Abstract
Glutamine, the most abundant amino acid in the bloodstream, is the preferred fuel source for enterocytes and plays a vital role in the maintenance of mucosal growth. The molecular mechanisms regulating the effects of glutamine on intestinal cell growth and survival are poorly understood. Here, we show that addition of glutamine (1 mmol/L) enhanced rat intestinal epithelial (RIE-1) cell growth; conversely, glutamine deprivation increased apoptosis as noted by increased DNA fragmentation and caspase-3 activity. To delineate signaling pathways involved in the effects of glutamine on intestinal cells, we assessed activation of extracellular signal-related kinase (ERK), protein kinase D (PKD) and phosphatidylinositol 3-kinase (PI3K)/Akt, which are important pathways in cell growth and survival. Addition of glutamine activated ERK and PKD in RIE-1 cells after a period of glutamine starvation; inhibition of ERK, but not PKD, increased cell apoptosis. Conversely, glutamine starvation alone increased phosphorylated Akt; inhibition of Akt enhanced RIE-1 cell DNA fragmentation. The role of ERK was further delineated using RIE-1 cells stably transfected with an inducible Ras. Apoptosis was significantly increased following ERK inhibition despite Ras activation. Taken together, these results identify a critical role for the ERK signaling pathways in glutamine-mediated intestinal homeostasis. Furthermore, activation of PI3K/Akt during periods of glutamine deprivation likely occurs as a protective mechanism to limit apoptosis associated with cellular stress. Importantly, our findings provide novel mechanistic insights into the anti-apoptotic effects of glutamine in the intestine.
Keywords: RIE-1 cells, enterocytes, extracellular signal-related kinase, apoptosis, Protein kinase D, cell proliferation, phosphatidylinositol 3-kinase
INTRODUCTION
The mucosal epithelium of the small bowel is a dynamic tissue that undergoes a continuous process of proliferation and self-renewal (25). This renewal process, consisting of intestinal cell proliferation, differentiation and eventual apoptosis (ie, programmed cell death), is tightly regulated by a variety of factors including luminal nutrients, hormones, growth factors and cytokines (30). Withdrawal of luminal nutrients (eg, use of total parenteral nutrition or liquid elemental diets) and growth factors or disuse of the small bowel, even for short periods of time, leads to atrophy of the epithelial layer and alterations in intestinal absorptive function (41). These findings suggest an important role for specific dietary components in promoting and maintaining intestinal epithelial health.
Glutamine, the most abundant free amino acid in the bloodstream, is the primary fuel source for enterocytes and is essential for gut homeostasis and health. Glutamine, traditionally termed a non-essential amino acid, is now considered “conditionally essential” for the small bowel mucosa since consumption exceeds the rate of production during high catabolic states (eg, trauma, sepsis, post-surgery). Glutamine is an essential component for numerous metabolic functions including acid-base homeostasis, gluconeogenesis, nitrogen transport and synthesis of proteins and nucleic acids (28). Enteral glutamine is thought to stimulate intestinal mucosal protein synthesis and protect against apoptosis. In vivo animal and clinical studies have shown that glutamine deprivation leads to villous atrophy, mucosal ulcerations and cell necrosis (19). Previous studies have demonstrated that the addition of glutamine to total parenteral nutrition (TPN) and liquid elemental diets reduces gut mucosal atrophy (8, 13). The molecular mechanisms by which glutamine promotes cell survival and prevents apoptosis in the small bowel mucosa have not been well defined.
Major signaling pathways that contribute to cell growth and survival include mitogen-activated protein kinases (MAPKs) and phosphatidylinositol 3-kinase (PI3K) (2, 9, 10, 24, 32). These pathways are activated in a cascade-like fashion by numerous growth factors. Once activated, they phosphorylate various downstream substrates eliciting specific cellular responses. At least three MAPKs have been identified in mammalian cells: extracellular signal-related kinase (ERK or p42/p44 MAPK), c-Jun amino-terminal kinase (JNK) and p38 MAPK (17). ERK is an important signal pathway for DNA synthesis, cell proliferation and anti-apoptosis in numerous cell lines (17, 32). JNK and p38 are considered to be stress-related kinases and activation often leads to apoptosis (18). PI3K is a ubiquitous lipid kinase comprising a large and complex family with multiple subunits and isoforms (2, 9, 10, 20, 43). Together these subunits catalyze upstream effectors which, in turn, phosphorylate (ie, activate) Akt kinase. Previous studies have shown that PI3K activation is closely associated with the proliferative activity of intestinal mucosa; treatment of mice with PI3K inhibitors (eg, wortmannin) blocked PI3K activity and attenuated intestinal mucosal proliferation associated with refeeding after a 48 hour fast (35). Protein kinase D (PKD) is a novel protein kinase which is structurally and functionally distinct from the PKC family (29, 39). Oxidative stress has been shown to activate PKD in intestinal epithelial cells and appears to play a protective role in cell survival (38). Although there are reports documenting the proliferative or protective effects of the MAPKs, PI3K and PKD in the intestine, little is known regarding the molecular mechanisms contributing to glutamine-mediated intestinal cell proliferation and survival. Therefore, the purpose of our current study was to investigate possible signal transduction pathways that are responsible for the effects of glutamine in intestinal cells.
MATERIALS & METHODS
Materials
The anti-phospho-ERK (1/2), anti-phospho-PKD (Ser916), anti-phospho-Akt, anti-phospho-JNK and anti-phospho-p38 antibodies and Cell Lysis Buffer were from Cell Signaling Technology (Beverly, MA). The secondary antibodies were from Pierce (Rockford, IL). The enhanced chemiluminenescence (ECL) system for Western immunoblot analysis was from Amersham (Arlington Heights, IL). The concentrated protein assay dye reagent was from Bio-Rad (Hercules, CA). Tissue culture media and reagents were from Mediatech (Herndon, VA). MEK inhibitors UO126 and PD98059 were from Promega (Madison, WI) and Biomol International (Plymouth Meeting, PA), respectively. PKCμ/PKD and non-target control (NTC) siRNA was synthesized by Custom SMARTPool siRNA Design Service of Dharmacon, Inc. (Lafayette, CO). Isopropyl-1-thio-β-D-galactopyranoside (IPTG) was purchased from Life Technologies, Inc (Gaithersburg, MD). The In Vitro Toxicology Assay Kit (Sulforhodamine B), the PI3K inhibitor wortmannin and all other reagents of molecular biology grade were purchased from Sigma Chemical Company (St. Louis, MO). The Cell Prolifation Kit and Cell Death Detection ELISAPlus were from Roche Applied Sciences (Indianapolis, IN). The Caspase-3 Activity Kit was from R&D Systems (Minneapolis, MN). Annexin V-FITC Apoptosis Detection Kit was from Calbiochem (San Diego, CA).
