Abstract
The immune system protects itself from autoreactivity by maintaining a balance between effector and Treg responses. Peripheral induction of Treg is one mechanism by which this balance may be maintained. Thus, it is important to understand factors that influence de novo generation of CD4+CD25+FOXP3+ Treg. Here, we focus on the effects of cytokines and the cell cycle inhibitor rapamycin. The cytokines IL-2 and IL-7, but not IL-4, increased initial activation induced FOXP3 expression, increased proliferation and sustained expression of FOXP3+ cells throughout the culture. Addition of rapamycin to cultures containing IL-2 further increased the frequency and absolute number of functional CD4+CD25+FOXP3+ Treg. This increase was not due to selective proliferation of FOXP3 cells, but was instead, the result of an increase in the frequency of FOXP3+ cells induced in G0 through delayed activation while the addition of IL-2 promoted survival and proliferation of the FOXP3+ population. Thus, combination of rapamycin and IL-2 may provide improved treatment options in transplantation and autoimmunity by promoting induction, survival, and expansion of functional iTreg from CD4+CD25− cells.
Keywords: FOXP3, IL-2, immune regulation, rapamycin, Treg
INTRODUCTION
It is well established that Treg play a key role in controlling the pathologic responses of autoreactive T cells [1]. Several types of Treg have been described in humans including thymically derived CD4+CD25+FOXP3+ natural Treg (nTreg) [2] and peripherally derived adaptive Treg. Adaptive Treg include IL-10 dependent Tr1 cells [3], TGFβ dependent Th3 cells [4], and a CD4+CD25+FOXP3+ population which arises de novo in the periphery [5–10]. In this paper we focus on the factors that enhance the peripheral generation of CD4+CD25+FOXP3+ T cells by utilizing an in vitro system from which CD4+CD25+FOXP3+ cells can be induced from CD4+CD25−FOXP3− population (iTreg) via anti-CD3/CD28 stimulation.
IL-2, IL-4 and IL-7 are common gamma chain cytokines that alter both effector and nTreg responses through various mechanisms [11]. IL-2 is required for both the function and proliferation of CD4+CD25+FOXP3+ nTR [12–14]. In comparison, IL-4 and IL-7 function as survival factors for nTreg, but are not required for function [15, 16]. In humans, FOXP3 expression occurs upon activation of CD4+ T cells [17–19] and addition of IL-2 in this setting increases expression levels of FOXP3 [19]. This indicates that the common gamma chain cytokines may influence expression of FOXP3 and that the induction, maintenance, or function of iTreg may be effected by these cytokines.
One of the agents known to alter T cell activation is rapamycin. It functions by binding the mammalian target of rapamycin (mTOR) FK506 binding protein complex leading to inhibition of mTOR protein kinase activity [20]. The effects of rapamycin are downstream of PI3 Kinase and Akt signaling allowing for efficient T cell receptor mediated activation [21] but limiting transition from G1 to S phase. Importantly, rapamycin does not block proliferation in all T cell subtypes. One of these sub-populations includes nTreg [22–25]. Thus, although rapamycin induces anergy in the majority of lymphocyte populations [26, 27], a subset of rapamycin-resistant T cells may respond to activation in a similar manner as nTreg.
To better understand the factors that enhance the development of adaptive Treg in vivo, we address the role of the common gamma chain cytokines and rapamycin in the induction of FOXP3 in CD4+FOXP3− T cells in vitro. We provide data suggesting that IL-2 and IL-7, but not IL-4, augment early induction of FOXP3 by enhancing activation. Generation of polyclonal iTreg is further enhanced when rapamycin is combined with IL-2. This occurs through delayed activation mediated by rapamycin and IL-2 supported proliferation of the resulting FOXP3+ population. These findings have implications regarding cell therapy and treatment options for GVHD and autoimmunity.
MATERIALS and METHODS
Subjects
Samples for this study were obtained from participants in the Benaroya Research Institute Immune Mediated Disease Registry. The study population was selected based on a lack of personal or family history of diabetes, autoimmunity, or asthma. In total, 42 samples were used for these studies. The number of samples used for each assay is indicated in figure legends. The research protocols were approved by the Benaroya Research Institute IRB.
Antibodies and reagents
BD Pharmingen (San Jose, CA) antibodies used include FITC conjugated anti-BrdU, PE conjugated CD25 and CD127, PerCP conjugated CD4, APC conjugated CD4 and CD25, purified anti-CD3 (UCH11) and anti-CD28 (CD28.2), and neutralizing anti-IL-10, anti-IL-2, anti-TGFβ and matching isotype controls. Intracellular AlexaFlour® 647 conjugated anti-FOXP3 (clone 259D) was purchased from Biolegend (San Diego, CA). CFSE was purchased from Invitrogen (Carlsbad, CA). IL-2 was purchased from Chiron (Emeryville, CA). IL-4 and IL-7 were purchased from BD Pharmingen (San Jose, CA). Rapamycin was purchased from Alexis Biochemicals (San Diego, CA).
Isolation of cells
Human peripheral blood was obtained from normal healthy donors and PBMC were prepared by centrifugation over Ficoll-Hypaque gradients. CD4+ T cells were purified by depletion of cells expressing CD8, CD11b, CD16, CD19, CD36, and CD56 with the CD4+ No-touch T cell isolation kit (Miltenyi Biotec, Auburn, CA). CD25− cells were isolated from total CD4+ T cells by negative selection with Miltenyi CD25 microbeads. Accessory cells were obtained by isolating the positive fraction of the CD4+ No-touch magnetic sort.
Induction of CD25+FOXP3+ T cells
CD4+CD25− selected T cells were activated with 5 μg/ml plate-bound anti-CD3 and 1μg/ml soluble anti-CD28 for 24 hours and then transferred to fresh plates. In selected experiments, CD4+CD25− T cells were stimulated with 5μg/ml soluble anti-CD3 bound to irradiated (5000 rad) accessory cells. IL-4 (10ng/ml), IL-7 (10ng/ml), rapamycin (100nM) and/or IL-2 (200 IU/ml) were added where indicated. Cultures were maintained for 10 days.
