions in immunized WT mice, uniquely allowing us to corroborate the results obtained with μMT mice in a system that was not potentially prejudiced by lack of B cells, a population with antigen presenting and effector capacities. This comparison yielded very similar conclusions about the preeminent role of CD4 T cells in protection. Differences we did observe between the WT and μMT mouse models were modest: First, replication-defective virus-immune WT mice that received anti-CD8 or control antibody restrained early HSV-2 replication in the genital tract slightly better than the analogous μMT mice. Second, all immunized μMT mice were slightly more susceptible to lethal HSV-2 infection than immunized WT mice regardless of the subsequent depletion protocol. Third and most conspicuous, immunized WT mice depleted of CD4 T cells developed less severe genital disease and neurologic signs on average than did unimmunized mice, whereas in μMT mice, CD4-depleted and unimmunized groups were indistinguishable.
One interpretation of the less severe genital disease in CD4-depleted WT mice is that immune CD8 T cells assist in protection of WT but not μMT mice. In support of this possibility, CD8-depleted WT mice showed some genital inflammation, unlike mice treated with control Ig. Nonetheless, inflammatory signs were equally mild in CD8-depleted μMT and WT mice, arguing against this possibility. Alternatively, immune serum antibody (still present in the immunized, CD4-depleted WT mice) assists in protection against genital and neurological disease. This interpretation is consistent with our prior observations (Morrison, Zhu, & Thebeau, 2001) and those of Dudley et al. (Dudley, Bourne, & Milligan, 2000) that passively transferred immune serum antibody 1) significantly reduces the incidence and severity of genital and neurological disease after HSV challenge of immunized μMT mice, and 2) helps reduce early challenge virus replication in the genital mucosa, but only in the presence of virus-immune T cells. Our results here suggest that the CD4 T cell subset is the critical component working with antibody to reduce mucosal replication of challenge virus: lower replication over the first 2 days post-challenge was observed only in immunized WT mice that still contained CD4 T cells (Fig. 1B). Even so, we cannot formally rule out an additional protective effect of CD8 T cells, particularly in view of the slight inflammation observed in the genital mucosa of CD8-depleted WT mice compared with control-depleted mice.
The interpretation that HSV-immune, CD8 T cells play a very limited role in protecting either the genital tract or the nervous system from HSV-2 infection depends upon evidence that they are induced by replication-defective virus, and that they mount normal responses in the context of a B cell-deficient mouse. CD8 T cells specific for HSV-2 are found in the genital lymph nodes and vaginal epithelium of mice after i.vag. immunization with attenuated HSV-2 (Milligan & Bernstein, 1997;Milligan & Bernstein, 1995a) or intranasal immunization with adenovirus expressing HSV-1 gB (Gallichan & Rosenthal, 1996). Immunization of WT and μMT mice with replication-defective virus also induces CD8 T cell responses (Morrison, Zhu, & Thebeau, 2001), and in preliminary experiments we have found higher percentages of activated CD8 T cells in genital lymph nodes after immunization or challenge of μMT mice (data not shown).
In μMT mice, CD8 T cell recruitment to the vaginal mucosa 20 hr after HSV challenge is reportedly normal (Parr & Parr, 2000), as are CD8 cytolytic responses to HSV (Deshpande, Zheng et al., 2000), and CD4 and CD8 responses to other viruses (Topham, Tripp et al., 1996;Asano & Ahmed, 1996). We had previously shown that CD8 T cells of μMT mice elicited by replication-defective virus produce IFNγ in normal amounts in response to challenge (Morrison, Zhu, & Thebeau, 2001). In preliminary experiments to further analyze T cell subsets in μMT mice, we have also found no differences in expression of early activation marker CD69 at 3 d after infection, or of CD25 (IL-2 receptor alpha chain) at 4 and 7 d after infection compared with WT mice. However, CD40L expression on CD8 T cells from μMT mice appears discernibly reduced (data not shown). This deficit or delay in CD40L expression potentially could have functional consequences (Kemball, Lee et al., 2006). Thus we must cautiously interpret our data to include the possibility of a minor contribution of replication-defective virus-immune CD8 T cells to protection of the genital mucosa and nervous system from HSV-2 infection and disease, as seen in WT mice but not in μMT mice containing potentially impaired CD8 T cells.