Cell culture and transfection
Rat intestinal epithelial (RIE-1) cells and RIE-iRas cells were used for all experiments. RIE-1 cells, a nontransformed, crypt-like cell line derived from rat jejunum, were a generous gift from Dr. Kenneth D. Brown (Cambridge Research Station, Babraham, Cambridge, U.K.) (4). RIE-1 cells, passages 15 – 35, were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS) in 5% CO2 at 37°C. RIE-iRas cells described by Sheng et al (33) have an inducible activated Ha-Rasval12 cDNA. The Ha-RasVal12 cDNA, generated by using LacSwitch eukaryotic expression system, is under the transcriptional control of the Lac operon in RIE-1 cells. RIE-iRas cells were used between passages 5 – 25 and maintained in DMEM containing 10% FBS, 400 μg/ml G418 sulphate and 150 μg/ml hygromycin B in 5% CO2 at 37°C. IPTG (5 mM) was used to induce Ha-ras expression. All experiments were conducted in serum-free media and performed on three separate occasions.
For siRNA studies, 3×106 RIE-1 cells were transfected by electroporation (400V, 500μF) using GenePulser XCell (Bio-Rad, Hercules, CA). Following electroporation, cells were seeded in 60mm dishes for 24 h prior to use in experiments. Cells were then washed with PBS and deprived of glutamine for 24 h; glutamine (1 mmol/L) or vehicle (control) was then readded and cells harvested 24 h later. All experiments were conducted in serum-free media.
Proteinextraction and Western blotting
RIE and RIE-iRas cells were plated (2×106 cells/100 mm plate) 24 h prior to experiments and grown to 90 – 95% confluence. Cells were then washed and deprived of glutamine for 24 h; glutamine (1 mmol/L) or vehicle (control) was then readded and cells were harvested over a time course (0-24 h). For inhibitor studies, cells were glutamine deprived for 24 h and then treated with MEK inhibitors (UO126 [10 μM] or PD98059 [20 μM]) or vehicle in the presence or absence of glutamine. After treatment, cells were washed with PBS and lysed using cell lysis buffer. Protein concentrations were calculated using the method described by Bradford (6). Equal amounts of protein were then resolved on NuPAGE Bis-Tris gels and electrophoretically transferred to polyvinylidene difluoride membranes; the membranes were incubated with primary antibodies overnight at 4°C followed by secondary antibodies conjugated with horseradish peroxidase as we have previously described (44). Membranes were developed using the ECLplus detection system.
MTT cell viability assay
Cell proliferation was determined by assaying the reduction of MTT to formazan as we have previously described (12). Briefly, cells were plated in 96-well plates (100 μl: 5,000 cells/well; 6 wells per treatment) 24 h before treatment. After a 24 h treatment, tetrazolium salts from the Cell Proliferation Kit were added and the production of blue formazan produced by viable cells was measured at an absorbance of 570nm (against a reference of 650 nm) per the manufacturer's protocol.
In vitro toxicology assay (Sulforhodamine B)
Total cellular biomass was determined by measuring the amount of Sulforhodamine B dye incorporated by viable cells as described previously (37). Briefly, cells were plated in 96-well plates (100 μl: 10,000 cells/well; 6 wells per treatment) 24 h before treatment. After a 24 h treatment, cells were fixed in 50% TCA at 4°C for 1 h, washed, and air dried. Plates were stained with Sulforhodamine B for 30 min followed by a 10% acetic acid wash.. The incorporated dye in viable cells was then solubilized in 10 mM Tris base and measured at an absorbance of 570 nm per the manufacturer's protocol.
DNA fragmentation ELISA assay
RIE-1 cells (1×105 cells/well; 4 wells per treatment) were seeded in 24-well plates for 24 h as we have previously described (44). Cells were treated with various combinations of media (+/− glutamine and +/− FBS). For inhibitor studies, RIE-1 cells were grown in the presence or absence of glutamine and treated with MEK inhibitors (UO126 [10 μM] or PD98059 [20 μM]) or vehicle for 24 h. RIE-iRas cells were maintained in the presence or absence of glutamine and treated with UO126 (10 μM) or vehicle and IPTG (5 mM). After treatment, both adherent and floating cells were centrifuged, lysed and the cell lysate added to streptavidin-coated 96-well plates. DNA fragmentation (a measure of apoptosis) was quantitated by examination of cytoplasmic histone-associated DNA fragments (mononucleosomes and oligonucleosomes) using a Cell Death Detection ELISAplus kit following the manufacturer's protocol.
Caspase-3 colorimetric assay
RIE-1 cells were seeded in 100 mm plates (2×106 cells/plate; 2 plates per treatment) for 24 h before treatment. Cells were maintained in the presence or absence of glutamine (1 mmol/L) for 24 h. Both adherent and floating cells were collected, washed with PBS and lysed. The cell lysate was then analyzed for caspase-3 activity by the addition of a caspase-specific peptide conjugated with the color reporter molecule p-nitroanaline. Caspase-3 activity (as a measure of apoptosis) was then quantitated spectrophotometrically at a wavelength of 405 nm per the manufacturer's protocol. Results were expressed as fold change.
Annexin V-FITC apoptosis assay
RIE-1 cells (1×106 cells/plate; 3 plates per treatment) were seeded in 60mm plates for 24 h. Cells were maintained in the presence or absence of glutamine (1 mmol/L) for 24 h. For inhibitor studies, RIE-1 cells were maintained in the presence or absence of glutamine and simultaneously treated with either UO126 (10 μM), PD98059 (20 μM), wortmannin (100 nM) or vehicle. Both adherent and floating cells were collected; 5×105 cells were double stained with propidium iodide (PI) and annexin V-fluorescein isothiocyanate (FITC) as we have described previously (21). Cells were immediately analyzed by a two-channel fluorescent-activated cell scanner (FACS Canto; Becton Dickinson Co, Franklin Lake, NJ) per the manufacturer's protocol. The total percentage of apoptotic cells was measured by counting the number of FITC+ and FITC+/PI+ stained cells by flow cytometry (20,000 events/plate).
Statistical analysis
All experiments were repeated at least three times, and data are reported as means ± SE. Absorbance of cell proliferation (cell growth) and DNA fragmentation (cell death) were analyzed using analysis of variance for a randomized complete block design with a factorial treatment arrangement. The factors were glutamine (absent or present) and UO126 or PD98059 (absent or present). For glutamine concentration studies, the two factors were glutamine concentration (5 levels + control) and incubation time (24 and 48 h). The block represented each set of experiments repeated 3 times. Bartlett's test was used to test homogeneity of 4 error terms (the interaction terms with the block). If all 4 error terms were homogeneous, they were pooled into one error term and the data were analyzed as a simple randomized complete block design using PROC GLM in SAS®, Release9.1 (31). If, on the other hand, all 4 error terms were not homogeneous, the data were analyzed as a three-factor mixed model using PROC MIXED with LSMEANS option and Satterthwaite approximation for the denominator degrees of freedom in SAS®. For the FBS study, absorbance of cell proliferation and DNA fragmentation were analyzed using analysis of variance for a two-factor factorial experiment with PROC GLM. The two factors were glutamine (absent or present) and FBS (absent or present). Caspase-3 activity was analyzed using a two-sample t test. Cell counts were analyzed using analysis of variance for a two-factor factorial experiment. The two factors were glutamine (absent or present) and incubation time (24 and 48 h). The main effects were assessed at the 0.05 level of significance. Since the interaction was expected, the interaction was assessed at the 0.15 level of significance. Fisher's least significant difference procedure was used for multiple comparisons with Bonferroni adjustment for the number of comparisons. When the numbers of measurements among blocks (or experiments) were unbalanced, the least square means was used for the multiple comparisons.