Suppression assays
Following a 9–10 day induction culture, cells were sorted based on expression of CD25 via a FACS Vantage (Becton Dickinson, San Jose, CA). CD4+CD25hi (2.5 × 103/well), CD4+CD25− (2.5 × 103/well) thawed autologous responder cells, or both (2.5 × 103 each/well) were cultured with irradiated accessory cells (2.5 × 104/well), 5 μg/ml soluble anti-CD3 and 2.5 μg/ml soluble anti-CD28. The ability of CD4+CD25hi cells to suppress proliferation of autologous responder cells was determined by 3H thymidine incorporation. For thymidine incorporation assays, 1 μCi 3H thymidine was added during the final 16 hrs of a 6–7 day assay and proliferation was measured by a scintillation counter (Wallac, Boston, MA).
BrdU Analysis
CD4+CD25− T cells were pulsed with 10μM BrdU (BD Biosciences, San Jose, CA) at different timepoints following activation. Cell cycle progression was determined by staining with anti-BrdU antibody as per the manufacturer’s instructions (BD Parmingen, San Jose, CA). Briefly, cells were fixed and permeabilized using BD Cytofix/Cytoperm and BD Cytoperm Plus buffers. Permeabilized cells were treated with DNase for 1 hr at 37°C prior to staining with FITC anti-BrdU antibody. 7-AAD was added to each sample 5 minutes prior to acquisition. Data was acquired on a FACS Caliber and analyzed using FlowJo (Tree Star, Ashland, OR) and Winlist software (Verity, Topsham, ME).
Statistical Analysis
All statistical analyses were performed using a paired Student t test unless otherwise noted.
RESULTS
Activation induced expression of FOXP3 is augmented by IL-2 and IL-7, but not IL-4
To determine whether selected common gamma chain cytokines influence the expression of FOXP3 upon stimulation in a population of CD4+FOXP3− T cells, we activated CD4+CD25− T cells with anti-CD3 and anti-CD28 in the presence of media alone, IL-2, IL-7 or IL-4 as depicted in Figure 1A. FOXP3 expression was detected within 24 hours of TCR mediated activation regardless of the media composition (p < 0.05). However no difference was seen between cultures containing media alone or those where cytokine was added at this early time point (Figure 1B). FOXP3 expression detected in the media, IL-7 and IL-4 cultures was not due to activation induced secretion of IL-2, as addition of blocking antibody for IL-2 did not significantly alter FOXP3 expression within the first 24 hours of culture (data not shown). However, by 48 hours, the cultures that contain IL-2 and IL-7, but not IL-4 demonstrate an increase in the frequency of FOXP3+ T cells compared to those with media alone (Figures 1C, D). The role of IL-2 is further demonstrated by the decrease in FOXP3 at 48 hours in the media only culture where FOXP3 expression is diminished when anti-IL-2 neutralizing antibodies are added (p < 0.05).
Figure 1. Induction of FOXP3 is associated with the level of early activation which is augmented by addition of IL-2 and IL-7.
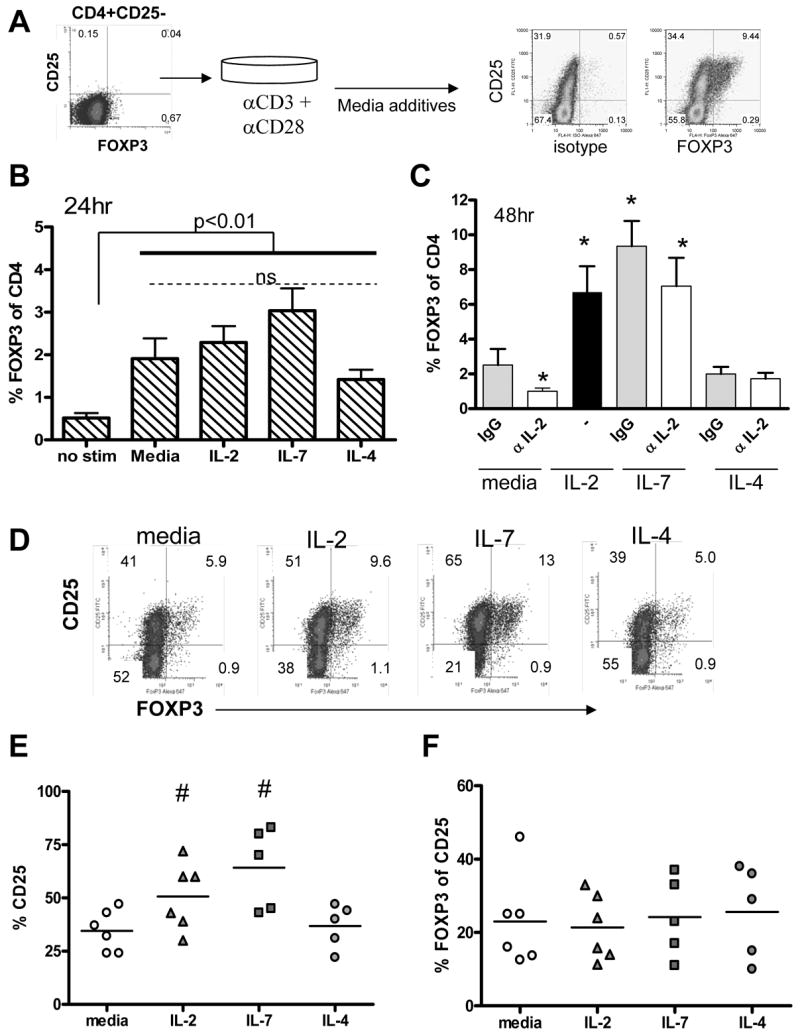
Negatively selected CD4+CD25− T cells were activated with irradiated APC and soluble anti-CD3 and anti-CD28 in the presence of media, 200 IU/ml IL-2, 10ng/ml IL-4 or 10ng/ml IL-7. The selected population and experimental design are depicted in (A) with staining controls. (B) FOXP3 expression was assessed using intracellular flow cytometry 24 hours following activation (n = 7 independent samples). All groups differed significantly from no stim (p < 0.01) while activated cultures did not differ (p > 0.05). (C) Saturating (5μg/ml) doses of neutralizing anti-IL-2 or control IgG were added to media, IL-7 and IL-4 cultures and FOXP3 expression was assessed at 48 hours. * denotes a significant difference from media alone (p < 0.05). (D) CD25 and FOXP3 co-expression were assessed 48 hours following activation in the presence of media, IL-2, IL-7 and IL-4. One representative sample of five is shown. (E) The percentage of CD4+ T cells expressing CD25 and (F) the percentage of CD25 cells expressing FOXP3 were analyzed for 5 samples. # denotes a significant difference from media alone (p < 0.05) as measured by an unpaired student’s t-test.