The prominent role of virus-immune CD4 T cells in protection leads to speculation about the protective effector mechanism. A large proportion of CD4 T cells in the genital lymph nodes stain IFNγ-positive during acute and recall responses to HSV (Milligan & Bernstein, 1995a), CD4 T cells are the main producers of IFNγ (Milligan & Bernstein, 1995a;Milligan & Bernstein, 1997), and IFNγ is known to be a critical component of the protective response to genital infection with HSV-2 (Asano & Ahmed, 1996;Milligan, Bernstein, & Bourne, 1998;Parr & Parr, 1999). In addition, we (Morrison, Zhu, & Thebeau, 2001) and others (Milligan, Dudley-McClain et al., 2004;Kolaitis, Doymaz et al., 1990) have demonstrated CD4 T cell cytolytic activity in the draining lymph nodes and vaginal epithelium soon after i.vag. HSV-2 infection. Either cytokine or cytolytic effector functions represent a plausible mechanism of CD4 T cell-mediated defense, though further experiments will be necessary to establish this point. Regardless of mechanism, replication-defective virus, used in a mouse model to vaccinate against HSV-2 infection, stimulates a strong protective effect mediated through CD4 T cells functioning in concert with, but to a large extent independently of providing help for, HSV-specific antibody. These results support development of vaccines that express viral proteins containing immunodominant CD4 epitopes to generate strong protective immunity.
Materials and Methods
Cells and viruses
A partially purified preparation of the ICP8−, replication-defective HSV-2 strain 5BlacZ (Da Costa, Bourne, Stanberry, & Knipe, 1997) was prepared for immunizations by high speed centrifugation of clarified supernatants of infected, ICP8-complementing S2 cells (Gao & Knipe, 1989) as previously described (Morrison, Da Costa, & Knipe, 1998). Stocks of HSV-2 strain G-6, a plaque-purified isolate of strain G, were grown in Vero cells and prepared as previously described (Morrison & Knipe, 1996). Titer of virus stocks was determined by standard plaque assay on S2 or Vero cell monolayers (Knipe & Spang, 1982).
Antibody production
Monoclonal antibodies specific for CD4 (GK1.5) or CD8 (53-6.72) were harvested from hybridoma culture supernatants of cells grown in Integra flasks (Integra Biosciences) according to the manufacturer’s recommendations. They were diluted to 1 mg/ml in sterile PBS for injection.
Animals and immunizations
BALB/c mice were purchased from the National Cancer Institute and were immunized at 6 wk of age. Mice bearing the μMT mutation (Igh6tm1Cgn) (Kitamura, Roes et al., 1991) were backcrossed 12 generations onto a BALB/c background in the Saint Louis University Department of Comparative Medicine. All animals were housed and treated in accordance with ALAC and Institutional guidelines. Mice were immunized s.c. in the hind flanks with 2x106 pfu of partially purified 5BlacZ in 20μl vol and rested for 4 wk before challenge.
