Abstract
Thrombospondin-1 is a potent physiologic regulator of nitric oxide driven blood flow and angiogenesis. This regulation requires the cell receptor CD47. In a murine model of skin graft survival we demonstrate that endogenous thrombospondin-1 limits graft survival via CD47 and targeting CD47 with antibodies or suppressing CD47 with a morpholino oligonucleotide enhances graft survival by blocking this pathway.
Background
Skin graft survival and healing requires rapid restoration of blood flow to the avascular graft. Failure or delay in the process of graft vascularization is a significant source of morbidity and mortality. One of the primary regulators of blood flow and vessel growth is nitric oxide (NO). The secreted protein thrombospondin-1 (TSP1) limits NO-stimulated blood flow and growth and composite tissue survival to ischemia. We herein demonstrate a role for TSP1 in regulating full thickness skin graft survival.
Methods and Results
Full thickness skin grafts (FTSG) consistently fail in wild type C57 Bl/6 mice but survive in mice lacking TSP1 or its receptor CD47. Ablation of the TSP1 receptor CD36, however, did not improve FTSG survival. Remarkably, wild type FTSG survived on TSP1 null or CD47 null mice, indicating that TSP1 expression in the wound bed is the primary determinant of graft survival. FTSG survival in wild type mice could be moderately improved by increasing NO flux, but graft survival was increased significantly through antibody blocking of TSP1 binding to CD47 or antisense morpholino oligonucleotide suppression of CD47.
Conclusions
TSP1 through CD47 limits skin graft survival. Blocking TSP1 binding or suppressing CD47 expression drastically increased graft survival. The therapeutic applications of this approach could include burn patients and the broader group of people requiring grafts or tissue flaps for closure and reconstruction of complex wounds of diverse etiologies.
Introduction
Burn injuries constitute a major source of mortality and morbidity 1. Among people aged 5 – 29 years, burns rank among the top 15 causes of morbidity and mortality worldwide. Over 2 million burn injuries are reported each year in the United States alone 2, 3. Survival from major burns has improved over the last century as a result of improved management of the cardiovascular collapse attendant to major burns and aggressive management of the burn wound and subsequent infection 4, 5. As a source of damaged and/or dead tissue, the burn injury requires aggressive debridement 6. However, this invariably creates massive open wounds, often with vital underlying structures exposed. Increasingly, aggressive surgical approaches with early tangential excision and wound closure are being applied 7, 8. Such therapeutic approaches represent a significant change in burn wound care and have lead to improvement in mortality rates of burn victims at a substantially lower cost 9. These approaches can also decrease the severity of hypertrophic scarring, joint contractures and stiffness, and promote faster rehabilitation 10. Irrespective of any other consideration, early healing is paramount for good aesthetic and functional recovery. Disruption of epidermal–mesenchymal communication due to a delay in epithelialization increases the frequency of developing fibrotic conditions such as scar hypertrophy and contractures. Autografts from uninjured skin remain the mainstay of treatment for the majority of patients 11. Skin grafting is also a primary approach to the management of complex and non-healing wounds of diverse etiology 12.
Maximizing skin graft survival and take is paramount to successful wound reconstruction. Numerous techniques and agents have been utilized in an attempt to maximize skin graft survival. These include special dressings and splints to prevent movement of the skin graft-wound bed interface 13. Growth factors and clotting related products, such as fibrin, have been placed in the interface between the skin graft and the wound bed 14. Yet substantial complications in healing remain, with significant numbers of patients requiring additional intervention due to skin graft failure and loss, and non-healing of burn wounds 15–17. Interventions to date have not addressed a fundamental problem, and determinant, of skin graft survival, namely the degree of vascularity within the wound bed. Following wounding, vascularity can increase by two primary methods: (1) the development of new blood vessels from an existing vascular network, a process termed angiogenesis; or (2) remodeling and recruitment of existing blood vessels within the wound bed. Control of angiogenesis represents a balance between factors that stimulate the process and inhibit the process. Recruitment and remodeling of an existing vascular network is an effect of vascular dilatation and alterations of regional perfusion.
Thrombospondin-1 (TSP1) was the first identified endogenous inhibitor of angiogenesis 18, 19. TSP1, a major secretory product of activated platelets, is over expressed in wound beds 20. TSP1 inhibits angiogenesis by modulating proliferation and migration of endothelial and vascular smooth muscle cells (VSMC) 19, 21, 22. Conversely, tumor driven angiogenesis has been found in several major cancers to be associated with decreased expression and production of TSP1 23. Recently, the TSP1 status of endothelial progenitor cells has been linked to their ability to enhance angiogenic responses 24
The bioactive gas nitric oxide (NO) is a major effector of vessel dilation and angiogenesis that was first identified as endothelial derived relaxing factor (EDRF) 25. NO functions in both paracrine and autocrine fashions to regulate a number of cell responses that occur on rather different time scales. NO regulates VSMC contractility and in so doing controls blood pressure and, by altering the caliber of resistance vessels, acutely regulates tissue perfusion 26, 27. NO also modulates a number of processes that, in toto, contribute to the healthy status of the cardiovascular system over an individual’s lifetime 28, 29.
Recently we reported that picomolar concentrations of TSP1 can effectively block the growth- and motility-promoting activities NO in endothelial and VSMC and that this inhibitory signal requires the TSP1 receptor CD47 30, 31. Furthermore, endogenous TSP1 limits the vasodilator activity of NO, and thereby limits tissue survival under ischemic conditions by blocking NO-dependent restoration of vascular perfusion 32. In the absence of TSP1, NO-driven signaling is enhanced, increasing tissue oxygenation. As a result, tissue necrosis in ischemic dorsal skin flaps is virtually eliminated 33. We now present evidence that TSP1 is limiting for full thickness skin graft (FTSG) survival. We further report that expression of TSP1 and its receptor CD47 within the wound bed, but not the skin graft, are major determinants of graft survival. Relevant to therapeutic applications, we show that functional blockade of TSP1 signaling at the wound bed-graft interface is sufficient to allow complete survival of FTSG.
Materials and Methods
Animals
C57BL/6 wild type, TSP1-null, CD47-null, and CD36-null mice were housed and maintained in a pathogen free environment and had ad libitum access to filtered water and standard rat chow. Handling and care of animals was in compliance with the guidelines established by the Animal Care and Use Committee of the National Cancer Institute, National Institutes of Health and of Washington University.
Reagents and cells
Human umbilical vein endothelial cells (HUVEC, Cambrex, Walkersville, MD) were maintained in endothelial cell growth medium (EM-GM) supplemented with the manufacturer’s additives (Cambrex) and 2% fetal calf serum (FCS) in 5% CO2 at 37° C. Cells were utilized within passages 4–9. Isosorbide dinitrate (ISDN), an exogenous NO donor, and N-nitro-L-arginine methyl ester (L-NAME), a competitive inhibitor of nitric oxide synthase (NOS), were purchased from Sigma (St. Louis, MO). A CD47 targeting morpholino oligonucleotide (5′-CGTCACAGGCAGGACCCACTGCCCA) and control mismatch morpholino were purchased from GeneTools (Philmonth, Oregon). The CD47 targeting morpholino recognized a sequence conserved between the murine and human mRNAs. A rat monoclonal antibody to murine CD47, Ab 301, was prepared as described 34. An isotype matched IgG2a control antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). An antibody to CD47, B6H12, was purified by protein G affinity chromatography (Pierce) from conditioned media of the respective hybridoma (American Type Culture Collection).
Full thickness skin graft model
Wild type, TSP1 null, CD47 null and CD36 null mice were matched for sex and age. Anesthesia was induced by and maintained with inhalation isoflurane. Body temperature was maintained at 37° C with a heating pad during the procedure. The dorsal surface was clipped of hair and depilated with Nair®. The skin was then cleansed with surgical soap and alcohol and the animals draped. Using sterile technique, a 1 × 1 cm FTSG incorporating the panniculus carnosus, subcutaneous tissues and skin were raised. Graft dimensions were marked on the animal skin surface with the aid of a template to insure consistency in dimension. Grafts were in the midline of the animal and secured with four interrupted 5-0 nylon sutures. Sutures were so placed as to include the fascia of the dorsal musculature to provide increased immobilization of grafts to the underlying wound bed. Animals were awakened and returned to individual cages and allowed ad libitum access to food and water. Trauma to FTSG was not encountered during the post-operative interval since grafts were located in the dorsal midline over the mid thoracic area and each animal was housed individually following surgery. On postoperative day three or seven, the animals were again anesthetized with inhalation isoflurane and skin graft survival evaluated.