To address whether IL-2 and IL-7 enhanced the expression of FOXP3 by increasing the number of activated T cells we examined the expression of CD25 in these cultures. Two days following activation two populations of CD25+ T cells are present within the culture: those that are FOXP3− and a subpopulation of CD25+ cells which co-express CD25 and FOXP3 (Figure 1D). Overall CD25 expression was increased in the presence of IL-2 and IL-7 in comparison to media alone or IL-4 (Figure 1E). Additionally, the expression of CD127, the high affinity IL-7 receptor, was high on the initial CD4+CD25− population and was down-modulated on all CD25+ T cells activated in vitro regardless of the media additive (data not shown). These findings show a role for IL-2 and IL-7 in increasing the number of T cells which become activated, but the relative frequency of CD25+ cells that co-expressed FOXP3 did not differ between groups at this early time-point (Figure 1F). Thus, expression of FOXP3 is associated with the level of overall activation which is enhanced in the presence of IL-2 and IL-7, but not IL-4.
IL-2 promotes proliferation of CD4+CD25+FOXP3+ T cells induced upon activation
IL-2 has been shown to be essential for nTreg proliferation [12, 13] suggesting the possibility that IL-2 and IL-7 may also influence survival and proliferation of iTreg. To address this possibility we utilized CFSE dye dilution to measure proliferation FOXP3+ cells at day 4, 7 and 10 in the presence of IL-2. In both the media alone and IL-2 cultures, FOXP3+ T cells were present in the undivided and proliferating population, and appear to proliferate in parallel with FOXP3− T cells (Figure 2A). Addition of IL-2 increased the frequency of FOXP3+ cells detected at all time-points tested. Moreover, the number of dividing cells was greater in the IL-2 culture. Thus, by day 10 of culture, addition of IL-2 significantly increased the frequency of FOXP3+ T cells in multiple samples (Figure 2B). Addition of IL-7 instead of IL-2 showed similar results (data not shown). Taken together, IL-2 increases the frequency of FOXP3+ T cells either by enhancing proliferation and/or survival of FOXP3+ T cells induced upon activation.
Figure 2. IL-2 promotes proliferation and survival of FOXP3+ T cells.
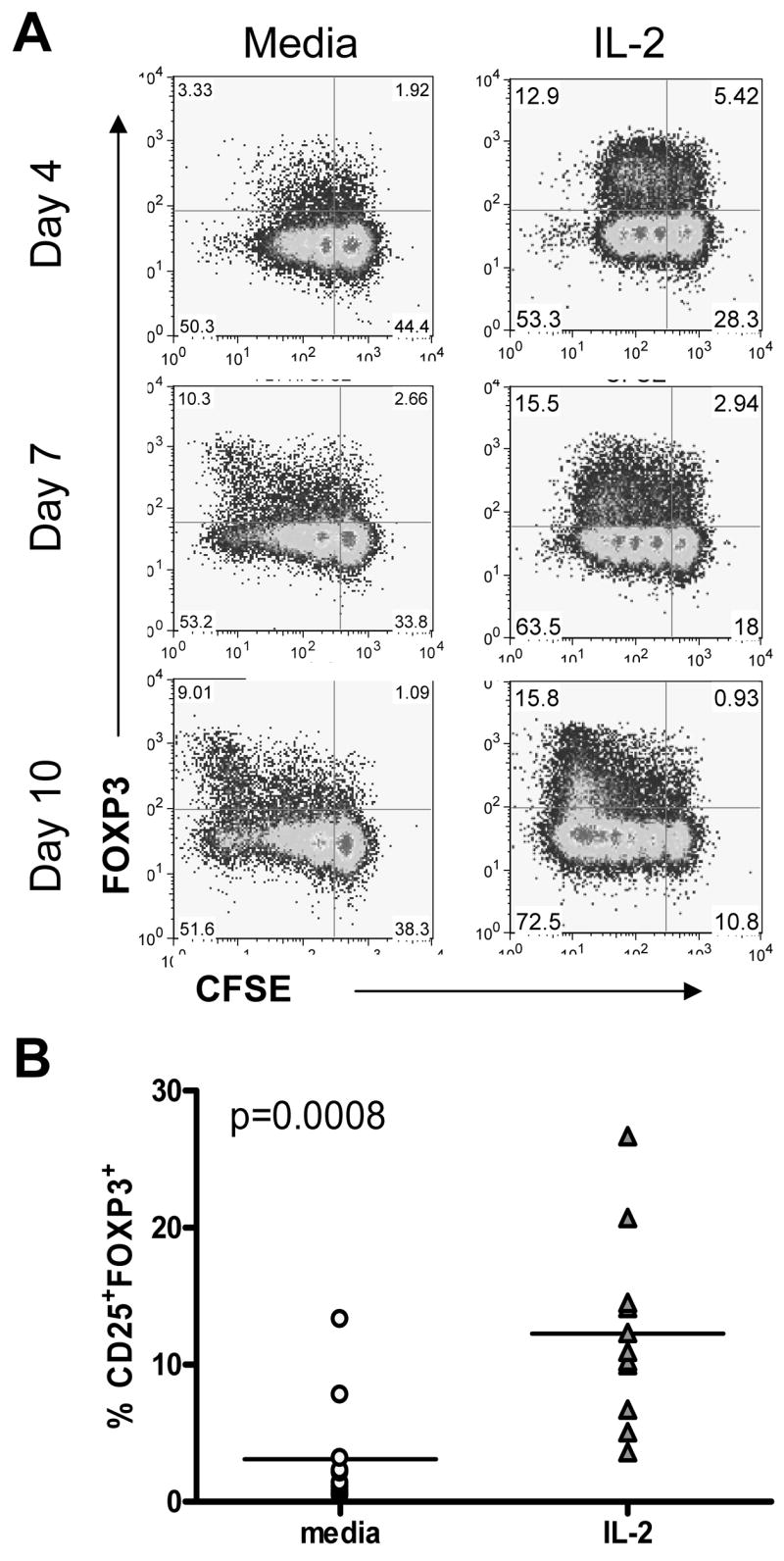
CD4+CD25− T cells were stained with CFSE and activated with irradiated APC and soluble anti-CD3 and anti-CD28 in the presence of media or 200 IU/ml IL-2. IL-2 was added on days 0, 3, and 6 (A). The frequency of CD25+FOXP3+ T cells was calculated 10 days following activation with irradiated APC and soluble anti-CD3 (n = 10) (B).