Depletions and challenge
One week and one day prior to challenge, mice were injected s.c. in the neck ruff with 3 mg hydroxyprogesterone (Depoprovera, UpJohn) in 100μl vol. Four days and one day prior to challenge mice received 0.3 mg monoclonal antibody in 0.3 ml i.p. Depletions were continued by antibody injections days 3 and 7 post-challenge. For challenge, mice were infected by i.vag. inoculation of 7.5x105 pfu G-6 in 5 μl vol. This represents 150 LD50 for BALB/c mice (Thebeau & Morrison, 2002). Challenge virus shed from the genital mucosa was quantified by swabbing vaginal vaults twice with calcium alginate swabs at 9 hr and days 1 through 5 post-infection. Duplicate swabs for each time point were frozen together in 1 ml PBS until plaque assays could be performed. Mice were monitored daily post-challenge for change in body weight, signs of disease and survival. Mice were weighed individually and mean change from initial body weight was calculated daily for each group. Disease scores were assigned in a blinded fashion based on the following scale: 0-no apparent signs of disease, 1-slight erythema and edema of the genitals, 2-prominent erythema and edema of the genitals, 3-severe erythema and edema with lesions on the genitals. Mean daily disease score ± SEM was calculated for each group. Virus replication in neural tissues was analyzed by dissection of brains, brainstems, and spinal cords from a cohort of mice 7 days after challenge. Tissues were stored frozen until use. For virus titer determination, the tissues were thawed and disrupted using a Mini-Bead Beater (BioSpec, Inc.) and diluted for standard plaque assay.
Flow cytometry
A cohort of mice was treated with monoclonal antibodies 3 days and 1 day prior to sacrifice. Splenocytes and genital lymph node cells were isolated and stained with anti-CD4 and anti-CD8 antibodies specific for different epitopes than the monoclonal antibodies used for depletions (Caltag). Flow cytofluorometric analyses revealed that 95% of CD4+ T cells were depleted and >98% of CD8+ T cells from each tissue (data not shown).
Statistics
Significance of difference in virus or antibody titers on individual days was determined by Student’s t test. Proportion of mice with hind-limb paralysis or surviving infection was compared using the Fisher exact method. The Kruskal-Wallis non-parametric test was used to assess the significance of difference in disease scores on individual days post-challenge.
Acknowledgments
I thank Rob Reass and Hong Wang for expert technical assistance, and David Leib, Sam Speck, Skip Virgin, Paul Olivo and their laboratories for helpful advice and discussion.
This work was supported by Public Health Service grant CA75052.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asano MS, Ahmed R. CD8 T cell memory in B cell-deficient mice. J Exp Med. 1996;183:2165–2174. doi: 10.1084/jem.183.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol. 2005;77:24–32. doi: 10.1189/jlb.0904486. [DOI] [PubMed] [Google Scholar]
- Bishop SA, Hill TJ. Herpes simplex virus infection and damage in the central nervous system: immunomodulation with adjuvant, cyclophosphamide and cyclosporin A. Arch Virol. 1991;116:57–68. doi: 10.1007/BF01319231. [DOI] [PubMed] [Google Scholar]
- Da Costa XJ, Bourne N, Stanberry LR, Knipe DM. Construction and characterization of a replication-defective herpes simplex virus 2 ICP8 mutant strain and its use in immunization studies in a guinea pig model of genital disease. Virology. 1997;232:1–12. doi: 10.1006/viro.1997.8564. [DOI] [PubMed] [Google Scholar]
- Da Costa XJ, Morrison LA, Knipe DM. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection. Virology. 2001;288:256–263. doi: 10.1006/viro.2001.1094. [DOI] [PubMed] [Google Scholar]
- Deshpande SP, Zheng M, Daheshia M, Rouse BT. Pathogenesis of herpes simplex virus-induced ocular immunoinflammatory lesions in B-cell-deficient mice. J Virol. 2000;74:3517–3524. doi: 10.1128/jvi.74.8.3517-3524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley KL, Bourne N, Milligan GN. Immune protection against HSV-2 in B-cell-deficient mice. Virology. 2000;270:454–463. doi: 10.1006/viro.2000.0298. [DOI] [PubMed] [Google Scholar]
