Abstract
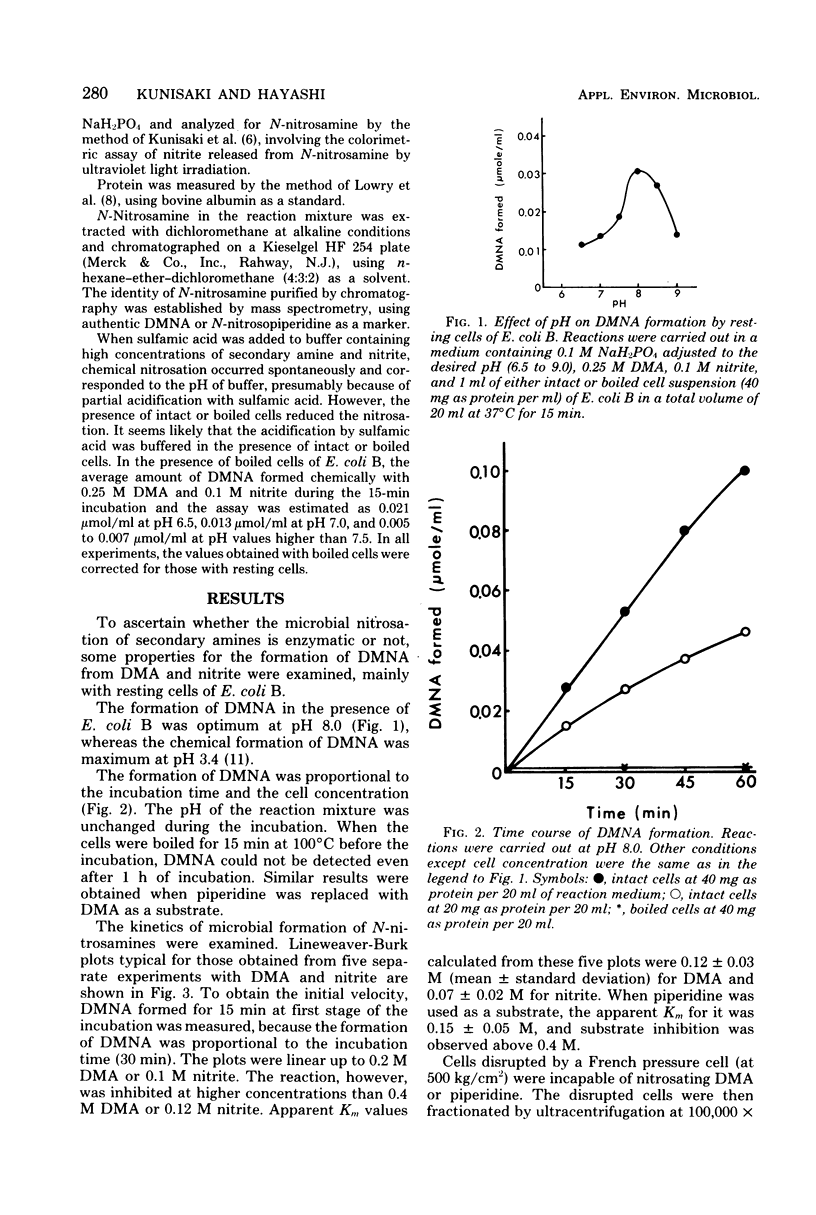
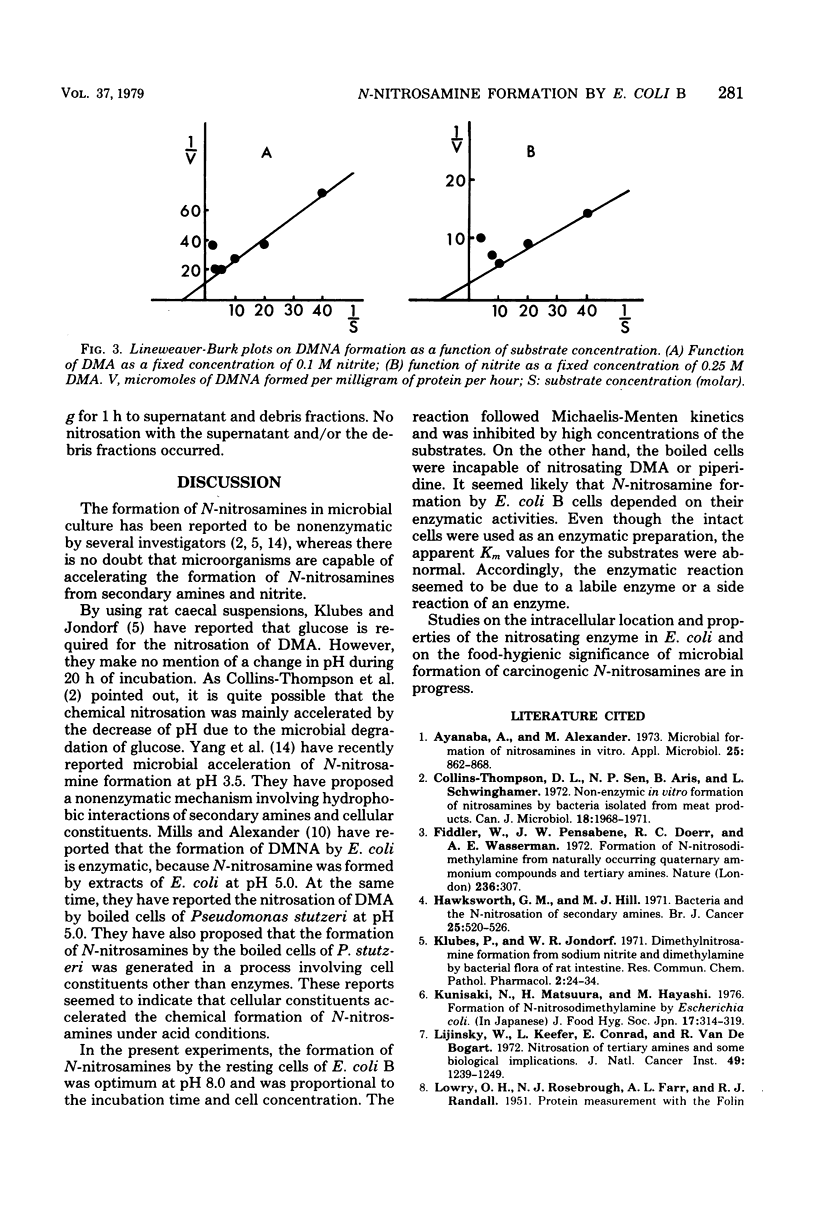
In the presence of resting cells of Escherichia coli B, the formation of N-nitrosamines from nitrite and secondary amines, such as dimethylamine and piperidine, was proportional to the incubation time and to the cell concentration. Optimum pH was 8.0. Boiled cells were incapable of nitrosating secondary amines. Although these experiments were carried out by using intact cells of E. coli B, the reaction followed Michaelis-Menten kinetics, and the apparent Km values calculated from Lineweaver-Burk plots were 0.12 +/- 0.03 M for dimethylamine and 0.07 +/- 0.02 M for nitrite. The apparent Km for piperidine was 0.15 +/- 0.05 M. The nitrosation was inhibited by high substrate concentrations. These results suggested that the formation of n-nitrosamines by resting cells of E. coli B apparently depends on their enzyme activities.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayanaba A., Alexander M. Microbial formation of nitrosamines in vitro. Appl Microbiol. 1973 Jun;25(6):862–868. doi: 10.1128/am.25.6.862-868.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins-Thompson D. L., Sen N. P., Aris B., Schwinghamer L. Non-enzymic in vitro formation of nitrosamines by bacteria isolated from meat products. Can J Microbiol. 1972 Dec;18(12):1968–1971. doi: 10.1139/m72-306. [DOI] [PubMed] [Google Scholar]
- Fiddler W., Pensabene J. W., Doerr R. C., Wasserman A. E. Formation of N-nitrosodimethylamine from naturally occurring quaternary ammonium compounds and tertiary amines. Nature. 1972 Apr 7;236(5345):307–307. doi: 10.1038/236307a0. [DOI] [PubMed] [Google Scholar]