Rapamycin plus IL-2 increases the absolute number of CD4+CD25+FOXP3+ T cells induced upon activation
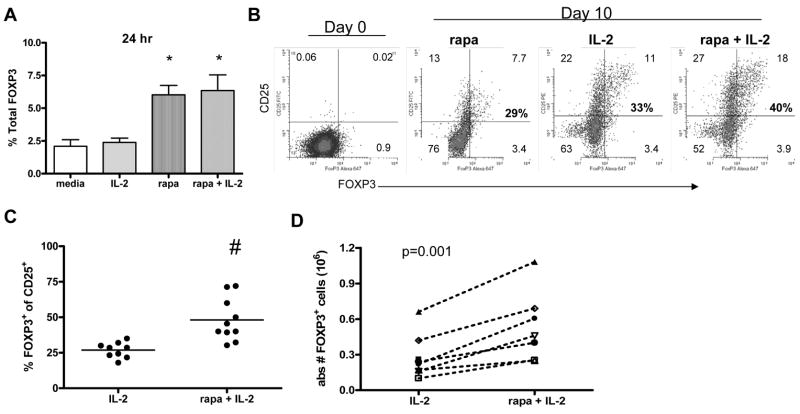
As shown in Figure 1, TCR (CD3/CD28) stimulation results in the expression of FOXP3 in a subset of CD4+ T cells. Thus, it is possible that agents that alter T cell activation may also influence induction of FOXP3. To address this hypothesis we used rapamycin, an agent known to inhibit cell cycle progression in a portion of T cells [21]. The influence of rapamycin on FOXP3 expression was examined both early and late in culture. We found that within 24 hours following activation, cultures containing rapamycin had a significant increase in the frequency of cells expressing FOXP3 (p < 0.01)(Figure 3A) and IL-2 did not have an additive effect. Due to the impaired T cell proliferation known to be a consequence of treatment with rapamycin, additional studies were performed in combination with IL-2.
Figure 3. Rapamycin plus IL-2 treatment promotes an increased absolute number of CD25+FOXP3+ T cells at the end of culture.
CD4+CD25− T cells were activated with plate bound anti-CD3 and soluble anti-CD28 in the presence of media alone, 200 IU/ml IL-2, 100nM rapamycin, or the combination of rapamcyin plus IL-2 added on day 0 of culture. (A) Twenty four hours following activation, the percentage of cells expressing FOXP3 was assessed using flow cytometry.* denotes a significant difference from media and IL-2 alone (p < 0.05). Ten days following activation, cultures were analyzed by flow cytometry. One representative experiment of three is shown in (B) and an average of 9–10 samples is shown in (C). # denotes a significant difference (p < 0.05) from IL-2 alone. The absolute number of CD25+FOXP3+ T cells was calculated using trypan blue live cell counts and flow cytometry for seven independent samples (D).
To measure the influence of rapamycin plus IL-2 on induction and proliferation of FOXP3+ cells, we activated CD4+CD25− T cells in the presence of IL-2 or the combination of rapamycin plus IL-2. We found that a mixture of both CD25+FOXP3−and CD25+FOXP3+ T cells was detected in both cultures ten days following activation (Figure 3B). However, the frequency of CD25+ T cells that co-expressed FOXP3 differed depending on the media additives. The highest frequency of CD25+FOXP3+ cells was seen in the rapamycin plus IL-2 culture. This pattern was consistently observed with multiple samples where the combination of rapamycin plus IL-2 induced a significantly greater frequency of iTreg (p < 0.05) as compared to IL-2 alone (Figure 3C). Additionally, the combination of rapamycin and IL-2 induced the greatest absolute number of iTreg as compared to IL-2 alone by day 10 of culture (p = 0.001) (Figure 3D). Taken together, combination of rapamycin and IL-2 induced an increased percentage and number of CD4+CD25+FOXP3+ T cells from CD25−FOXP3− cells in comparison to IL-2 alone.
Rapamycin plus IL-2 does not alter the function of CD4+CD25+FOXP3+ iTreg
FOXP3 has been reported to be both an activation marker and a transcription factor associated with regulatory cells [17–19, 30]. Thus, it was important to determine whether the FOXP3 populations induced in these culture systems were functional. To measure the regulatory potential of cells cultured in the presence of IL-2 or rapamycin plus IL-2, CD25hi T cells (Figure 4A) were sorted ten days following activation and co-cultured with autologous CD4+CD25− responder cells in a suppression assay. CD25hi sorted cells expressed FOXP3 and were CD127lo, a surface marker associated with functional nTreg [28, 29]. These responses were compared to induction in the presence of media alone. Regardless of the culture condition, sorted CD25hi T cells inhibited proliferation of autologous responder cells in a dose dependent manner (Figure 4B). This is true not only for IL-2 and rapamycin cultures, but also IL-4 and IL-7 cultures (data not shown). For each culture, suppression was contact dependent and TGFβ and IL-10 independent and sorted CD25− T cells failed to suppress responses (data not shown). Although potency appears to be weaker for the iTreg isolated from media or IL-2 than the rapamycin plus IL-2 iTreg, these differences in the potency of suppression correlated with the frequency of FOXP3 expressing cells within the CD25hi sorted population, as shown in parentheses in the legend of Figure 4B and in Figure 4C. Thus, the frequency of FOXP3+ cells directly correlates with regulatory function and these CD25hiFOXP3+ regulatory cells can be induced in the presence of either IL-2 or rapamycin plus IL-2.