- Eis-Hubinger AM, Schmidt DS, Schneweis KE. Anti-glycoprotein B monoclonal antibody protects T cell-depleted mice against herpes simplex virus infection by inhibition of virus replication at the inoculated mucous membranes. J Gen Virol. 1993;74:379–385. doi: 10.1099/0022-1317-74-3-379. [DOI] [PubMed] [Google Scholar]
- Field AJ, Gottsch JD. Persisting epithelial herpes simplex keratitis while on cyclosporin-A ointment. Aust N Z J Ophthalmol. 1995;23:333–334. doi: 10.1111/j.1442-9071.1995.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Gallichan WS, Rosenthal KL. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J Exp Med. 1996;184:1879–1890. doi: 10.1084/jem.184.5.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Knipe DM. Genetic evidence for multiple nuclear functions of the herpes simplex virus ICP8 DNA-binding protein. J Virol. 1989;63:5258–5267. doi: 10.1128/jvi.63.12.5258-5267.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harandi AM, Svennerholm B, Holmgren J, Eriksson K. Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J Gen Virol. 2001;82:845–853. doi: 10.1099/0022-1317-82-4-845. [DOI] [PubMed] [Google Scholar]
- Homann D, Tishon A, Berger DP, Weigle WO, von Herrath MG, Oldstone MB. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamali A, Nunn AJ, Mulder DW, Van Dyck E, Dobbins JG, Whitworth JA. Seroprevalence and incidence of genital ulcer infections in a rural Ugandan population. Sex Transm Infect. 1999;75:98–102. doi: 10.1136/sti.75.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemball CC, Lee ED, Szomolanyi-Tsuda E, Pearson TC, Larsen CP, Lukacher AE. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J Immunol. 2006;176:1814–1824. doi: 10.4049/jimmunol.176.3.1814. [DOI] [PubMed] [Google Scholar]
- Kimberlin DW, Whitley RJ. Neonatal herpes: what have we learned. Semin Pediatr Infect Dis. 2005;16:7–16. doi: 10.1053/j.spid.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Knipe DM, Spang AE. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982;43:314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaitis G, Doymaz M, Rouse BT. Demonstration of MHC class II-restricted cytotoxic T lymphocytes in mice against herpes simplex virus. Immunology. 1990;71:101–106. [PMC free article] [PubMed] [Google Scholar]
- Kuklin NA, Daheshia M, Chun S, Rouse BT. Role of mucosal immunity in herpes simplex virus infection. J Immunol. 1998;160:5998–6003. [PubMed] [Google Scholar]
- Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MR, Brais LJ, Evelegh MJ. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J Gen Virol. 1990;71:1497–1504. doi: 10.1099/0022-1317-71-7-1497. [DOI] [PubMed] [Google Scholar]
- McDermott MR, Goldsmith CH, Rosenthal KL, Brais LJ. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J Infect Dis. 1989;159:460–466. doi: 10.1093/infdis/159.3.460. [DOI] [PubMed] [Google Scholar]
- McDermott MR, Smiley JR, Leslie P, Brais J, Rudzroga HE, Bienenstock J. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J Virol. 1984;51:747–753. doi: 10.1128/jvi.51.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKendall RR, Klassen T, Baringer JR. Host defenses in herpes simplex infections of the nervous system: effect of antibody on disease and viral spread. Infect Immun. 1979;23:305–311. doi: 10.1128/iai.23.2.305-311.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology. 1995a;212:481–489. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology. 1995b;206:234–241. doi: 10.1016/s0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- Milligan GN, Bernstein DI, Bourne N. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J Immunol. 1998;160:6093–6100. [PubMed] [Google Scholar]