RESULTS
Glutamine supplementation stimulates cell growth and prevents apoptosis
We first determined the effects of varying glutamine concentrations on cell growth. Physiological concentrations of glutamine in the small intestine are reported to be approximately 1 mmol/L (11, 16, 27); supplementation of glutamine at 1 mmol/L showed a significant increase in RIE-1 cell proliferation and was therefore used for all subsequent experiments (data not shown).
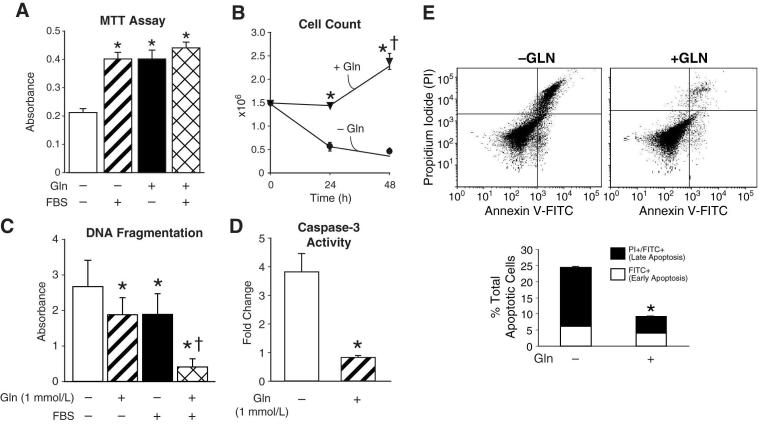
Next, we compared cell proliferation of cells deprived of glutamine and FBS with cells grown in the presence of either glutamine or FBS (5%) in order to establish that FBS starved cells were viable and able to proliferate, thus eliminating any possible effects of other growth factors on cell survival. In glutamine and serum starved cells, proliferation was significantly reduced when compared to glutamine, FBS or a combination of both factors (Fig. 1A). There was no statistical difference in cell proliferation of cells grown in either glutamine alone, FBS alone or glutamine and FBS. To confirm the effect of glutamine on cell proliferation, we next determined total cell numbers. RIE-1 cells (1.5×106) were plated and grown in the presence or absence of glutamine for 24 and 48 h. The media was removed, live cells were gently washed twice with PBS, trypsinized, and counted using a Coulter Cell Counter (Fig. 1B). Cell numbers were unchanged at 24 h in cells maintained in glutamine; however, at 48 h there was an approximate 1.6-fold increase in cell numbers. Conversely, glutamine-deprived cells had an approximate 3- and 4-fold decrease in cell numbers at 24 and 48 h, respectively.
Figure 1. Glutamine deprivation reduces cell growth and increases apoptosis.
(A) RIE-1 cells were maintained in DMEM with or without glutamine (1 mmol/L) and FBS (5%). Cell proliferation was measured by MTT assay (* = p<0.05 vs. control (Gln-deprivation)). (B) RIE-1 cells were grown in the presence or absence of glutamine for 24 and 48 h. Cells were then trypsinized and counted using a Coulter Particle Counter (* = p<0.05 vs. control [Gln-deprivation] at 24 and 48 h; † = p<0.05 vs. Gln-treated at 24 h). (C) RIE-1 cells were maintained in the presence or absence of glutamine (1 mmol/L) and FBS (5%) for 24 h. DNA fragmentation ELISA was then performed as a measure of apoptosis (* = p<0.05 vs. control [Gln-deprivation]; † = p<0.05 vs. control, Gln-treated and Gln-deprived/FBS treated). (D) RIE-1 cells were maintained in the presence or absence of glutamine for 24 h. Caspase-3 activity was then measured as a marker of apoptosis. Results are expressed as fold-change compared to cells maintained in the presence of glutamine (* = p<0.05 vs. control [Gln-deprived]). (E) RIE-1 cells were maintained in the presence or absence of glutamine for 24 h. Following treatment, the total percentage of apoptotic cells were determined by annexin V-FITC staining (* = p<0.05 vs. control [Gln-deprivation]). (Results are from three separate experiments; experiments were conducted in serum-free media unless otherwise noted).
Previous studies have demonstrated that glutamine deprivation induces apoptosis (22, 23). Therefore, we examined DNA fragmentation as a marker of apoptosis in the presence or absence of glutamine and FBS. Glutamine deprivation significantly increased apoptosis when compared to cells grown in the presence of glutamine (Fig. 1C). There was no statistical difference in levels of apoptosis when cells were grown in the presence of either glutamine or FBS; however, cells grown in the presence of both factors had significantly lower levels of apoptosis suggesting a synergistic effect of these two growth factors on RIE-1 cell survival. We next analyzed caspase-3 levels to further confirm increased apoptosis in glutamine-starved cells. Caspase-3 is an intracellular cysteine protease that upon activation by apoptotic signals, acts on downstream targets resulting in cell death (22). Glutamine-deprived cells had an approximate 3.5-fold increase in caspase-3 levels when compared to cells maintained in the presence of glutamine (Fig. 1D). Finally, we analyzed apoptosis by annexin V-FITC staining. RIE-1 cells were double stained with annexin V-FITC and propidium iodide and analyzed by flow cytometry. Early apoptotic cells are stained with annexin V-FITC whereas late apoptotic cells stain with both annexin V-FITC and propidium iodide. RIE-1 cells maintained with or without glutamine demonstrated a similar percentage of cells undergoing early apoptosis (annexin V-FITC staining only; 6.2% vs. 4.0%); however, the percentage of cells staining for both annexin V-FITC and propidium iodide (late apoptosis) was significantly increased with glutamine deprivation compared to cells maintained in glutamine (18.2 % vs. 5.1%) (Fig. 1E). Taken together, our results confirm that physiologic concentration of glutamine stimulates RIE-1 cell growth and that glutamine deprivation induces apoptosis in RIE-1 cells.