Figure 4. Rapamycin plus IL-2 treatment results in enhanced regulatory function of the CD25+ population due to an increased number of FOXP3+ cells.
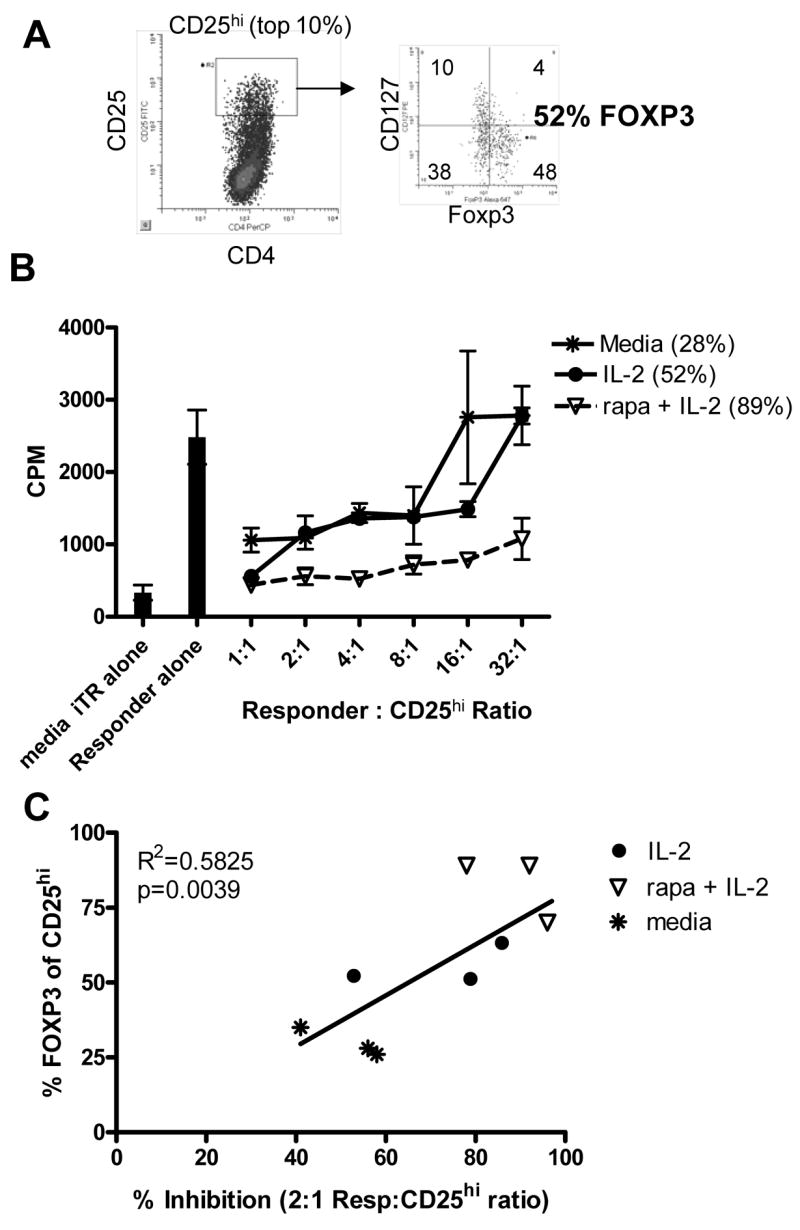
CD4+CD25− T cells were activated in the presence of IL-2 or the combination of rapamycin plus IL-2. Following 10 days in culture, CD25hi T cells were sorted and placed in a suppression assay as described in Materials and Methods. Prior to sorting, an aliquot of cells were co-stained with CD127 and FOXP3 (A). The percentage of sorted CD25hi T cells that express FOXP3 is shown in parentheses in the legend (B). One representative experiment of 3 is shown. (C) The percentage of CD25hi T cells expressing FOXP3 on day 10 was compared to % inhibition at a 2:1 Responder:CD25hi ratio using linear regression analysis.
Cytokines including IL-10 and TGFβ have been shown to influence the induction of regulatory populations [3, 4, 31]. To determine whether culture with rapamycin alters the cytokine microenvironment, we assayed supernatants during culture. Culture with rapamycin plus IL-2 did not alter the cytokine phenotype or promote secretion of TGFβ in our culture system (data not shown).
Rapamycin plus IL-2 treatment does not selectively inhibit proliferation of all CD25+FOXP3− T cells
Others have shown that rapamycin preferentially promotes expansion of nTreg from total CD4+ T cells [22, 24]. One model to explain how the combination of Rapamycin plus IL-2 enhances induction and proliferation of iTreg, is that there is selective proliferation of cells that have up-regulated FOXP3 upon activation with a parallel inhibition of proliferation in the FOXP3− subset. To directly test this hypothesis, we compared BrdU incorporation of the FOXP3− and FOXP3+ subpopulations in IL-2 and rapamycin plus IL-2 cultures 3 days following activation. FOXP3 expression was detected in all phases of cell cycle and both FOXP3− and FOXP3+ T cells incorporated BrdU (Figure 5). The increased percentage of FOXP3+ T cells in S phase most likely reflects the observation that FOXP3 is expressed in activated CD25+ T cells while FOXP3− T cells comprise both activated CD25+FOXP3− T cells and cells that have not upregulated CD25. Thus, FOXP3 is induced upon activation and is expressed in activated and proliferating cells. Rapamycin does not inhibit proliferation of all FOXP3− T cells, but instead, influences the frequency of FOXP3+ T cells induced upon activation.
Figure 5. Rapamycin plus IL-2 treatment does not selectively inhibit proliferation of all CD25+FOXP3− T cells.