Treatment groups and protocols
The groups of animals utilized and the treatments received as indicated either at the time of skin grafting and/or during the post-operative interval are summarized in Figure 1. Animals treated with ISDN (1 mg/ml) or L-NAME (0.5 mg/ml) had ad libitum access to drinking water containing the given concentrations of agents during the postoperative interval. Animals treated with a CD47 monoclonal antibody (Ab clone 301) or an isotype matched control antibody (Ig2α) underwent injection of 40 µg delivered as 10 µl of a 4 mg/ml stock in 100 µl of PBS with equal volumes injected between the wound bed and graft prior to suturing the graft in place. Animals treated with a CD47 morpholino oligonucleotide underwent injection of the FTSG and wound bed with 10 µmol/L in 250 µl of PBS with 125µl volumes injected in the graft and wound bed, respectively prior to suturing the graft in place.
Figure 1. Treatment and experimental groups.
The indicated groups and numbers of animals on a C57BL/6 background were utilized and received treatments as indicated either at the time of skin grafting and/or during the post-operative interval. Treatments included isosorbide dinitrate (ISDN) or L-NAME (L-N) in the drinking water, or treatment of skin grafts and wound beds using a CD47 blocking antibody (Ab 301) or an isotype matched control antibody (IgG2α) or a CD47 oligonucleotide morpholino (morph). Additional control mice were included in experimental groups.
Estimation of survival area in FTSG
The necrotic areas of the FTSG were determined by color, refill, eschar, and the pin-prick test. The outlines of viable and nonviable areas were traced using transparent film, and the area of necrosis cut from the template and the area of skin graft necrosis versus total skin graft area determined by comparison of the original template weight to the weight of the cut template utilizing the method described by Ueda 35. Skin graft survival area was presented as the ration of graft survival on post-operative day three or seven divided by the original graft area multiplied by 100%. Additionally, digital images were then acquired using an Olympus C5500 digital camera at a fixed distance (20 cm perpendicular to the animal) under standard room lighting with autoflash and macro settings engaged and uploaded for image analysis of tissue survival with a standard software program (Image-Pro Plus, Media Cybernetics, Inc., Silver Spring, MD). Animals were euthanized and skin grafts and wound beds excised, fixed in 10% paraformaldehyde and processed for routine histology. Independent review of representative histologic sections from FTSG was performed to assess levels of tissue necrosis and inflammation providing another assessment of FTSG survival.
Laser Doppler analysis of tissue perfusion
Animals were secured supine as dictated by the anatomic area analyzed. Core temperature was monitored via rectal probe and maintained at 37° C by a heated stage. Anesthesia was maintained with 1.5% isoflurane and a 50:50 mixture of room air and oxygen. The following scanner parameters were employed using a Moor Instruments LDI2-2λ imager: scan area − 1.6 × 2.5 cm; scan speed − 4 ms/pixel, scan time 1 min 54 sec, override distance 25 cm. Measurement of the flux of blood was determined by the formula flux = blood × area−1 × time−1. Pre-operative baseline perfusion data was obtained, FTSG elevated and sutured in place and post-operative scanning initiated at the indicated time points.
Western analysis of CD47
HUVEC were plated in 12-well culture plates (5 × 104 cells/well) (Nunc, Denmark) in full growth medium and treated over 48 h with indicated doses of CD47 and control morpholino. Cells were subsequently washed twice with PBS and lysed immediately in 1X SDS sample buffer containing 10 µg/mL leupeptin, 10 µg/mL aprotinin, 1 mM Na3VO4, and 40 mM NaF. Lysates prepared in the SDS sample buffer described above were electrophoresed in 4–12% BisTris NuPAGE gels and transferred to PVDF membranes prior to immunoblotting with a monoclonal antibody to CD47 (clone B6H12, Lab Visions, Inc, Fremont, CA). The membrane was stripped and reprobed with a monoclonal antibody against β-actin (Sigma-Aldrich, St. Louis, MO).
Histology
Full thickness skin grafts and graft wound beds were excised, fixed in 10% buffered formaldehyde, paraffin embedded and sectioned at a thickness of 5 µm. Sections were then stained with hematoxylin and eosin (H + E) according to standard procedures. Review of each slide was performed by an independent pathologist blinded to the origin of each tissue slide.
Wound bed vascular index
Skin graft wound beds were assessed at indicated time points (72 h or 7 days post-operatively) for visible alterations in vascularity under 5x magnification. An arbitrary though strictly applied definition of countable vessels was employed to highlight both individual vessels and vascular ramifications. In any given vascular plexus visible by 5x magnification a vessel was defined as that segment traversing two branches. Visible vessels without ramifications and branches were counted once. Treatment status and genetic background of tissue images was not known by the reviewer. Results are expressed as vessels per cm2.
Mitochondrial viability assay
Mitochondrial viability of full thickness skin grafts as a correlate of tissue viability was assessed by the reduction of a tetrazolium salt to water insoluble colored formazan crystals through mitochondrial metabolism. Full thickness skin grafts from wild type and TSP1 null mice were weighed and incubated in 3 ml PBS supplemented 1:10 with 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium (MTT, Promega) for 3 h in the dark at 37° C. Samples were removed, washed with distilled water and blotted dry. The formazan salt was extracted in 3 ml of 2-propranol for 6 h in the dark at 37° C. Absorbance for 200 µl aliqouts were determined at 450 nm on a microplate reader. Samples were dried at 90° C overnight and weighed again. Results were expressed as absorbance normalized to tissue dry weight.
Statistics
Results are presented as the mean ± SD of a total of 196 animals distributed as indicated above including the described treatments groups and controls (see also Figure 1 and Figure Legends). Significance was calculated with Student’s t test or where appropriate with one-way ANOVA using a standard soft ware package (Origin) with p values as indicated.