- Hawksworth G. M., Hill M. J. Bacteria and the N-nitrosation of secondary amines. Br J Cancer. 1971 Sep;25(3):520–526. doi: 10.1038/bjc.1971.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klubes P., Jondorf W. R. Dimethylnitrosamine formation from sodium nitrite and dimethylamine by bacterial flora of rat intestine. Res Commun Chem Pathol Pharmacol. 1971 Jan;2(1):24–34. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lijinsky W., Keefer L., Conrad E., Van de Bogart R. Nitrosation of tertiary amines and some biologic implications. J Natl Cancer Inst. 1972 Nov;49(5):1239–1249. [PubMed] [Google Scholar]
- MAGEE P. N., BARNES J. M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br J Cancer. 1956 Mar;10(1):114–122. doi: 10.1038/bjc.1956.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A. L., Alexander M. N-Nitrosamine formation by cultures of several microorganisms. Appl Environ Microbiol. 1976 Jun;31(6):892–895. doi: 10.1128/aem.31.6.892-895.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirvish S. S. Kinetics of dimethylamine nitrosation in relation to nitrosamine carcinogenesis. J Natl Cancer Inst. 1970 Mar;44(3):633–639. [PubMed] [Google Scholar]
- Sander J. Nitrosaminsynthese durch Bakterien. Hoppe Seylers Z Physiol Chem. 1968 Apr;349(4):429–432. [PubMed] [Google Scholar]
- Yang H. S., Okun J. D., Archer M. C. Nonenzymatic microbial acceleration of nitrosamine formation. J Agric Food Chem. 1977 Sep-Oct;25(5):1181–1183. doi: 10.1021/jf60213a025. [DOI] [PubMed] [Google Scholar]