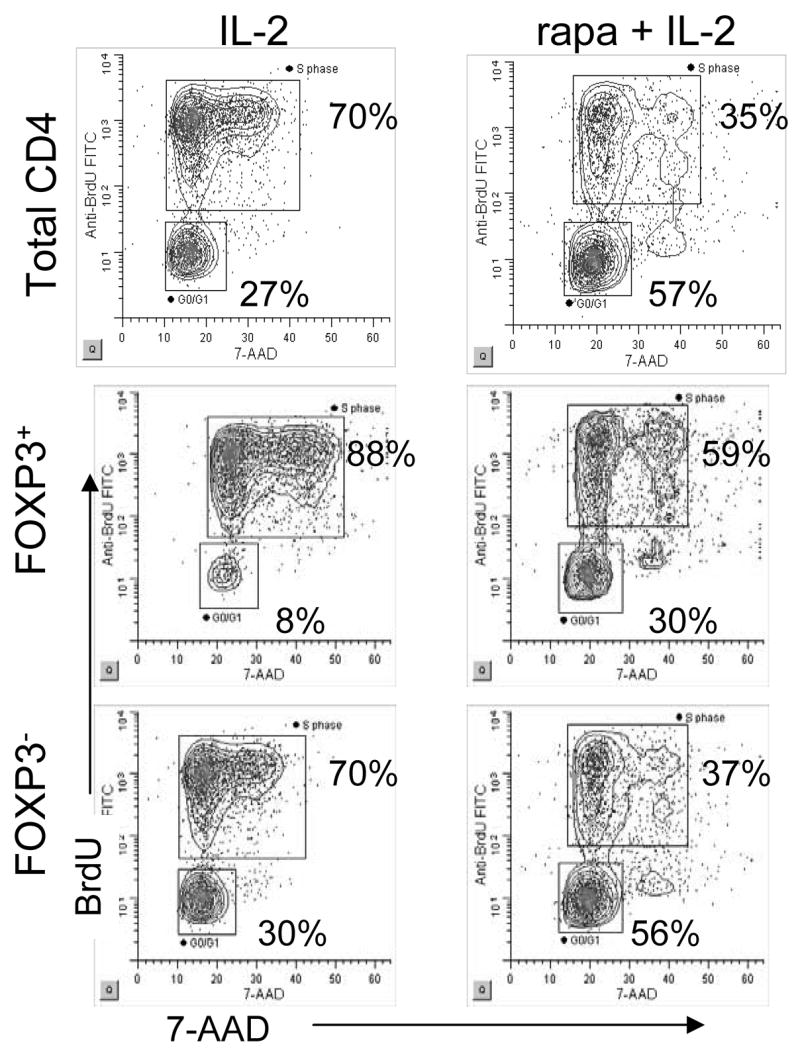
CD4+CD25− T cells were activated with plate bound anti-CD3 and soluble anti-CD28 in the presence of 200 IU/ml IL-2 or the combination of rapamcyin plus IL-2 added on day 0 of culture. Three days following activation, cultures were pulsed with BrdU and cell cycle analysis was performed as described in Materials and Methods. Cells that are 7-AADlo and BrdU− are in G0/G1. BrdU+ cells are in S phase and BrdU−7-AADhi cells are in M phase.
Rapamycin plus IL-2 prolongs activation thereby increasing the frequency of proliferating FOXP3+ T cells
A second model to explain how treatment of CD4+CD25− T cells with rapamycin plus IL-2 results in an increased absolute number of FOXP3+ T cells is that rapamycin plus IL-2 may alter activation in a manner that increases induction of FOXP3+ cells capable of proliferating in response to IL-2. To address this hypothesis, we activated CFSE labeled cells and co-stained with CD25 and FOXP3 (Figure 6). All dividing cells expressed CD25 (data not shown). By day 3, FOXP3 was induced in the undivided population in both cultures, however, cell division was only observed in the IL-2 culture. Of note, a higher frequency of FOXP3 expressing cells was observed in the undivided population of the rapamycin plus IL-2 culture. Five days following activation, the IL-2 culture displayed greater proliferation than the rapamycin plus IL-2 culture and most FOXP3+ T cells had divided. In comparison, the rapamycin plus IL-2 culture contained a higher percentage of FOXP3+ T cells that remained in the undivided population and this increased percentage of FOXP3+ cells was maintained through-out each cell division. Taken together, proliferation of both the CD4+FOXP3− and FOXP3+ populations is reduced in the presence of rapamycin plus IL-2 as compared to culture with IL-2 only, yet, the absolute number of FOXP3+ CD4 T cells is increased, demonstrating that the relative increase in FOXP3+ T cells in the presence of IL-2 and rapamycin is not solely due to inhibition of expansion of FOXP3− T cells, but instead, is in part due to the augmentation of FOXP3 expression as depicted in a Figure 7.
Figure 6. Rapamycin plus IL-2 treatment increases the frequency of FOXP3+ T cells induced upon activation.
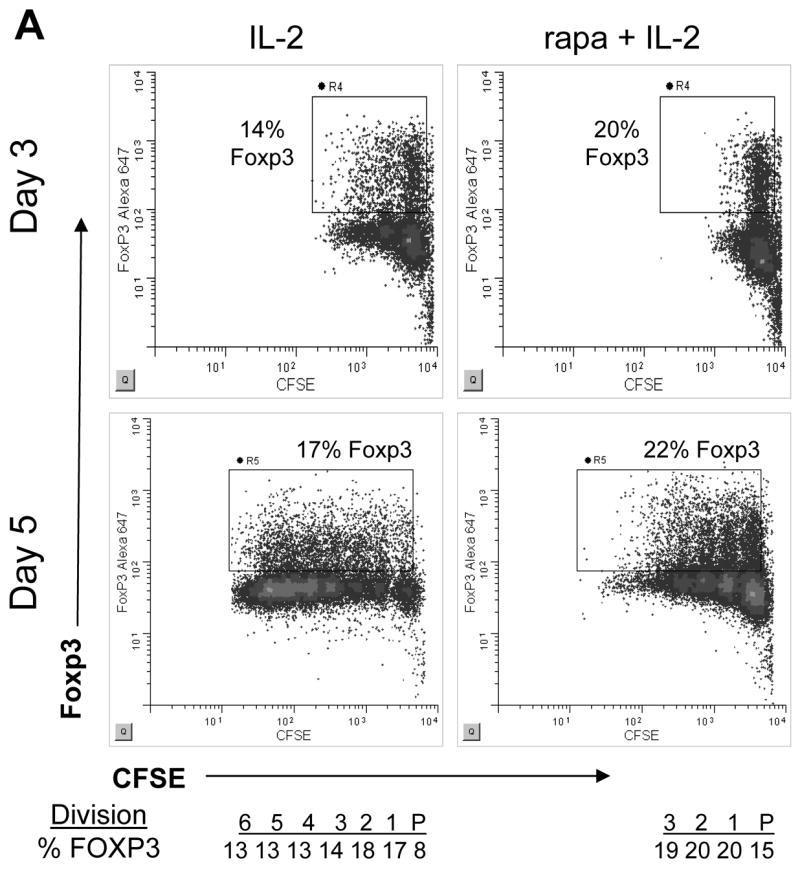
CD4+CD25− T cells were stained with CFSE and activated with plate bound anti-CD3 and soluble anti-CD28 in the presence of IL-2 or rapamycin plus IL-2. (A) Cultures were analyzed on days 3 and 5 for CFSE dye dilution, CD25 and FOXP3 expression. One representative sample of 5 is shown. The frequency of FOXP3+ cells in each cell division is shown below the day 5 analysis.