Results
Thrombospondin-1 limits FTSG survival
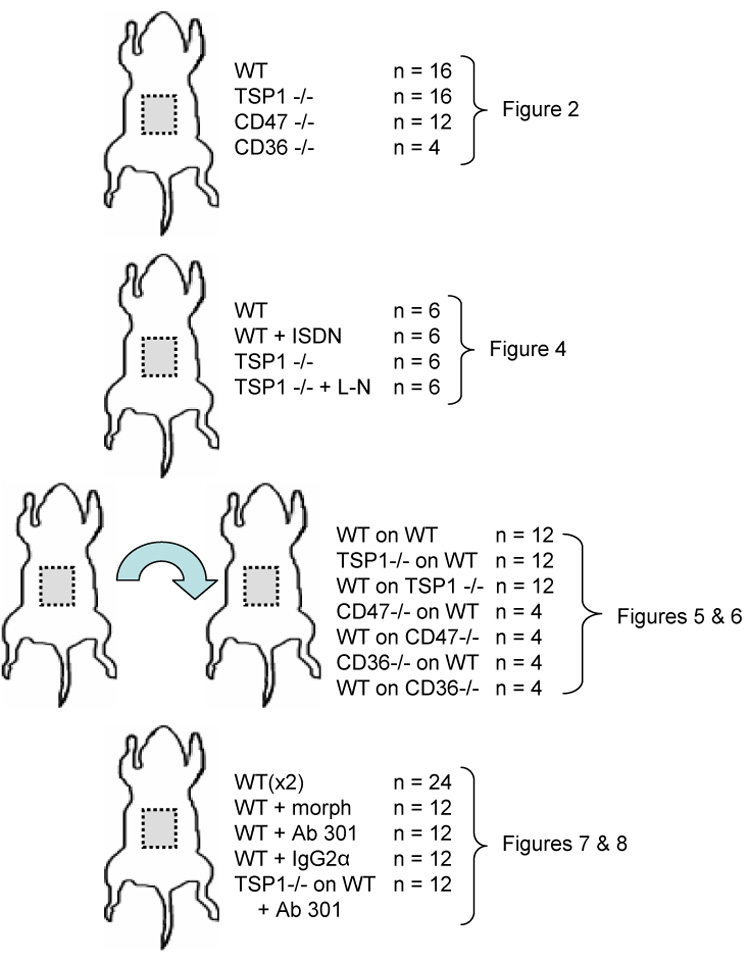
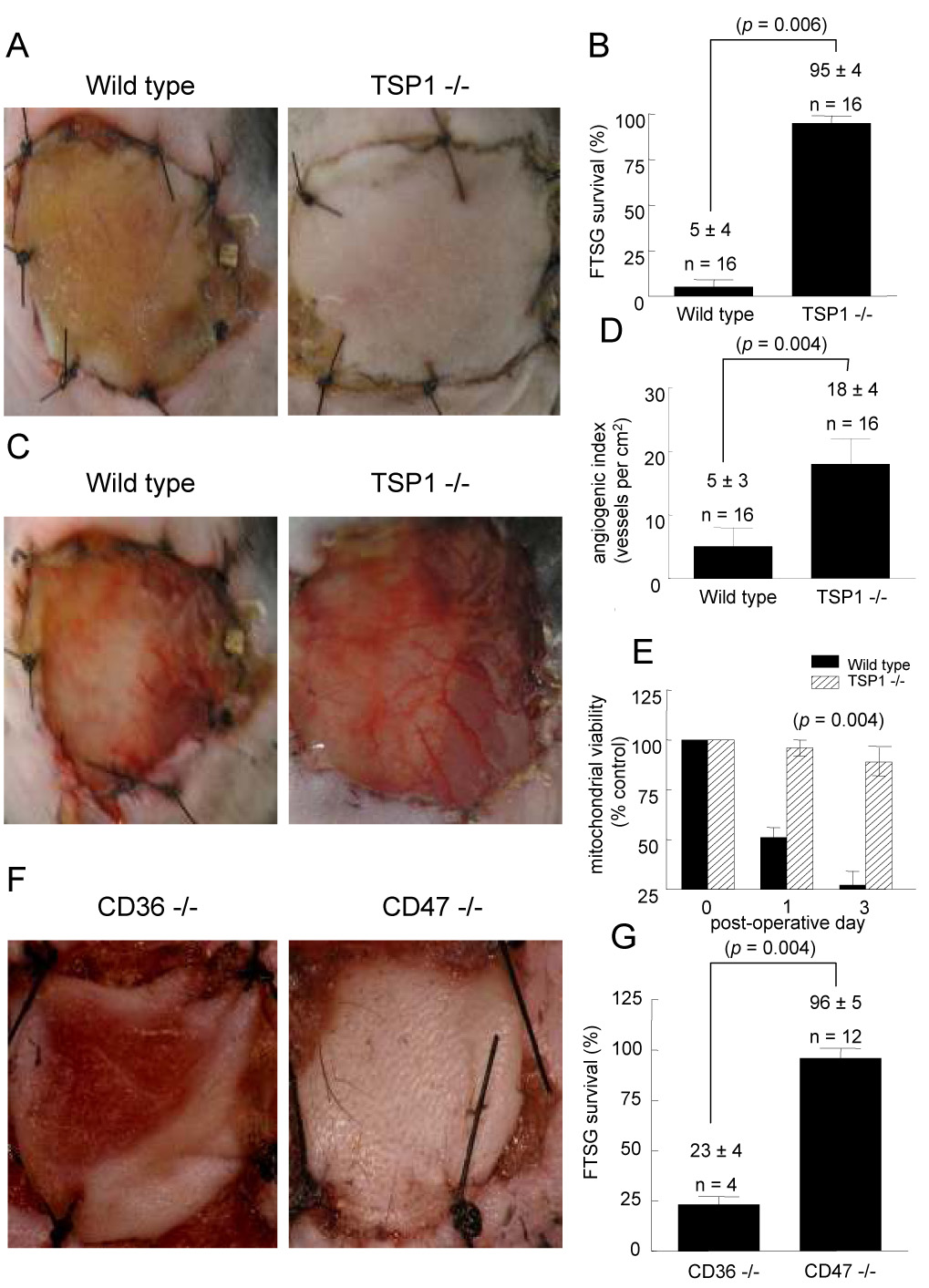
FTSG in both wild type (Fig. 2A, B) and CD36 null animals (Fig. 2F, G) demonstrated 95 ± 6 and 77 ± 6 % necrosis respectively. In contrast FTSG in TSP1 (Fig. 2A, B) and CD47 null animals (Fig. 2F, G) showed almost complete survival (95 ± 4 and 96 ± 12% respectively). Analysis of wound bed vascularity at 72 hours post skin grafting demonstrated significantly increased numbers of visible vessels in those wounds beds lacking endogenous TSP1 compared to wild type (Fig. 2C, D). Similarly, wound bed analysis at 72 hours of CD47 null mice demonstrated increased vascularity compared to CD36 null wound beds (data not shown). Consistent with increased graft survival and take, mitochondrial viability was found to be significantly greater in TSP1 null FTSG at 24 and 72 hours post-operatively compared to wild type (Fig. 1E). Laser Doppler analysis of FTSG survival was also performed in a series of wild type and TSP1-null animals on post-operative days 5 and 10 (Fig. 3A, B). In the absence of TSP1 significant perfusion was found at both time points compared to wild type FTSG supporting clinical and histologic analysis of graft survival.
Figure 2. Endogenous TSP1 is limiting for full thickness skin graft survival.
Age and sex matched C57BL/6 wild type (A, B), TSP1-null (C, D), CD47-null and CD36-null mice (F, G) underwent autologous FTSG to the dorsal back. Graft survival and wound bed vascularity was measured on post-operative day 3. Mitochondrial viability of wild type and TSP1-null FTSG units was determined at the indicated time points (E). Results represent the mean ± SD. p = 0.006 (B) and 0.004 (D, G) and 0.004 versus wild type on day 1 and 3 respectively (E).
Figure 3. Endogenous TSP1 limits reperfusion of FTSG.
Age and sex matched C57BL/6 wild type and TSP1-null mice underwent FTSG and at the indicated post-operative time points laser Doppler analysis of tissue perfusion was performed (A, B). The following scanner parameters were employed: scan area − 1.6 × 2.5 cm; scan speed − 4 ms/pixel, scan time 1 min 54 sec, override distance 25 cm. Measurement the flux of blood was determined by the formula flux = blood × area−1 × time−1. Pre-operative baseline perfusion data was obtained, FTSG elevated and sutured in place and post-operative scanning initiated at the indicated time points. Results represent the mean ± SD of 6 mice from each background. p = 0.004 and 0.002 versus wild type on post-operative day 5 and 10 respectively.