Glutamine increases expression of phosphorylated ERK and PKD(916); phosphorylated Akt levels are suppressed
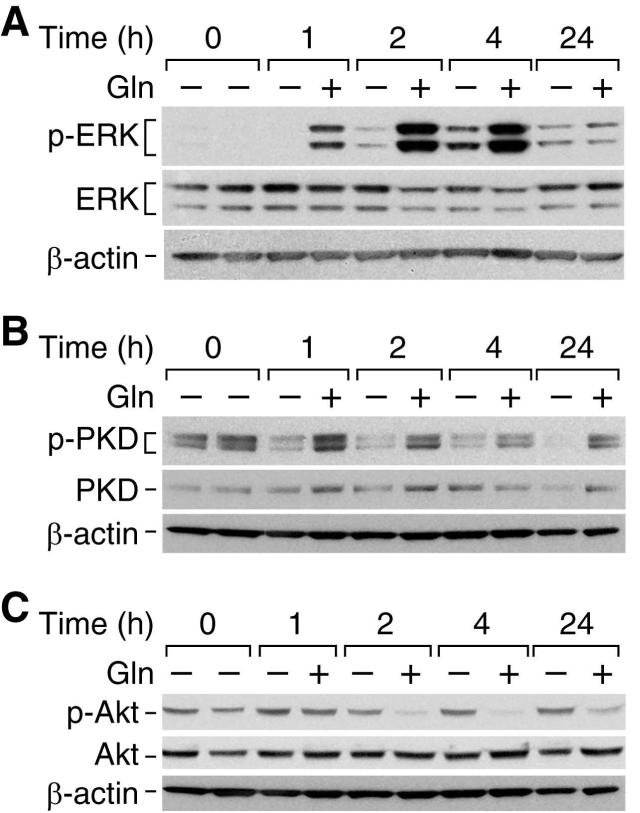
To investigate the molecular mechanisms responsible for the proliferative effects of glutamine in the intestine, we determined effects of glutamine on the activation of signaling pathways known to be either proliferative and/or protective in the gut. RIE-1 cells were deprived of glutamine for 24 h and then supplemented with glutamine (1 mmol/L) or vehicle (control) for a further 24 h. Glutamine supplementation increased phosphorylated ERK at 1, 2 and 4 h (Fig. 2A). There were no changes in total ERK or β-actin levels (protein loading controls). Additionally, PKD, a novel class of proteins that has been shown to be important in cell survival and proliferation, was activated by glutamine supplementation. Phosphorylated PKD(916) levels were increased at 1, 2, 4 and 24 h with minimal to no changes in total PKD levels or β-actin levels (Fig. 2B). In both instances, the experiment was repeated at 8 and 12 h time points with a similar induction of phosphorylated ERK and phosphorylated PKD after glutamine treatment (data not shown).
Figure 2. Glutamine-induced protein changes following 24 h starvation.
Following 24 h glutamine-deprivation, RIE-1 cells were treated with glutamine (1 mmol/L) or vehicle and protein collected over a time course. Protein phosphorylation was detected by Western blotting using anti-phospho-ERK (Thr202/Tyr 204) (A), anti-phospho-PKD (Ser916) (B), and anti-phospho-Akt (Ser473) (C) antibodies. The membranes were stripped and reprobed with anti-ERK, anti-PKD and anti-Akt antibodies, respectively to assess total protein levels. Finally, membranes were stripped and probed with β-actin as a loading control. (Representative Western blots shown following 3 separate experiments; all experiments were conducted in serum-free media).
The PI3K/Akt pathway has been reported to play a vital role in cell survival and proliferation (9, 10, 24). Following 24 h of glutamine starvation, RIE-1 cells refed with vehicle (ie, no glutamine) demonstrated increased levels of phosphorylated Akt at all time points (Fig. 2C). Conversely, levels of phosphorylated Akt decreased following the addition of glutamine starting at 2 h and remained decreased at all subsequent time points. Total Akt and β-actin levels were unchanged.
The p38 and JNK signaling pathways have been implicated in apoptotic signaling (18). However, in multiple experiments, we noted minimal to no changes in phosphorylated p38 or JNK levels in either the control or re-fed cells (data not shown).
Inhibition of PI3K/Akt following glutamine-deprivation increases apoptosis
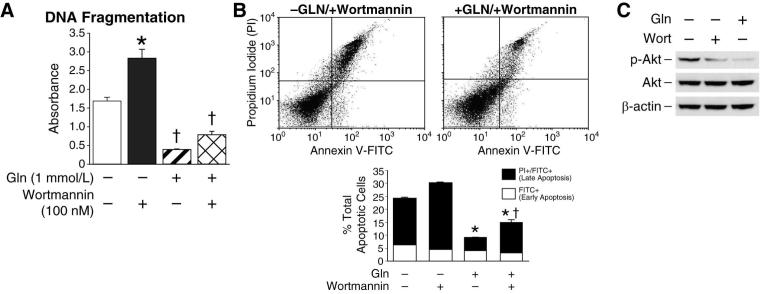
Glutamine-deprived RIE-1 cells demonstrated increased phosphorylation of Akt following prolonged (>24 h) starvation. To examine the role of Akt in RIE-1 cell survival, cells were treated with the selective PI3K inhibitor wortmannin. RIE-1 cells were maintained in the presence or absence of glutamine for 24 h and then treated with wortmannin (100 nM) or vehicle for a further 3 h; DNA fragmentation was then assessed. Similar to our previous results, glutamine deprivation significantly increased DNA fragmentation compared with cells maintained in glutamine; inhibition of PI3K with wortmannin further enhanced DNA fragmentation compared with glutamine deprivation alone (Fig. 3A). The addition of wortmannin to RIE-1 cells maintained in glutamine did not increase apoptosis compared to cells treated with glutamine and vehicle. To confirm apoptosis, cells were analyzed by annexin V flow cytometry. Glutamine-deprived RIE-1 cells treated with wortmannin (100 nM) demonstrated a significant increase in the percentage of cells staining for annexin V-FITC and propidium iodide (late apoptosis) compared to glutamine-deprived only (25.7% vs. 18.2%, respectively) and cells maintained in glutamine (25.7% vs. 5.1%, respectively) (Fig. 3B). Levels of apoptosis, as measured by annexin V flow cytometry, were similar to levels detected by DNA fragmentation. Western blotting confirmed that phosphorylated Akt was inhibited following wortmannin treatment (Fig. 3C). These results suggest that cellular stress (ie, glutamine deprivation) induces Akt in intestinal cells, likely as a protective mechanism to limit cell death; inhibition of PI3K/Akt enhances apoptosis. The PI3K/Akt pathway does not appear to be mediating the proliferative effects of glutamine as noted by the inhibition of phosphorylated Akt expression with glutamine treatment.
Figure 3. Akt inhibition increases RIE-1 cell apoptosis following glutamine-deprivation.
(A) RIE-1 cells were maintained in the presence or absence of glutamine (1 mmol/L) for 24 h. Cells were then treated with the PI3K inhibitor wortmannin (100 nM) or vehicle for an additional 3 h; DNA fragmentation ELISA was then performed (* = p<0.05 vs. control [Gln-deprived]; † = p<0.05 vs. Gln-deprived + wortmannin). (B) RIE-1 cells were maintained in the presence or absence of glutamine for 24 h. Cells were then treated with wortamannin (100 nM) or vehicle for an additional 3 h. Following treatment, the total percentage of apoptotic cells were determined by annexin V-FITC staining (* = p<0.05 vs. control [Gln-deprivation]; † = p<0.05 vs. Gln-deprivation + wortmannin). (C) RIE-1 cells were maintained in the presence or absence of glutamine for 24 h. Cells were then treated with wortmannin (100 nM) or vehicle for an additional 3 h. Akt phosphorylation was detected by Western blotting using anti-phospho-Akt (Ser473) antibody. The membranes were stripped and re-probed with anti-Akt antibody to measure total Akt protein levels. Membranes were probed with β-actin as loading control. All experiments (A – C) were performed in serum-free media. (Western blots were representative of 3 separate experiments. Results from DNA fragmentation ELISA and annexin V flow cytometry were performed in triplicate on three separate occasions).