Figure 7. Schematic of proposed mechanism for enhanced generation of iTreg in the presence of rapamycin plus IL-2.
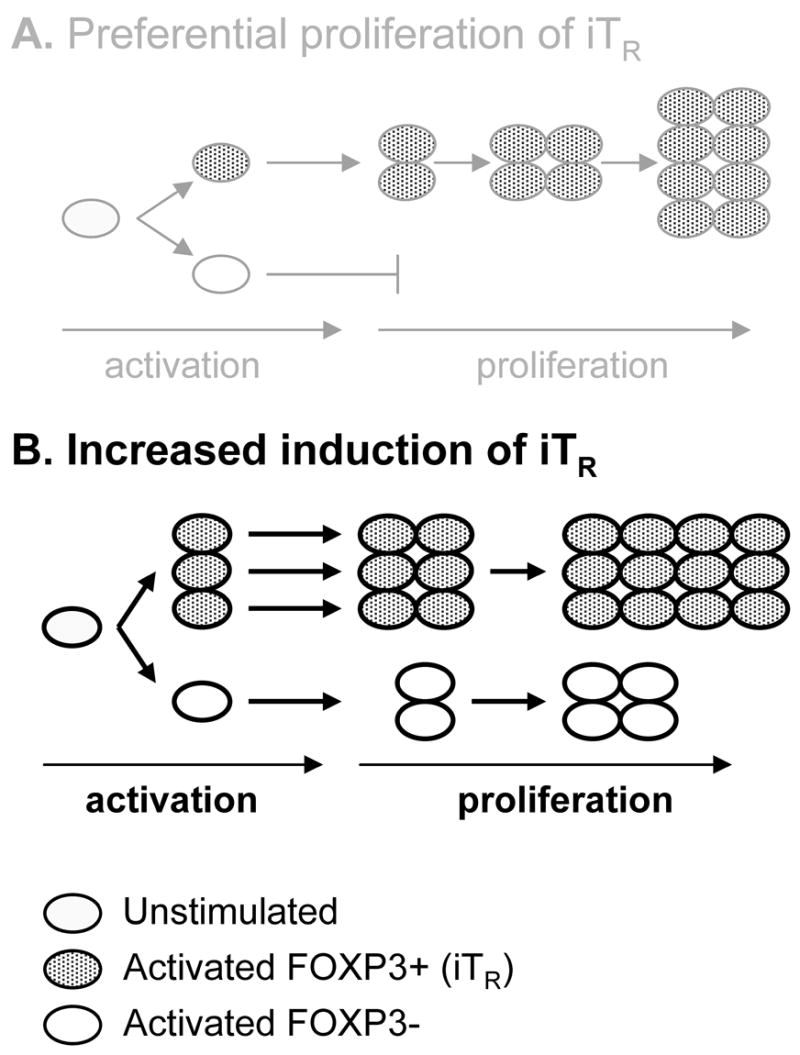
Rapamycin plus IL-2 does not cause (A) preferential proliferation of cells that express FOXP3 upon activation, but instead, causes (B) increased induction of FOXP3 by altered activation followed by proliferation.
DISCUSSION
IL-2, IL-4, IL-7 and IL-15 have been shown to preserve optimal regulatory potency of nTreg [11]. In addition, nTreg display an increased growth potential in the presence of rapamycin plus IL-2, resulting in a selective expansion of FOXP3+ nTreg [23, 24]. However, factors that influence FOXP3 induction are not well understood. CD4+CD25+FOXP3+ can be induced in vitro and in the periphery in vivo [7–10;32]. We and others have shown that FOXP3 expression can be induced upon TCR mediated activation of human CD4+CD25− T cells [7, 17–19]. In this paper, we address the role of the common gamma chain cytokines IL-2, IL-7 and IL-4 and the cell cycle inhibitor rapamycin on the induction and persistence of FOXP3 in CD4+CD25− T cells upon activation.
We demonstrate here that IL-2 and IL-7, but not IL-4, influence the induction of FOXP3 following activation, both at 48 hours and after 10 days in culture. IL-2 and IL-7 increased the frequency of activated CD25+ T cells at an early time point in these cultures, this increase in CD25+ T cells correlates with an increase in the frequency of FOXP3+ cells, suggesting that IL-2 and IL-7 alone do not induce FOXP3, but act in a co-stimulatory capacity to enhance the number T cells which achieve full activation. Further exposure to these cytokines promoted proliferation of the CD25+FOXP3+ T cells in parallel with CD25−FOXP3− T cells, indicating they play a role in providing survival signals to the newly induced Treg as well as the FOXP3- T cells. These finding implicate the common gamma chain cytokines in the regulation of both nTreg, and adaptive Treg.