Modulation of FTSG survival through nitric oxide regulation
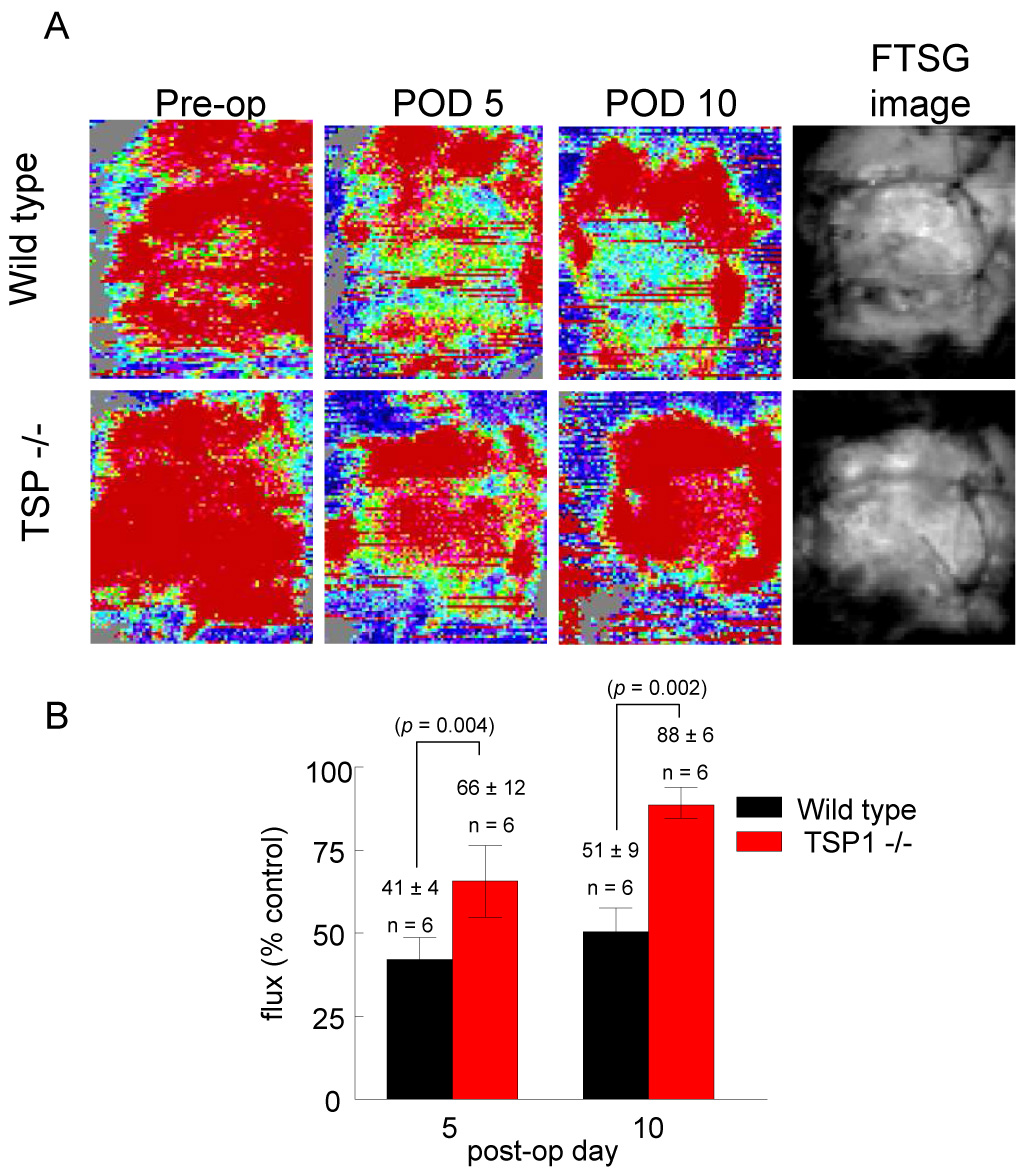
Wild type FTSG survival increased significantly (62 ± 8%) on supplementation of the drinking water post-operatively with the NO releasing drug ISDN (Fig. 4A, B). FTSG survival, already approaching 100%, in TSP1-null grafts was not substantially altered by ISDN. Conversely, inhibiting endogenous NO synthesis through L-NAME administration in the drinking water during the post-operative interval decreased TSP1-null graft survival (58 ± 6%) (Fig. 4C, D).
Figure 4. Exogenous nitric oxide improves FTSG survival in wild type animals.
Age and sex matched wild type and TSP1-null mice underwent autologous FTSG to the dorsal back and received ISDN (1 mg/ml) (A, B) or L-NAME (0.5 mg/ml) in the drinking water post-operatively (C, D). Graft survival was evaluated on post-operative day 7. Results represent the mean ± SD of 6 age and sex matched animals in each group. p = 0.002 (B) and 0.024 (D) versus respective controls.
Wound bed TSP1 or CD47 are limiting for FTSG survival
To examine the role in graft survival of TSP1 expression in the graft versus the wound bed, wild type skin grafts were transplanted onto TSP1-null wound beds (recipients), and TSP1-null grafts were transplanted onto wild type wounds. At 72 hours post-operatively, survival was significantly greater in wild type FTSG placed on TSP1-null wound beds (97 ± 6%) (Fig. 5A, B). Remarkably, TSP1-null FTSG placed on wild type wound beds all underwent significant necrosis at 72 hours (37 ± 5%), as did a series of control wild type FTSG placed on wild type wound beds. These findings again correlated with wound bed vascularity. TSP1-null wound beds demonstrated increased vascularity despite the presence of wild type FTSG, whereas wild type wound beds showed minimal vascularity despite the presence of TSP1-null FTSG (data not shown). In other experiments FTSG from wild type animals were transplanted to CD47-null wound beds and, as on TSP1-null wound beds, demonstrated dramatically increased graft survival (92 ± 8%) (Fig. 6A). In contrast CD47-null FTSG placed on wild type wounds demonstrated nearly total necrosis, similar to TSP1-null grafts transplanted to wild type wounds. Wild type FTSG on CD36-null wound beds all showed substantial necrosis and not unexpectedly, CD36-null grafts on wild type wounds experienced substantial necrosis (85 ± 4 and 74 ± 3% respectively) (Fig. 6B). Analysis of wound bed vascularity at 7 days post grafting found significantly increased vascularity in both TSP1-null (47 ± 7) and CD47-null (68 ± 14) wound beds regardless of the genotype of the skin graft applied (Fig. 6C) as compared to wild type (21 ± 10) and CD36-null (20 ± 7) wound beds. Interestingly, extension of the post-operative interval at which wound bed analysis was performed from 72 hours to 7 days greatly enhanced the mean vessel count in TSP1-null but not wild type wound beds (see Fig. 2D versus Fig. 6C).
Figure 5. Wound bed TSP1 determines FTSG survival.
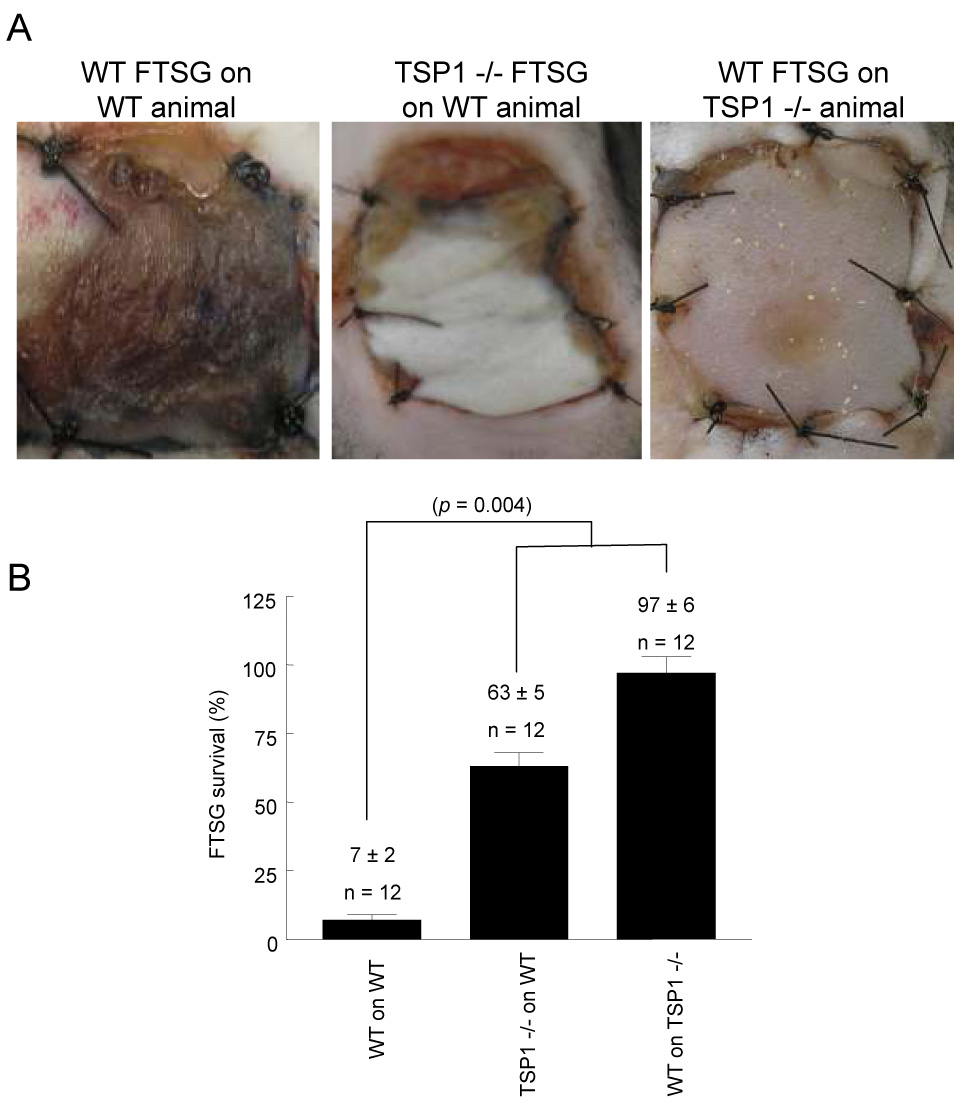
Age and sex matched wild type and TSP1-null mice underwent cross allograft FTSG to the dorsal back (A, B). Graft survival was measured on post-operative day 7. Results represent the mean ± SD of 12 pairs of animals. p = 0.004 (B) versus wild type on wild type, one-way ANOVA.