ERK, but not PKD, inhibition increases RIE-1 cell apoptosis
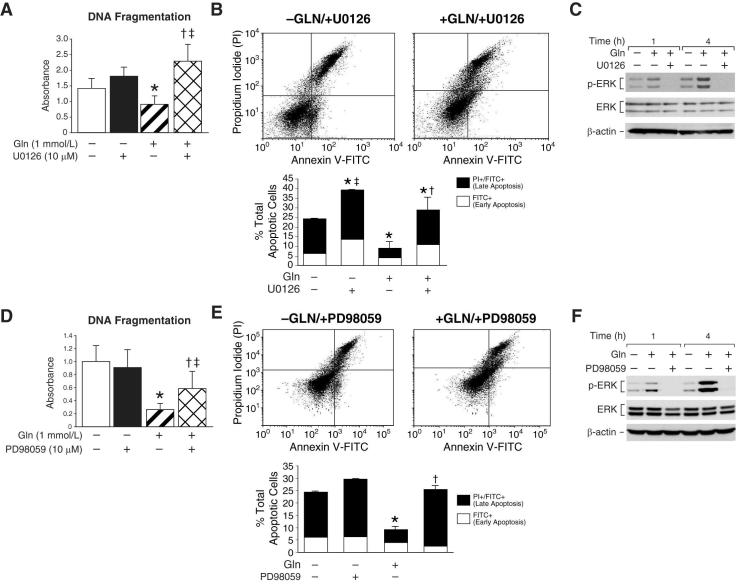
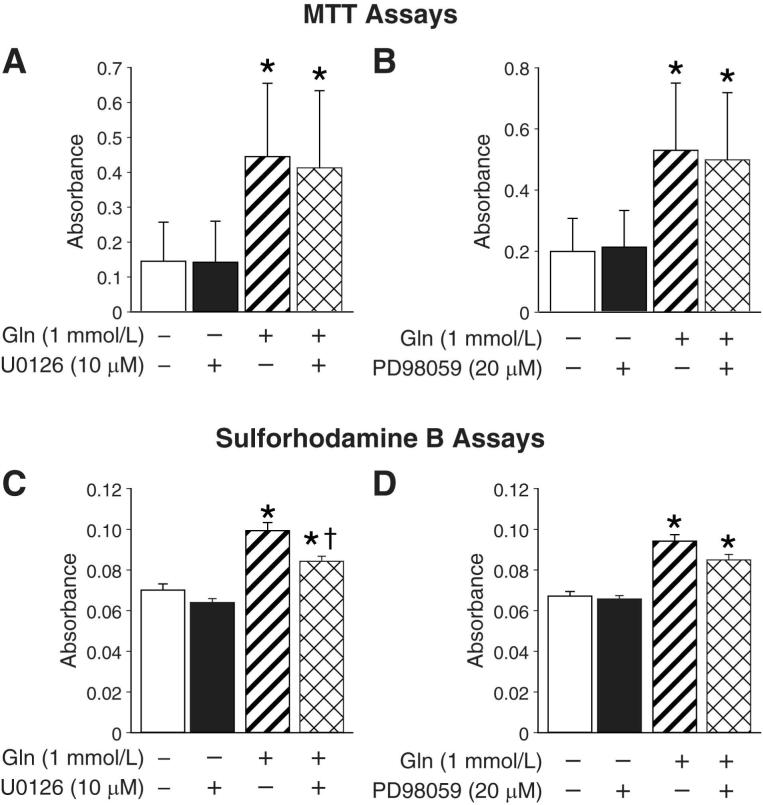
The strong activation of ERK by glutamine treatment led us to postulate that this protein may play a critical role in intestinal cell survival mediated by glutamine. To test this hypothesis, RIE-1 cells were treated with the selective MEK inhibitors UO126 or PD98059. First, cells were maintained in the presence or absence of glutamine and treated with UO126 (10 μM) or vehicle (DMSO). After 24 h, DNA fragmentation was measured. In the presence of glutamine, UO126 treatment significantly increased apoptosis when compared to vehicle-treated RIE-1 cells (Fig. 4A). Glutamine-deprived cells treated with UO126 also demonstrated increased DNA fragmentation when compared to glutamine-deprived cells alone; however, when the two UO126 treatment groups were compared, RIE-1 cells maintained in glutamine demonstrated significantly higher levels of DNA fragmentation. Increased apoptosis was confirmed by annexin V flow cytometry. RIE-1 cells maintained in the presence of glutamine and treated with UO126 demonstrated a significant increase in the percentage of cells undergoing apoptosis when compared to cells maintained in glutamine only (29% vs. 91%, respectively) (Fig. 4B); the overall percentage of apoptotic cells was similar to that of glutamine-deprived cells. Western blotting confirmed that phosphorylated ERK was blocked in UO126 treated cells (Fig. 4C). To confirm these results, we repeated the experiments using the MEK inhibitor PD98059. Similar to our results with UO126, the addition of PD98059 (20 μM) in the presence of glutamine significantly increased DNA fragmentation (Fig. 4D). Increased apoptosis was confirmed with annexin V flow cytometry. Again, the percentage of cells undergoing late apoptosis was increased in cells maintained in glutamine treated with PD98059 compared to cells maintained in the presence of glutamine only (21.4% vs. 5.1%, respectively) (Fig. 4E). Western blotting confirmed that phosphorylated ERK was blocked in PD98059 treated cells (Fig. 4F).
Figure 4. ERK inhibition induces apoptosis in glutamine treated RIE-1 cells.
(A) RIE-1 cells were maintained in the presence or absence of glutamine (1 mmol/L) and treated with UO126 (10 μM) or vehicle for 24 h; DNA fragmentation ELISA was then performed (* = p<0.05 vs. control [Gln-deprived]; † = p<0.05 vs. Gln-treated without UO126; ‡ = p<0.05 vs. Gln-deprived + UO126). (B) RIE-1 cells were again maintained in the presence or absence of glutamine (1 mmol/L) and treated with UO126 (10 μM) or vehicle for 24 h. Following treatment, the total percentage of apoptotic cells were determined by annexin V-FITC staining (* = p<0.05 vs. control [Gln-deprivation]; † = p<0.05 vs. Gln-treated alone; ‡ = p<0.05 vs. Gln-treated + UO126). (C) RIE-1 cells were maintained in the presence or absence of glutamine (1 mmol/L) for 24 h. Cells were then treated with UO126 (10 μM) or vehicle for a further 4 h. Cells were lysed and protein collected at 1 and 4 h. Protein phosphorylation was detected by Western blotting using anti-phospho-ERK (Thr202/Tyr204) antibody. Membranes were stripped and re-probed with anti-ERK antibody to measure total ERK protein levels and β-actin as loading control. (D) Similar to experiments in A, cells maintained in the presence or absence of glutamine were treated with PD98059 (20 μM) or vehicle for 24 h; DNA fragmentation ELISA was performed (* = p<0.05 vs. control [Gln-deprived]; † = p<0.05 vs. Gln-treated without PD98059; ‡ = p<0.05 vs. Gln-deprived + PD98059). (E) Similar to experiments in B, cells were maintained in the presence or absence of glutamine and simultaneously treated with PD98059 (20 μM) for 24 h. Following treatment, the total percentage of apoptotic cells were determined by annexin V-FITC staining (* = p<0.05 vs. control [Gln-deprivation]; †= p<0.05 vs. Gln-treated without PD98059). (F) RIE-1 cells were maintained in the presence or absence of glutamine for 24 h. Cells were then treated with PD98059 (20 μM) for a further 4h. ERK phosphorylation was detected by Western blotting using anti-phospo-ERK antibody. The membranes were stripped and re-probed with anti-ERK antibody to measure total ERK protein levels and β-actin as loading control. (Western blots in C and F were representative of 3 separate experiments. Results from DNA fragmentation ELISA and annexin V flow cytometry were performed in triplicate on three separate occasions).