Rapamycin alters activation of T cells [21, 27], resulting in anergy mediated through blockade of mTOR [33], which has been shown to favor the proliferation of nTreg [23]. We find that treatment of CD4+CD25− T cells with rapamycin increases the frequency of CD25+ T cells expressing FOXP3 within 24 hours, and that activation of CD4+CD25− T cells in the presence of both rapamycin and IL-2 increased the frequency and absolute number of iTreg at the end of a 10 day culture. We tested two hypotheses proposed to explain how activation of CD4+CD25− T cells in the presence of rapamycin plus IL-2 resulted in an increased absolute number of FOXP3+ T cells. First we addressed the possibility that rapamycin exposure resulted in preferential expansion of the FOXP3+ population. By following proliferation over time with CSFE we were able to establish that although the initial kinetics of proliferation were delayed in cultures containing rapamycin. Both the CD25+FOXP3− and CD25+FOXP3+ T cells experienced this delay in proliferation and proliferated in parallel indicating that rapamycin resistant cells are not exclusively FOXP3+ T cells. However, rapamycin resistant cells were enriched in cells expressing FOXP3. To address the second model, that rapamycin preferentially induces FOXP3 we examined the early activation events in these cultures. By utilizing CSFE and BrdU we demonstrate that the enrichment for FOXP3 occurs early, prior to cell division and even in the cell population that remains in G0. These findings suggest that the altered activation profile that results from exposure to rapamycin enhances induction of FOXP3 - a finding supported by the observation that FOXP3 message is increased in CD4+CD25− T cells activated in the presence of rapamycin [34].
In mice, treatment with rapamycin promotes de novo induction of FOXP3+ allo-antigen specific T cells that protect from GVHD [10]. Moreover, nTreg survival and proliferation is preserved in vitro in the presence of rapamycin and IL-2 [22–24]. Both thymic and peripheral Treg may control harmful autoimmune responses in vivo in humans. In fact, induction of FOXP3+ T cells has been observed in vivo in human memory T cell populations proliferating in response to antigen [32, 35]. Combined treatment with rapamycin plus IL-2 may increase induction of FOXP3+ iTreg from CD25−FOXP3− T cells, the most abundant CD4+ T cell population in human peripheral blood.
Control of auto-aggressive immune responses in the periphery is essential to prevent autoimmune manifestations [36]. In many autoimmune diseases, Treg play a key role in controlling these deleterious responses as demonstrated in both mouse models and human immune deficiencies where loss of molecules essential for Treg survival and function lead to pathology [14, 37, 38]. In mouse models, autoimmunity can be both prevented and treated by adoptive transfer of competent Treg. Thus, therapies designed to rescue or enhance Treg function and number may be efficacious in humans. Alone, rapamycin functions as an immunosuppressant [21] and IL-2 supports proliferation of Treg [39]. Based on data shown here and generated by others, we suggest that combination of rapamycin plus IL-2 may be used in vitro to generate therapeutic numbers of iTreg for cellular therapy or be used in combination in vivo to induce and/or enhance peripheral tolerance.
Acknowledgments
The authors would like to thank K. Arugamanathan for cell sorting, Megan Tatum for technical assistance, and Steven Ziegler and Paul Bollyky for their critical review of this manuscript. In addition, we wish to acknowledge the staff of the BRI Translational Research program for subject recruitment and sample management. This work was supported by a JDRF Grant to the Center for Cell Therapy at UCSF and a NIDDK R01 to JHB.
Abbreviations
- iTreg
induced Treg
- rapa
rapamycin
- nTreg
natural Treg
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Bluestone JA, Boehmer H. Regulatory T cells. Semin Immunol. 2006;18:77. doi: 10.1016/j.smim.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiang S, Camara N, Lombardi G, Lechler RI. Induction of allopeptide-specific human CD4+CD25+ regulatory T cells ex vivo. Blood. 2003;102:2180–2186. doi: 10.1182/blood-2003-04-1164. [DOI] [PubMed] [Google Scholar]
- 6.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, Ziegler SF. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25- T cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+ Proc Natl Acad Sci U S A. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, Von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 10.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–1732. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yates J, Rovis F, Mitchell P, Afzali B, Tsang JS, Garin M, Lechler R, Lombardi G, Garden O. The maintenance of human CD4+CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro. Int Immunol. 2007;19:785–799. doi: 10.1093/intimm/dxm047. [DOI] [PubMed] [Google Scholar]
- 12.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 13.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 14.Aoki CA, Roifman CM, Lian ZX, Bowlus CL, Norman GL, Shoenfeld Y, Mackay IR, Gershwin ME. IL-2 receptor alpha deficiency and features of primary biliary cirrhosis. J Autoimmun. 2006;27:50–53. doi: 10.1016/j.jaut.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Harnaha J, Machen J, Wright M, Lakomy R, Styche A, Trucco M, Makaroun S, Giannoukakis N. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes. 2006;55:158–170. [PubMed] [Google Scholar]
- 16.Maerten P, Shen C, Bullens DM, Van Assche G, Van Gool S, Geboes K, Rutgeerts P, Ceuppens JL. Effects of interleukin 4 on CD25+CD4+ regulatory T cell function. J Autoimmun. 2005;25:112–120. doi: 10.1016/j.jaut.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 18.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4(+) T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 20.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 21.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 22.Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 23.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 24.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 25.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, Roncarolo MG. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–49. [PubMed] [Google Scholar]
- 26.Nikolaeva N, Bemelman FJ, Yong SL, van Lier RA, ten Berge IJ. Rapamycin does not induce anergy but inhibits expansion and differentiation of alloreactive human T cells. Transplantation. 2006;81:445–454. doi: 10.1097/01.tp.0000194860.21533.b9. [DOI] [PubMed] [Google Scholar]
- 27.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 28.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, de St Groth BF, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4(+) T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St GB. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 31.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+ J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 32.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 34.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, Ayyoub M. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177:944–949. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 35.Jankovic D, Kullberg MC, Feng CG, Goldszmid RS, Collazo CM, Wilson M, Wynn TA, Kamanaka M, Flavell RA, Sher A. Conventional T-bet(+)Foxp3(-) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med. 2007;204:273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbas AK, Lohr J, Knoechel B. Balancing autoaggressive and protective T cell responses. J Autoimmun. 2007;28:59–61. doi: 10.1016/j.jaut.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. CD4+CD25+ naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J Autoimmun. 2007;29:195–202. doi: 10.1016/j.jaut.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Lan RY, Mackay IR, Gershwin ME. Regulatory T cells in the prevention of mucosal inflammatory diseases: Patrolling the border. J Autoimmun. 2007;29:272–280. doi: 10.1016/j.jaut.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]