- Milligan GN, Dudley-McClain KL, Young CG, Chu CF. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection of mice. J Reprod Immunol. 2004;61:115–127. doi: 10.1016/j.jri.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Da Costa XJ, Knipe DM. Influence of mucosal and parenteral immunization with a replication-defective mutant of HSV-2 on immune responses and protection from genital challenge. Virology. 1998;243:178–187. doi: 10.1006/viro.1998.9047. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Knipe DM. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology. 1996;220:402–413. doi: 10.1006/viro.1996.0328. [DOI] [PubMed] [Google Scholar]
- Morrison LA, Zhu L, Thebeau LG. Vaccine-induced serum immunoglobin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J Virol. 2001;75:1195–1204. doi: 10.1128/JVI.75.3.1195-1204.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obasi A, Mosha F, Quigley M, Sekirassa Z, Gibbs T, Munguti K, Todd J, Grosskurth H, Mayaud P, Changalucha J, Brown D, Mabey D, Hayes R. Antibody to herpes simplex virus type 2 as a marker of sexual risk behavior in rural Tanzania. J Infect Dis. 1999;179:16–24. doi: 10.1086/314555. [DOI] [PubMed] [Google Scholar]
- Openshaw H, Asher LV, Wohlenberg C, Sekizawa T, Notkins AL. Acute and latent infection of sensory ganglia with herpes simplex virus: immune control and virus reactivation. J Gen Virol. 1979;44:205–215. doi: 10.1099/0022-1317-44-1-205. [DOI] [PubMed] [Google Scholar]
- Parr EL, Bozzola JJ, Parr MB. Immunity to vaginal infection by herpes simplex virus type 2 in adult mice: characterization of the immunoglobulins in vaginal mucus. J Reprod Immunol. 1998;38:15–30. doi: 10.1016/s0165-0378(97)00081-8. [DOI] [PubMed] [Google Scholar]
- Parr EL, Parr MB. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J Virol. 1998a;72:5137–5145. doi: 10.1128/jvi.72.6.5137-5145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr MB, Kepple L, McDermott MR, Drew MD, Bozzola JJ, Parr EL. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab Invest. 1994;70:369–380. [PubMed] [Google Scholar]
- Parr MB, Parr EL. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J Virol. 1998b;72:2677–2685. doi: 10.1128/jvi.72.4.2677-2685.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282–294. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- Parr MB, Parr EL. Immunity to vaginal herpes simplex virus-2 infection in B-cell knockout mice. Immunology. 2000;101:126–131. doi: 10.1046/j.1365-2567.2000.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt DS, Eis-Hubinger AM, Schneweis KE. The role of the immune system in establishment of herpes simplex virus latency--studies using CD4+ T-cell depleted mice. Arch Virol. 1993;133:179–187. doi: 10.1007/BF01309753. [DOI] [PubMed] [Google Scholar]
- Schneweis KE, Brado M, Ebers B, Friedrich A, Olbrich M, Schuler W. Immunological mechanisms giving rise to latency of herpes simplex virus in the spinal ganglia of the mouse. Med Microbiol Immunol. 1988;177:1–8. doi: 10.1007/BF00190305. [DOI] [PubMed] [Google Scholar]
- Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- Thebeau LG, Morrison LA. B7 costimulation plays an important role in protection from herpes simplex virus type 2-mediated pathology. Virology. 2002;76:2563–2566. doi: 10.1128/jvi.76.5.2563-2566.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Hamilton-Easton AM, Sarawar SR, Doherty PC. Quantitative analysis of the influenza virus-specific CD4+ T cell memory in the absence of B cells and Ig. J Immunol. 1996;157:2947–2952. [PubMed] [Google Scholar]
- van Lint A, Ayers M, Brooks AG, Coles RM, Heath WR, Carbone FR. Herpes simplex virus-specific CD8+ T cells can clear established lytic infections from skin and nerves and can partially limit the early spread of virus after cutaneous inoculation. J Immunol. 2004;172:392–397. doi: 10.4049/jimmunol.172.1.392. [DOI] [PubMed] [Google Scholar]
- Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- Xu F, Lee FK, Morrow RA, Sternberg MR, Luther KE, Dubin G, Markowitz LE. Seroprevalence of herpes simplex virus type 1 in children in the United States. J Pediatr. 2007;151:374–377. doi: 10.1016/j.jpeds.2007.04.065. [DOI] [PubMed] [Google Scholar]