PKD(916) activation was observed in RIE-1 cells following glutamine treatment. To better delineate the possible role of PKD(916) in RIE-1 cell survival, cells were transfected with small interfering RNA (siRNA) targeting PKCμ/PKD. Glutamine treatment decreased levels of DNA fragmentation despite PKD siRNA transfection; inhibition of PKD following siRNA transfection was confirmed by Western blotting although there was variability in the levels of protein suppression (see supplemental Figure 1).
RIE-1 cell proliferation is not affected by ERK inhibition
To examine the role of ERK inhibition on cell proliferation, RIE-1 cells were maintained in the presence or absence of glutamine and treated with either UO126 (10 μM), PD98059 (20 μM) or vehicle for 24 h. Cell proliferation was analyzed by MTT assay. Cells maintained in media containing glutamine and treated with UO126 or PD98059 did not show a significant difference in cell proliferation when compared to cells maintained in glutamine and treated with vehicle (Figs. 5A & 5B, respectively). Glutamine-deprived cells, regardless of the treatment (inhibitor or vehicle), showed a statistically significant decrease in cell proliferation. These results were confirmed by conducting identical experiments and measuring total cellular biomass (a marker of cell proliferation) using a Sulforhodamine B assay (Figs. 5C & 5D, respectively). Similar to our results with MTT assay, the addition of either UO126 or PD98059 to cells maintained in glutamine did not significantly reduce total cellular biomass. These results suggest that, although ERK plays an important role in preventing apoptosis, it has a minimal role in promoting cell proliferation in RIE-1 cells supplemented with glutamine.
Figure 5. ERK inhibition does not affect glutamine-mediated RIE-1 cell proliferation.
(A) RIE-1 cells were maintained in the presence or absence of glutamine (1 mmol/L) and treated with UO126 (10 μM) or vehicle for 24 h. MTT cell proliferation assays were then performed (* = p<0.05 vs. Gln-deprived cells ±UO126). (B) Similar experiments were performed using PD98059 (20 μM) (* = p<0.05 vs. Gln-deprived cells ± PD98059). (C) RIE-1 cells were maintained in the presence or absence of glutamine (1 mmol/L) and treated with UO126 (10 μM) or vehicle for 24 h. Total cellular biomass was determined by sulforhodamine B assay (* = p<0.05 vs. Gln-deprived cells ± UO126; † = p<0.05 vs. Gln-treated alone). (D) Experiments were repeated using PD98059 (20 μM) (* = p<0.05 vs. Gln-deprived cells ± PD98059). (All experiments were performed in serum-free media on 3 separate occasions).
ERK inhibition increases RIE-iRAS cell apoptosis
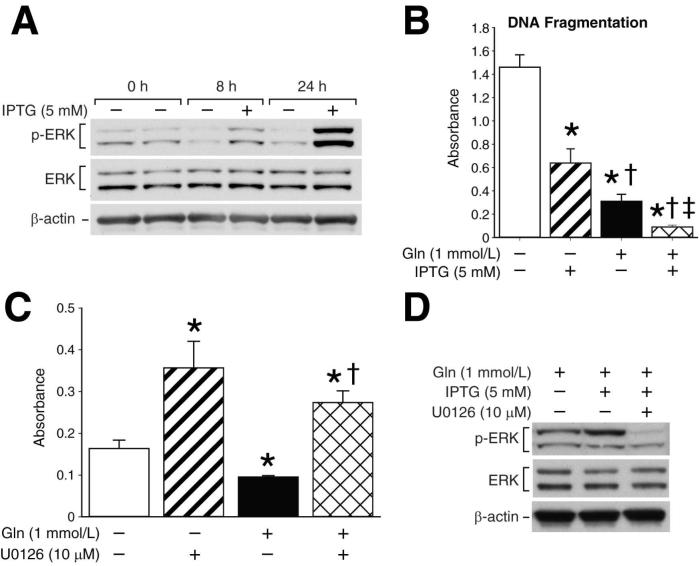
Finally, to further delineate the role of ERK in glutamine-mediated intestinal cell survival, we utilized RIE-iRas cells which have been engineered to express Ha-ras when treated with IPTG (33, 34); induction of Ha-ras increases ERK in a cascade-like fashion. Western blotting confirmed increased levels of phosphorylated ERK following IPTG treatment at 8 and 24 h (Fig. 6A). Based on our previous results, we hypothesized that activation of ERK following IPTG treatment in RIE-iRas cells would promote cell survival even in the absence of glutamine. To test this hypothesis, RIE-iRas cells were maintained in the presence or absence of glutamine and treated with IPTG (5 mM) or vehicle for 24 h. Increased DNA fragmentation was noted in glutamine-deprived RIE-iRas cells; the addition of IPTG significantly reduced apoptosis (Fig. 6B). In the presence of glutamine, the addition of IPTG significantly decreased apoptosis compared untreated cells (Fig. 6B). To further confirm the role of ERK in intestinal cell survival, we next treated RIE-iRas cells with UO126 in conjunction with IPTG. Cells were maintained in the presence or absence of glutamine and pretreated with UO126 (10 μM) or vehicle (DMSO); after 30 min, cells were treated with IPTG (5 mM) and DNA fragmentation was measured at 24 h. UO126 treatment significantly increased DNA fragmentation despite the presence of glutamine and IPTG (Fig. 6C). ERK inhibition was confirmed by Western blotting (Fig. 6D). Thus, by utilizing RIE-iRas cells with inducible Ha-ras, we have demonstrated that the induction of ERK protects intestinal cells from apoptosis despite the absence of glutamine. These results further confirm a critical role for ERK in glutamine-mediated intestinal cell survival.
Figure 6. Inhibition of Ha-ras induced ERK increases RIE-iRas cell apoptosis.