Figure 6. Wound bed CD47 is limiting for FTSG survival.
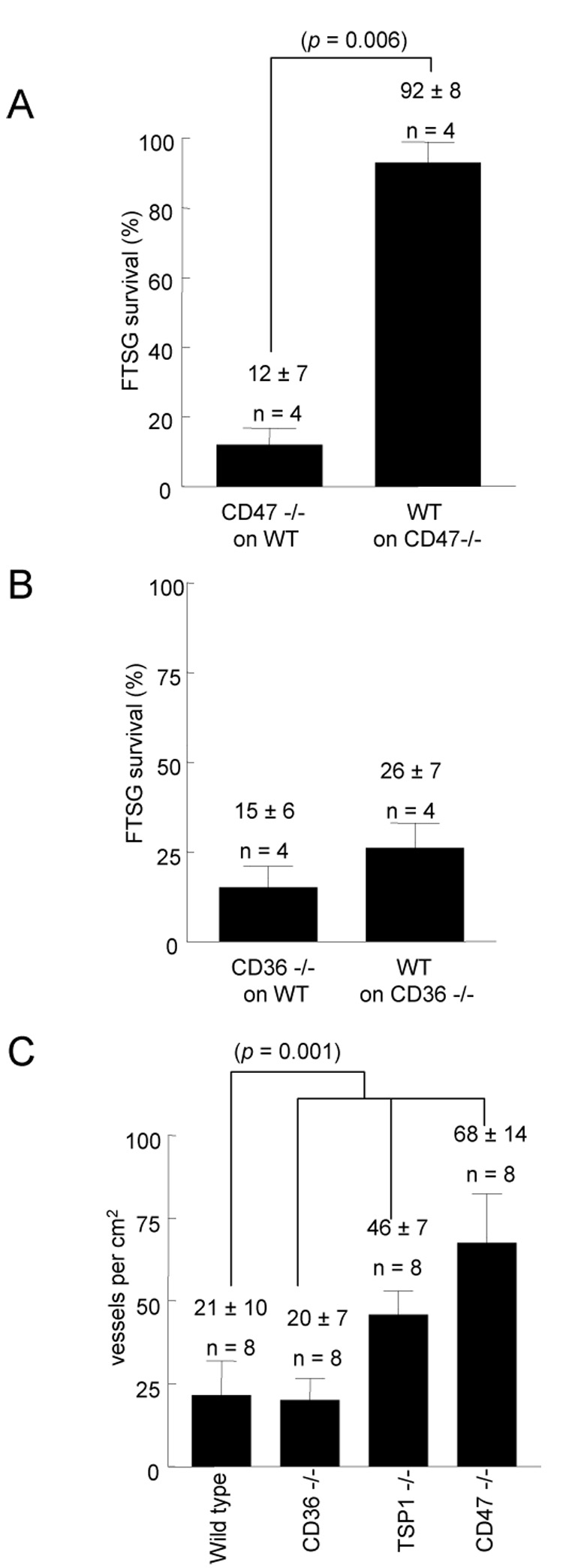
Age and sex matched CD47 and CD36-null mice underwent cross allograft FTSG to the dorsal back (A, B). Graft survival was measured at 72 h. Results represent the mean ± SD of 4 pairs of animals. Quantification of vascularity in wild type, TSP1-null, CD47-null and CD36-null wound beds was made on postoperative day 7 and expressed per cm sq of wound bed surface area (C). p = 0.006 (A) versus respective controls. p = 0.001 (C) versus wild type, one-way ANOVA.
Suppression of CD47 expression is sufficient to increase FTSG survival
Wild type FTSG and wound beds were treated with either a CD47 antisense morpholino oligonucleotide or a mismatched control morpholino (10 µM) at the time of graft elevation (10 µmol/L in 250 µl of PBS with 125µl volumes injected in the graft and wound bed, respectively). All grafts treated with the CD47 morpholino demonstrated dramatically increased survival (79 ± 5%) and were comparable to results obtained in TSP1 null and CD47 null animals (Fig. 7A, B). Conversely, control morpholino treated grafts and grafts treated with delivery vehicle alone demonstrated significant graft necrosis and loss (data not shown). Effective dose-dependent suppression of CD47 with the same morpholino was demonstrated in HUVEC (Fig. 7C) and previously in VSMC 33. Effective tissue suppression of CD47 through local delivery via syringe injection has also been demonstrated 33. Control morpholino treated endothelial cells (and VSMC, see (33)) did not show suppression of CD47. H & E staining of morpholino treated wild type FTSG found essentially normal tissue architecture without evidence of necrosis and increased vessel density (Fig. 7D). These histologic findings were paralleled in TSP1 null skin grafts (data not shown). Conversely, untreated wild type FTSG showed loss of epidermis and hair follicles with ulceration, necrosis of dermal collagen, inflammatory cell infiltration, and decreased vascular density (Fig 7E).
Figure 7. CD47 suppression increases FTSG survival.
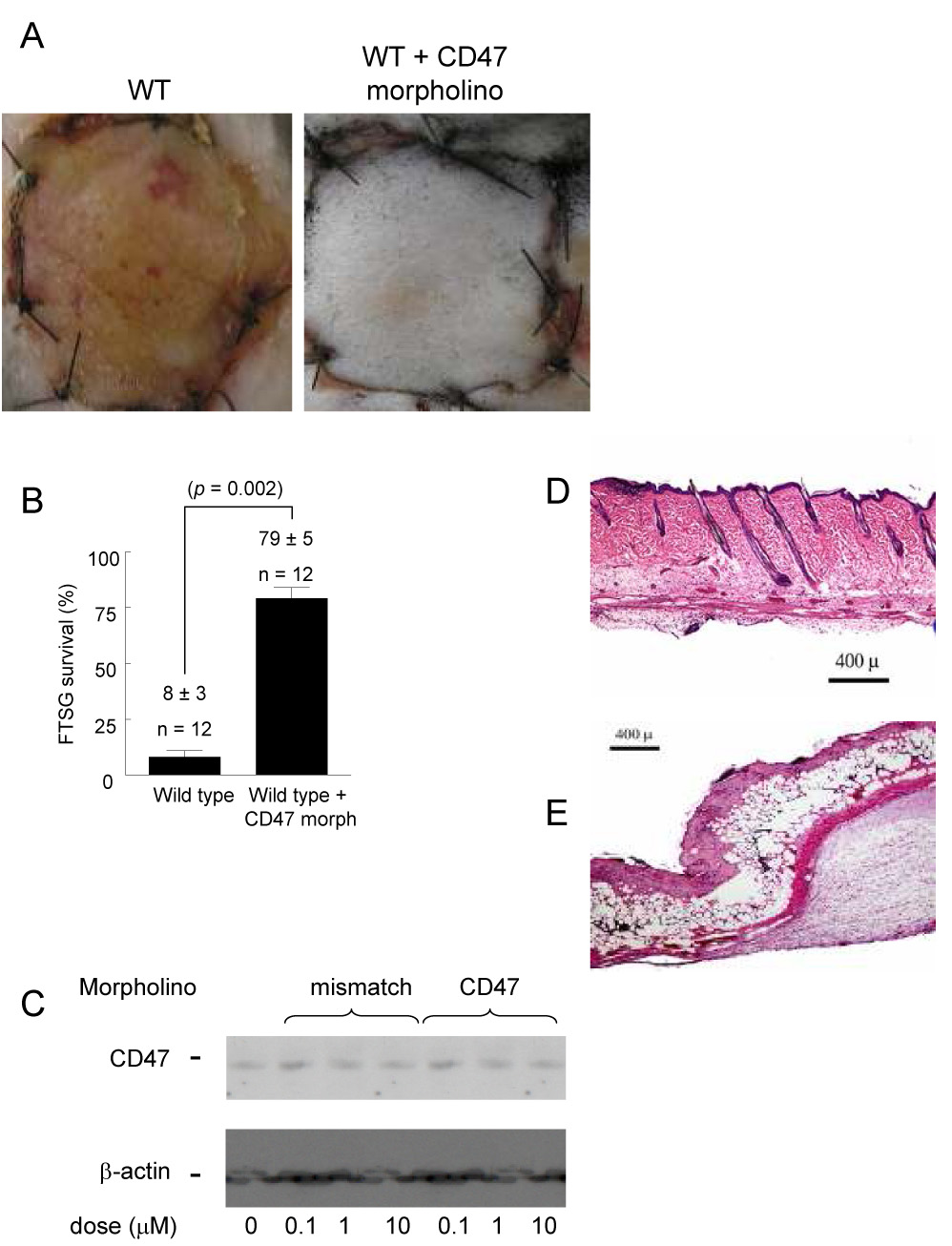
Wild type FTSG and wound beds were infiltrated with a CD47 morpholino (10 µM in 100 µl PBS to the graft and wound bed) or mismatch control and grafts survival determined at 7 days (A, B). Results represent the mean ± SD of 12 pairs of animals. HUVEC were treated in standard growth medium with a CD47 or control morpholino (0.1 – 10 µM) for 48 hours and cell lysates prepared. Blots were developed with a CD47 specific antibody (clone B6H12). Results presented are a representative blot from 3 separate experiments (C). H & E staining of CD47 morpholino treated (D) versus untreated wild type FTSG (E). p = 0.002 (B) versus control.