(A) RIE-iRas cells were maintained in the presence of glutamine with or without IPTG (5 mM) for 24 h. IPTG induces Ha-ras which in turn activates ERK expression. ERK phosphorylation was detected by Western blotting using anti-phospho-ERK (Thr202/Tyr204) antibody. Membranes were stripped and re-probed with anti-ERK antibody to assess total protein levels. Membranes were then probed with β-actin as a loading control. (B) RIE-iRAS cells were maintained in the presence or absence of glutamine and stimulated with IPTG (5 mM) or vehicle for 24 h; DNA fragmentation ELISA was then performed (* = p<0.05 vs. control; †= p<0.05 vs. Gln-deprived + IPTG; ‡ = p<0.05 vs. Gln-treated without IPTG). (C) RIE-iRas cells were maintained in the presence or absence of glutamine and pre-treated with UO126 (10 μM) or vehicle. Following 30 min of pretreatment, cells were subsequently treated with IPTG for a further 24 h; DNA fragmentation was then performed (* = p<0.05 vs. control [Gln-deprived + IPTG]; †= p<0.05 vs. Gln-treated + IPTG). (D) RIE-iRas cells were maintained in the presence of glutamine with or without IPTG (5 mM). Cells were simultaneously treated with UO126 (10 μM) or vehicle for 24 h. Cells were then collected and ERK phosphorylation was assessed by Western blotting using anti-phospho-ERK antibody. Membranes were stripped and re-probed with anti-ERK antibody to assess total protein levels and β-actin as a loading control. All experiments were performed in serum-free media. (Western blots shown in A and D are representative of 3 separate experiments. DNA Fragmentation ELISA results are from 3 separately performed experiments).
DISCUSSION
Despite numerous experimental and clinical studies demonstrating the importance of glutamine in the intestine, the molecular mechanisms by which it enhances intestinal cell growth have not been clearly elucidated. In our current study, we have demonstrated that activation of ERK is an important contributor to glutamine-mediated intestinal cell survival. Our findings implicating ERK as a critical signaling pathway for the effects of glutamine provide important mechanistic insights into the anti-apoptotic effects of glutamine in the intestine.
The MAPKs represent an important signal transduction pathway for the effects of growth factors and other proteins which ultimately affect cell proliferation, survival or apoptosis. We have demonstrated that glutamine treatment following a period of starvation results in the rapid activation of ERK, a MAPK protein which has been implicated in numerous and important pathways of the intestine including proliferation, cell survival and differentiation. Rhoades et al (27) previously reported ERK activation following a short period of glutamine treatment in IEC-6 cells. Our findings demonstrate that ERK activation occus following prolonged starvation. Furthermore, we have shown that activation of ERK functions as a cell survival mechanism to prevent apoptosis as opposed to enhancing proliferation. The anti-apoptotic effects of ERK mediated by glutamine concur with recent findings by Bhattacharya et al (3), who showed that inhibition of ERK increased apoptosis in polyamine depleted IEC-6 cells. Others have shown that ERK activation plays a critical anti-apoptotic role in intestinal cells during periods of cellular stress (14, 45, 46). Similar findings of increased ERK activation and the prevention of apoptosis have been reported using non-intestinal cell lines. For example, ERK activation protects renal epithelial cells and cardiomyocytes against oxidative injury and cell death (1, 15). Furthermore, Holmstrom et al (14) have demonstrated that ERK, in conjunction with NF-κB, protects Jurkat cells from Fas-induced apoptosis. Our in vitro results suggest that ERK activation, mediated by glutamine, plays a predominantly anti-apoptotic role in intestinal cells which are further supported by Sheng et al (35) who showed that increase levels of activated ERK did not correlate with increased proliferation or alter cell cycle progression in RIE cells.
The critical role for ERK activation in intestinal cells was further established using a novel RIE cell line stably transfected with an inducible Ras, thus leading to constitutive ERK activation. Inhibition of ERK by selective MEK inhibitors significantly increased apoptosis in these cells despite treatment with glutamine or the activation of Ras. Surprisingly, we did not note activation of either JNK or p38 in intestinal cells following glutamine treatment. Although JNK and p38 are recognized as the stress related kinases, it appears that ERK is the predominant MAPK signaling protein which contributes to the increased cell survival with glutamine treatment. Taken together, these results demonstrate that ERK plays a significant role in glutamine-mediated intestinal cell survival.
The PI3K/Akt pathway is critical for survival and proliferation of numerous cell types. We have previously shown that activation of PI3K/Akt is important for small bowel and colon mucosal proliferation after fasting and subsequent refeeding (35, 42). Our initial hypothesis was that glutamine-mediated survival occurred through the activation of PI3K. In contrast, we found that glutamine deprivation increased Akt activation; conversely, levels of activated Akt were decreased with glutamine supplementation. The addition of the selective PI3K inhibitor wortmannin further increased RIE-1 cell apoptosis in glutamine-deprived cells. These results indicate that PI3K activation occurs with nutrient deprivation, likely as a protective mechanism to limit further apoptosis associated with cellular stress. Although PI3K/Akt is commonly activated by a plethora of growth factors, similar to our results in intestinal cells, this activation can occur during periods of cell stress. Zhou et al (46) demonstrated that the PI3K/Akt pathway was activated in RIE-1 cells exposed to H202. In addition, the PI3K/Akt pathway interacts with the mammalian target of rapamycin (mTOR) pathway to regulate important cellular functions during periods of nutrient deprivation (eg, amino acid deprivation) (26). Our laboratory is currently delineating the potential role of the PI3K/Akt/mTOR pathway in glutamine-deprived intestinal cell survival.
Glutamine supplementation also activated phosphorylated PKD(916). PKD activation occurs in response to a number of stimuli and appears to play a role in cell proliferation and protecting cells from apoptosis during oxidative stress (38-40). Other investigators have reported that PKD activation stimulates the ERK pathway (5, 7, 36); however, we did not observe changes in PKD(916) levels with ERK inhibition. Furthermore, we did not consistently detect changes in PKD(744/748) with glutamine treatment. Activation of serine 744 and 748 (the so called “activation loop”) has been reported to be a prerequisite for full PKD kinase activity and PKD does not autophoshorylate its own activation loop (40). Further studies will be necessary to correlate our observed findings of increased PKD(916) activation in glutamine-mediated intestinal cell survival. Although PKD(916) is activated with glutamine treatment, this activation appears not to be required for enhanced cell survival.
In conclusion, we have demonstrated a critical role for ERK in glutamine-mediated intestinal cell survival; inhibition of ERK increased levels of apoptosis. Conversely, the induction of ERK in RIE-iRas cells significantly decreased apoptosis with glutamine deprivation; this protective effect was further enhanced with glutamine. Moreover, we have demonstrated an important role for the PI3K/Akt pathway during periods of intestinal cell nutrient deprivation. Further studies will be directed towards identifying the counterbalance effects of the ERK and PI3K/Akt pathways in intestinal cell survival. Importantly, our findings provide novel insights into the molecular mechanisms regulating the gut protective effects of glutamine, a commonly used supplement to enteral feedings. These results provide a better understanding of signaling pathways activated and their roles during periods of intestinal cell stress.