CD47 blockade increases survival of FTSG
A monoclonal antibody to murine CD47, Ab clone 301, (40 µg delivered as 10 µl of a 4 mg/ml stock in 100 µl of PBS delivered as 50 µl to the skin graft and wound bed, respectively) when infiltrated into wild type FTSG and wound beds increased survival of wild type FTSG from only 5 ± 2% to 82 ± 4% (Fig. 8A, B). FTSG and wound beds infiltrated with vehicle (PBS) (data not shown) or an isotype matched control IgG2a antibody showed no increase in graft survival (8 ± 5%). FTSG transplanted from TSP1-null animals were placed upon wild type wound beds pretreated with monoclonal antibody (Ab 301) and demonstrated increased graft survival (95 ± 4%) as compared to grafts placed on untreated wound beds (Fig 8B). H & E of antibody treated wild type FTSG demonstrated normal architecture without ulceration and minimal inflammatory cell infiltration (Fig. 8C).
Figure 8. CD47 ligation with monoclonal antibody increases wild type autologous FTSG survival.
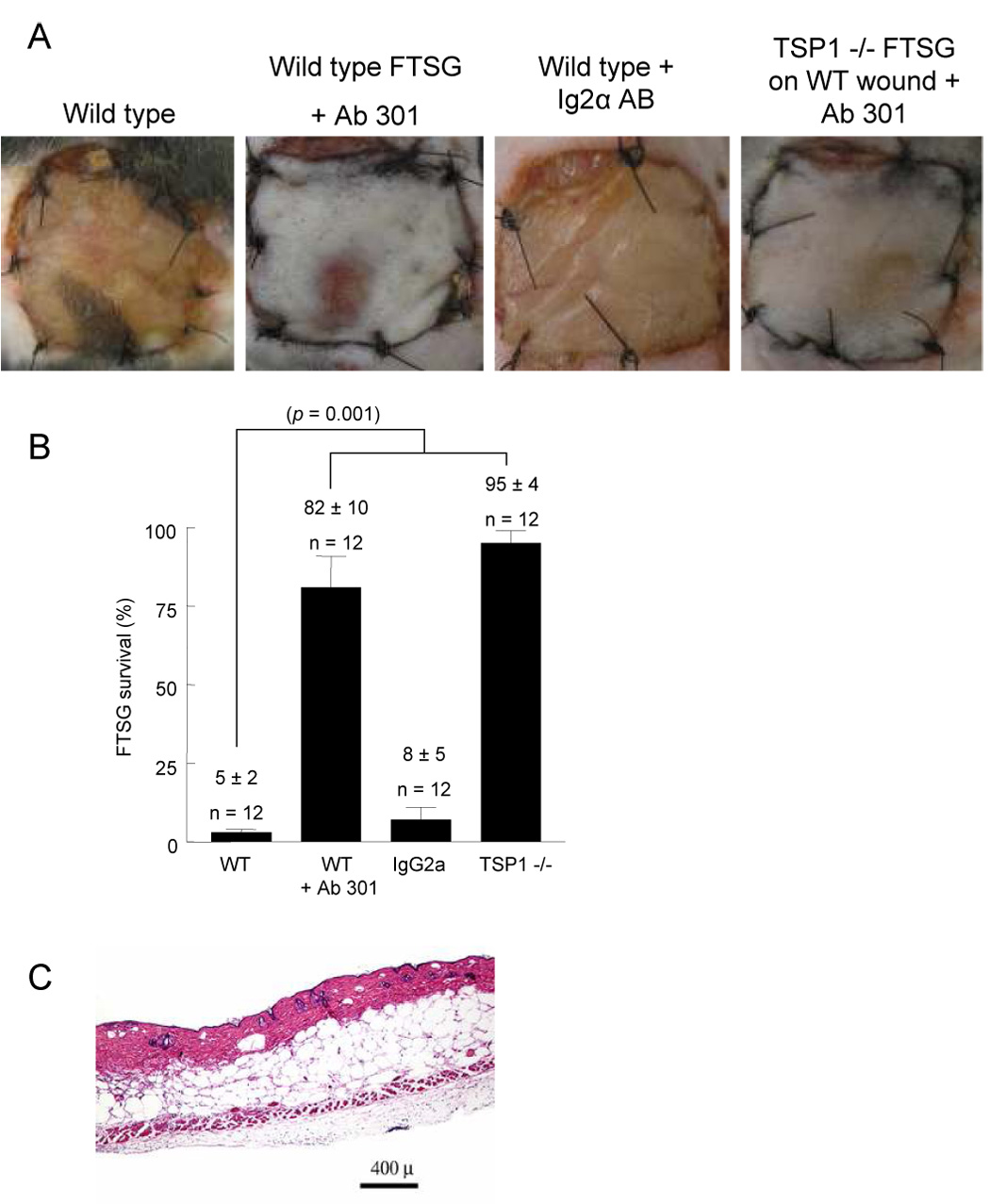
Age and sex matched wild type and TSP1-null mice underwent allograft FTSG to the dorsal back (A, B). Infiltration of FTSG and wound beds with Ab 301 (40 µg in 200 µl PBS) or an isotype matched control antibody (IgG2α) was performed prior to graft suturing. Results represent the mean ± SD of 12 animals in the indicated groups. H & E staining of wild type FTSG treated with a TSP1 monoclonal antibody clone Ab 301 (C). p = 0.001 (B) versus wild type, one-way ANOVA.
Discussion
Skin consists of two tissue layers - a keratinized epidermis and a deeper layer of dermis. Appendages including hair and glands are derived from the epidermis and project into the dermis. Skin serves as a protective barrier, and any break in it must be rapidly and efficiently repaired. A temporary repair is achieved in the form of a clot. Inflammatory cells, fibroblasts and capillaries invade the clot and form granulation tissue while the epithelial edges migrate to cover the wound surface. The current standard for wound closure following excision of a deep partial or full thickness burn is the application of a skin graft. Recently skin substitutes and engineered products have been employed with some success to minimize the need for skin grafts 36, 37. These methods of wound closure all require rapid restoration of perfusion to the grafted elements. Restoration of tissue perfusion is important to wound healing and tissue survival under a range of conditions 38, 39. L-arginine, the precursor for NO production, has been reported to be useful in resuscitation of burn wound patients 40 and in increasing survival and re-perfusion of ischemic tissues 41, 42. NO has also been shown to enhance ischemic tissue survival 43–45
The present report demonstrates for the first time a critical role for TSP1 in controlling survival of FTSG by limiting the beneficial effects of NO. In the absence of endogenous TSP1, or its receptor CD47, significant increases in survival of FTSG were achieved. These findings parallel our recent reports that TSP1 regulates soft tissue survival under ischemic conditions in a NO-dependent manner 32, 33. Random myocutaneous flaps were found to experience more than 50% increase in survival in the absence of endogenous TSP1 32. Random myocutaneous flaps, though ischemic, retain partial perfusion through vascular networks at the base of the flap. In contrast FTSG initially lack any vascular connections. Therefore, FTSG survival requires rapid angiogenic and vascular remodeling responses. Analysis of wound beds at 72 hours and 7 days post-operatively in TSP1-null animals suggests that the absence of TSP1 results in rapid vascular remodeling of the wound bed. These findings are consistent with similar data obtained in wound beds of random ischemic myocutaneous flaps, and in muscle units of hind limbs following proximal vascular ligation 32, 33 supporting a role for TSP1 in regulating vascular remodeling to hypoxic/ischemic stress in addition to its known long term regulation of angiogenesis 46.
CD47 is the critical target for controlling vascular responses to ischemia. This concept is clearly demonstrated here and in our earlier study in less severe models of tissue ischemia 33. Our results also demonstrate that the previously-identified anti-angiogenic TSP1 receptor, CD36, plays a minimal role in limiting FTSG survival. These results support our findings that ischemic composite tissue survival is also not limited by CD36 33. These in vivo findings complement our recent findings that CD47 is necessary for TSP1 abrogation of the pro-angiogenic effects of NO in vitro, and that direct ligation of CD47 effectively blocks NO signaling 47. Importantly for therapeutic application of these findings, antibody blockade of CD47 or morpholino suppression of CD47 increases survival of FTSG (and composite tissue units 33). Ab 301, a monoclonal antibody that recognizes murine CD47, and a CD47 morpholino, when infiltrated into wild type FTSG and wound beds promoted tissue survival to nearly the same extent as genetic deletion of CD47 or TSP1. Both the morpholino and Ab 301 are target specific 33, yet the ability to completely block or suppress CD47 with these agents in vivo is likely incomplete. Although we cannot determine the precise extent to which CD47 and CD47 signaling responses are neutralized by these agents, our results in animals clearly confirm they are efficacious within the zspecific models employed.
Our data show that it is the presence or absence of TSP1 and CD47 in the wound bed, not the graft, which ultimately dictates graft survival. Vascular index data from wild type wound beds treated with a morpholino or Ab 301 and wound beds from CD47- and TSP1-null animals found substantial increases in visible vasculature suggesting that the TSP1-CD47 interaction limits acute vascular remodeling. Additional support for the primacy of the wound bed in determining FTSG survival was provided by cross-allograft transplant experiments. Regardless of the TSP1 or CD47 status of the FTSG itself, improved graft survival occurred if the wound bed was either inherently TSP1- or CD47-null or rendered effectively so through antibody ligation of or morpholino suppression of CD47. Preliminary experiments with autograft transplants of wild type FTSG to wild type wound beds in which only the wound beds were treated with a CD47 monoclonal antibody or CD47 morpholino similarly demonstrated increased survival of skin grafts (data not shown). Therefore, skin graft survival is primarily determined by the status of the wound bed vis a vis functioning CD47 and TSP1. Interfering with TSP1-CD47 interactions or decreasing the total number of such interactions through temporary receptor suppression is sufficient to significantly enhance skin graft survival and take. Since all animals were extensively backcrossed to the same C57Bl/6 background, T cell rejection would not be expected to be a complicating factor.
TSP1 has also been found to be important in tumor growth and metastasis 46, 48. Overexpression of TSP1 in cancer cells has been found to slow their growth and spread 49. Though TSP1-null animal do not demonstrate increased rates of tumor formation, crossing TSP1-null mice with tumor prone mice such as p53 nulls, enhances tumor progression 50, 51. Targeting either TSP1 or CD47 for the brief intervals employed in the present studies would not be expected to increase rates of tumor formation in the treated tissues. Also, the protocols of this study were based on local-regional application of the therapeutic agents, making systemic side effects less likely. It remains to be seen if targeting TSP1 and CD47 for extended lengths of time increases tumor formation rates.
The clinical implications of the data presented herein are substantial. Skin graft necrosis and loss is a major source of morbidity and mortality worldwide. Though we found a modest increase in FTSG survival could be obtained by elevating tissue NO through orally administered ISDN, systemic delivery of such agents may have potential untoward side effects. More importantly, we demonstrate that engagement of CD47 with monoclonal antibody or suppression using a specific morpholino can dramatically improve FTSG survival. This again illustrates the underlying principle that TSP1-CD47 interactions limit the responsive range of NO whether it is produced endogenously or administered exogenously as a therapeutic agent. Because the morpholino we used was designed to hybridize with a sequence conserved in the human CD47 and was demonstrated to suppress CD47 in cultured human cells 32, this morpholino can be further tested for immediate clinical applications in improving skin graft survival and outcomes from burn wounds. A number of function modifying antibodies that recognize human CD47 are currently available and, with proper humanization, could also warrant clinical testing. Importantly, both therapeutic agents can be delivered directly to the wound bed, minimizing potential systemic side effects. Additionally, these therapeutic agents may have a role in the treatment of burns irrespective of skin grafting. A major impediment to proper healing of burn wounds is their initial ischemic state 10, 52. Given the demonstrated benefit of anti-CD47 therapy on ischemic tissue survival, direct application to the ischemic burn wound may promote increased perfusion of the burn wound and thereby limit the extent of tissue death, decreasing the overall magnitude, duration and need for reconstruction of the burn injury 53, 54.
Acknowledgments
SOURCES OF FUNDING This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (D.D.R., M.T., D.A.W.) and NIH grants HL54390 and GM57573 (W.A.F.).
Footnotes
DISCLOSURE W.A.F. is president of Vasculox, Inc.
References
- 1.Wong L, Munster AM. New techniques in burn wound management. Surg Clin North Am. 1993;73(2):363–371. doi: 10.1016/s0039-6109(16)45987-6. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EACP, Miller TR. Incidence and Economic Burden of Injuries in the United States. New York: Oxford University Press; 2006. [Google Scholar]
- 3.Quinney B, McGwin G, Jr, Cross JM, et al. Thermal burn fatalities in the workplace, United States, 1992 to 1999. J Burn Care Rehabil. 2002;23(5):305–310. doi: 10.1097/00004630-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Atiyeh BS, Gunn SW, Hayek SN. State of the art in burn treatment. World J Surg. 2005;29(2):131–148. doi: 10.1007/s00268-004-1082-2. [DOI] [PubMed] [Google Scholar]
- 5.Nolan WB. Acute management of thermal injury. Ann Plast Surg. 1981;7(3):243–251. [PubMed] [Google Scholar]
- 6.Quinby WC, Jr, Burke JF, Bondoc CC. Primary excision and immediate wound closure. Intensive Care Med. 1981;7(2):71–76. doi: 10.1007/BF01687263. [DOI] [PubMed] [Google Scholar]
- 7.Shuck JM. The use of homografts in burn therapy. Surg Clin North Am. 1970;50(6):1325–1335. doi: 10.1016/s0039-6109(16)39291-x. [DOI] [PubMed] [Google Scholar]
- 8.Oshima H, Inoue H, Matsuzaki K, et al. Permanent restoration of human skin treated with cultured epithelium grafting--wound healing by stem cell based tissue engineering. Hum Cell. 2002;15(3):118–128. doi: 10.1111/j.1749-0774.2002.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 9.Zhu ZX, Xu XG, Li WP, et al. Experience of 14 years of emergency reconstruction of electrical injuries. Burns. 2003;29(1):65–72. doi: 10.1016/s0305-4179(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 10.Pruitt BA., Jr The burn patient: II. Later care and complications of thermal injury. Curr Probl Surg. 1979;16(5):1–95. doi: 10.1016/s0011-3840(79)80009-x. [DOI] [PubMed] [Google Scholar]
- 11.Boyce ST, Warden GD. Principles and practices for treatment of cutaneous wounds with cultured skin substitutes. Am J Surg. 2002;183(4):445–456. doi: 10.1016/s0002-9610(02)00813-9. [DOI] [PubMed] [Google Scholar]
- 12.Hierner R, Degreef H, Vranckx JJ, et al. Skin grafting and wound healing-the "dermatoplastic team approach". Clin Dermatol. 2005;23(4):343–352. doi: 10.1016/j.clindermatol.2004.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Danikas D, Fullerton JK, Smith CE, Milner SM. A new method for securing skin grafts in pediatric patients. Plast Reconstr Surg. 2003;111(1):489. doi: 10.1097/00006534-200301000-00091. [DOI] [PubMed] [Google Scholar]
- 14.Sierra DH, Eberhardt AW, Lemons JE. Failure characteristics of multiple-component fibrin-based adhesives. J Biomed Mater Res. 2002;59(1):1–11. doi: 10.1002/jbm.1210. [DOI] [PubMed] [Google Scholar]
- 15.Subrahmanyam M. Early tangential excision and skin grafting of moderate burns is superior to honey dressing: a prospective randomised trial. Burns. 1999;25(8):729–731. doi: 10.1016/s0305-4179(99)00063-7. [DOI] [PubMed] [Google Scholar]
- 16.Unal S, Ersoz G, Demirkan F, et al. Analysis of skin-graft loss due to infection: infection-related graft loss. Ann Plast Surg. 2005;55(1):102–106. doi: 10.1097/01.sap.0000164531.23770.60. [DOI] [PubMed] [Google Scholar]
- 17.McCampbell B, Wasif N, Rabbitts A, et al. Diabetes and burns: retrospective cohort study. J Burn Care Rehabil. 2002;23(3):157–166. doi: 10.1097/00004630-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Good DJ, Polverini PJ, Rastinejad F, et al. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taraboletti G, Roberts D, Liotta LA, Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reed MJ, Puolakkainen P, Lane TF, et al. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41(10):1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- 21.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12(5):634–640. doi: 10.1016/s0955-0674(00)00143-5. [DOI] [PubMed] [Google Scholar]
- 22.Isenberg JS, Calzada MJ, Zhou L, et al. Endogenous thrombospondin-1 is not necessary for proliferation but is permissive for vascular smooth muscle cell responses to platelet-derived growth factor. Matrix Biol. 2005;24(2):110–123. doi: 10.1016/j.matbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Lawler J, Detmar M. Tumor progression: the effects of thrombospondin-1 and -2. Int J Biochem Cell Biol. 2004;36(6):1038–1045. doi: 10.1016/j.biocel.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Ii M, Takenaka H, Asai J, et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98(5):697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 25.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53(4 Pt 1):503–514. [PubMed] [Google Scholar]
- 26.Isenberg JS. Nitric oxide modulation of early angiogenesis. Microsurgery. 2004;24(5):385–391. doi: 10.1002/micr.20051. [DOI] [PubMed] [Google Scholar]
- 27.Bolz SS, Vogel L, Sollinger D, et al. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation. 2003;107(24):3081–3087. doi: 10.1161/01.CIR.0000074202.19612.8C. [DOI] [PubMed] [Google Scholar]
- 28.McAllister RM, Laughlin MH. Vascular nitric oxide: effects of physical activity, importance for health. Essays Biochem. 2006;42:119–131. doi: 10.1042/bse0420119. [DOI] [PubMed] [Google Scholar]
- 29.Rush JW, Denniss SG, Graham DA. Vascular nitric oxide and oxidative stress: determinants of endothelial adaptations to cardiovascular disease and to physical activity. Can J Appl Physiol. 2005;30(4):442–474. doi: 10.1139/h05-133. [DOI] [PubMed] [Google Scholar]
- 30.Isenberg JS, Ridnour LA, Perruccio EM, et al. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005;102(37):13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71(4):785–793. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 32.Isenberg JS, Hyodo F, Matsumoto K, et al. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood. 2007;109(5):1945–1952. doi: 10.1182/blood-2006-08-041368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isenberg JS, Romeo MJ, Abu-Asab M, et al. Increasing survival of ischemic tissue by targeting CD47. Circ Res. 2007;100(5):712–720. doi: 10.1161/01.RES.0000259579.35787.4e. [DOI] [PubMed] [Google Scholar]
- 34.Lindberg FP, Gresham HD, Schwarz E, Brown EJ. Molecular cloning of integrin-associated protein: an immunoglobulin family member with multiple membrane-spanning domains implicated in alpha v beta 3-dependent ligand binding. J Cell Biol. 1993;123(2):485–496. doi: 10.1083/jcb.123.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ueda K, Nozawa M, Miyasaka M, et al. Sulfatide protects rat skin flaps against ischemia-reperfusion injury. J Surg Res. 1998;80(2):200–204. doi: 10.1006/jsre.1998.5443. [DOI] [PubMed] [Google Scholar]
- 36.Wood FM, Kolybaba ML, Allen P. The use of cultured epithelial autograft in the treatment of major burn injuries: a critical review of the literature. Burns. 2006;32(4):395–401. doi: 10.1016/j.burns.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Atiyeh BS, Hayek SN, Gunn SW. New technologies for burn wound closure and healing--review of the literature. Burns. 2005;31(8):944–956. doi: 10.1016/j.burns.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 38.Hochberg J, Raman M, Cilento E, et al. Development and evaluation of an in vivo mouse model for studying myocutaneous flap microcirculation and viability before and after suturing or stapling. Int J Microcirc Clin Exp. 1994;14(1–2):67–72. doi: 10.1159/000178209. [DOI] [PubMed] [Google Scholar]
- 39.Israeli D, Zhang WX, Senderoff DM, et al. Use of urokinase during secondary ischemia in experimental skin flaps. Ann Plast Surg. 1994;32(3):305–309. doi: 10.1097/00000637-199403000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Yan H, Peng X, Huang Y, et al. Effects of early enteral arginine supplementation on resuscitation of severe burn patients. Burns. 2007;33(2):179–184. doi: 10.1016/j.burns.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Komorowska-Timek E, Timek TA, Brevetti LS, et al. The effect of single administration of vascular endothelial growth factor or L-arginine on necrosis and vasculature of the epigastric flap in the rat model. Br J Plast Surg. 2004;57(4):317–325. doi: 10.1016/j.bjps.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 42.Cordeiro PG, Mastorakos DP, Hu QY, Kirschner RE. The protective effect of L-arginine on ischemia-reperfusion injury in rat skin flaps. Plast Reconstr Surg. 1997;100(5):1227–1233. doi: 10.1097/00006534-199710000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Gatti JE, Brousseau DA, Silverman DG, LaRossa D. Intravenous nitroglycerin as a means of improving ischemic tissue hemodynamics and survival. Ann Plast Surg. 1986;16(6):521–526. doi: 10.1097/00000637-198606000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Gribbe O, Samuelson UE, Wiklund NP. Effects of nitric oxide synthase inhibition on blood flow and survival in experimental skin flaps. J Plast Reconstr Aesthet Surg. 2007;60(3):287–293. doi: 10.1016/j.bjps.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 45.Topp SG, Zhang F, Chatterjee T, Lineaweaver WC. Role of nitric oxide in surgical flap survival. J Am Coll Surg. 2005;201(4):628–639. doi: 10.1016/j.jamcollsurg.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Bornstein P, Agah A, Kyriakides TR. The role of thrombospondins 1 and 2 in the regulation of cell-matrix interactions, collagen fibril formation, and the response to injury. Int J Biochem Cell Biol. 2004;36(6):1115–1125. doi: 10.1016/j.biocel.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Isenberg JS, Ridnour LA, Dimitry J, et al. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281(36):26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 48.Adams JC. Thrombospondin-1. Int J Biochem Cell Biol. 1997;29(6):861–865. doi: 10.1016/s1357-2725(96)00171-9. [DOI] [PubMed] [Google Scholar]
- 49.Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, et al. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994;54(24):6504–6511. [PubMed] [Google Scholar]
- 50.Lawler J, Miao WM, Duquette M, et al. Thrombospondin-1 gene expression affects survival and tumor spectrum of p53-deficient mice. Am J Pathol. 2001;159(5):1949–1956. doi: 10.1016/S0002-9440(10)63042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ren B, Yee KO, Lawler J, Khosravi-Far R. Regulation of tumor angiogenesis by thrombospondin-1. Biochim Biophys Acta. 2006;1765(2):178–188. doi: 10.1016/j.bbcan.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 52.Foley FD. Pathology of cutaneous burns. Surg Clin North Am. 1970;50(6):1201–1210. doi: 10.1016/s0039-6109(16)39280-5. [DOI] [PubMed] [Google Scholar]
- 53.Pruitt BA., Jr Complications of thermal injury. Clin Plast Surg. 1974;1(4):667–691. [PubMed] [Google Scholar]
- 54.Wang HJ, Chen TM, Chow LS, et al. Recipient bed vascularity and the survival of ischaemic flaps. Br J Plast Surg. 1997;50(4):266–271. doi: 10.1016/s0007-1226(97)91158-9. [DOI] [PubMed] [Google Scholar]