Supplementary Material
(A) RIE-1 cells were transfected with PKD siRNA (100 nM) or non-targeting control (NTC) siRNA (100 nM) 24 h prior to experiments. Cells were then maintained in the presence or absence of glutamine (1 mmol/L) for an additional 24 h; DNA fragmentation ELISA was then performed (* = p < 0.05 vs. control {Gln-deprived, NTC transfection}; † = p < 0.05 vs. Gln treated, NTC transfection). (B) RIE-1 cells were transfected with either PKD siRNA or non-targeting control (NTC) 24 h prior to experiments. Following transfection, cells were maintained in the presence or absence of glutamine for an additional 24 h. Western blots were performed to detect phosphorylated PKD(916) (p-PKD(916)) and total PKD; β-actin was used as a loading control.
ACKNOWLEDGMENTS
The authors would like to thank Karen Martin for manuscript preparation and Tatsuo Ucihda for assistance with statistical analysis. This work was supported by grants RO1CA104748, R01DK48498, P01DK35608, and T32DK07639 from the National Institutes of Health and a Jeane B. Kempner Scholar award (to SDL).
REFERENCES
- 1.Aikawa R, Komuro I, Yamazaki T, Zou Y, Kudoh S, Tanaka M, Shiojima I, Hiroi Y, Yazaki Y. Oxidative stress activates extracellular signal-regulated kinases through Src and Ras in cultured cardiac myocytes of neonatal rats. J Clin Invest. 1997;100:1813–1821. doi: 10.1172/JCI119709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson RA, Boronenkov IV, Doughman SD, Kunz J, Loijens JC. Phosphatidylinositol phosphate kinases, a multifaceted family of signaling enzymes. The Journal of biological chemistry. 1999;274:9907–9910. doi: 10.1074/jbc.274.15.9907. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya S, Ray RM, Johnson LR. Prevention of TNF-alpha-induced apoptosis in polyamine-depleted IEC-6 cells is mediated through the activation of ERK1/2. Am J Physiol Gastrointest Liver Physiol. 2004;286:G479–490. doi: 10.1152/ajpgi.00342.2003. [DOI] [PubMed] [Google Scholar]
- 4.Blay J, Brown KD. Characterization of an epithelioid cell line derived from rat small intestine: demonstration of cytokeratin filaments. Cell Biol Int Rep. 1984;8:551–560. doi: 10.1016/0309-1651(84)90054-7. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MD, Soltoff SP. P2X7 receptors activate protein kinase D and p42/p44 mitogen-activated protein kinase (MAPK) downstream of protein kinase C. Biochem J. 2002;366:745–755. doi: 10.1042/BJ20020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 7.Brandlin I, Hubner S, Eiseler T, Martinez-Moya M, Horschinek A, Hausser A, Link G, Rupp S, Storz P, Pfizenmaier K, Johannes FJ. Protein kinase C (PKC)eta-mediated PKC mu activation modulates ERK and JNK signal pathways. The Journal of biological chemistry. 2002;277:6490–6496. doi: 10.1074/jbc.M106083200. [DOI] [PubMed] [Google Scholar]
- 8.Buchman AL, Moukarzel AA, Bhuta S, Belle M, Ament ME, Eckhert CD, Hollander D, Gornbein J, Kopple JD, Vijayaroghavan SR. Parenteral nutrition is associated with intestinal morphologic and functional changes in humans. Jpen. 1995;19:453–460. doi: 10.1177/0148607195019006453. [DOI] [PubMed] [Google Scholar]
- 9.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 10.Carpenter CL, Cantley LC. Phosphoinositide kinases. Curr Opin Cell Biol. 1996;8:153–158. doi: 10.1016/s0955-0674(96)80060-3. [DOI] [PubMed] [Google Scholar]
- 11.Curi R, Lagranha CJ, Doi SQ, Sellitti DF, Procopio J, Pithon-Curi TC. Glutamine-dependent changes in gene expression and protein activity. Cell Biochem Funct. 2005;23:77–84. doi: 10.1002/cbf.1165. [DOI] [PubMed] [Google Scholar]
- 12.Ehlers RA, Kim S, Zhang Y, Ethridge RT, Murrilo C, Hellmich MR, Evans DB, Townsend CM, Jr., Mark Evers B. Gut peptide receptor expression in human pancreatic cancers. Ann Surg. 2000;231:838–848. doi: 10.1097/00000658-200006000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox AD, Kripke SA, De Paula J, Berman JM, Settle RG, Rombeau JL. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. Jpen. 1988;12:325–331. doi: 10.1177/0148607188012004325. [DOI] [PubMed] [Google Scholar]
- 14.Holmstrom TH, Tran SE, Johnson VL, Ahn NG, Chow SC, Eriksson JE. Inhibition of mitogen-activated kinase signaling sensitizes HeLa cells to Fas receptor-mediated apoptosis. Molecular and cellular biology. 1999;19:5991–6002. doi: 10.1128/mcb.19.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung CC, Ichimura T, Stevens JL, Bonventre JV. Protection of renal epithelial cells against oxidative injury by endoplasmic reticulum stress preconditioning is mediated by ERK1/2 activation. The Journal of biological chemistry. 2003;278:29317–29326. doi: 10.1074/jbc.M302368200. [DOI] [PubMed] [Google Scholar]
- 16.Ko TC, Beauchamp RD, Townsend CM, Jr., Thompson JC. Glutamine is essential for epidermal growth factor-stimulated intestinal cell proliferation. Surgery. 1993;114:147–153. discussion 153-144. [PubMed] [Google Scholar]
- 17.Kohno M, Pouyssegur J. Pharmacological inhibitors of the ERK signaling pathway: application as anticancer drugs. Prog Cell Cycle Res. 2003;5:219–224. [PubMed] [Google Scholar]
- 18.Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 19.Labow BI, Souba WW. Glutamine. World J Surg. 2000;24:1503–1513. doi: 10.1007/s002680010269. [DOI] [PubMed] [Google Scholar]
- 20.Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 21.Litvak DA, Evers BM, Hwang KO, Hellmich MR, Ko TC, Townsend CM., Jr. Butyrate-induced differentiation of Caco-2 cells is associated with apoptosis and early induction of p21Waf1/Cip1 and p27Kip1. Surgery. 1998;124:161–169. discussion 169-170. [PubMed] [Google Scholar]
- 22.Papaconstantinou HT, Chung DH, Zhang W, Ansari NH, Hellmich MR, Townsend CM, Jr., Ko TC. Prevention of mucosal atrophy: role of glutamine and caspases in apoptosis in intestinal epithelial cells. J Gastrointest Surg. 2000;4:416–423. doi: 10.1016/s1091-255x(00)80022-0. [DOI] [PubMed] [Google Scholar]
- 23.Papaconstantinou HT, Hwang KO, Rajaraman S, Hellmich MR, Townsend CM, Jr., Ko TC. Glutamine deprivation induces apoptosis in intestinal epithelial cells. Surgery. 1998;124:152–159. discussion 159-160. [PubMed] [Google Scholar]
- 24.Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139:809–815. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development (Cambridge, England) 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 26.Reiling JH, Sabatini DM. Stress and mTORture signaling. Oncogene. 2006;25:6373–6383. doi: 10.1038/sj.onc.1209889. [DOI] [PubMed] [Google Scholar]
- 27.Rhoads JM, Argenzio RA, Chen W, Rippe RA, Westwick JK, Cox AD, Berschneider HM, Brenner DA. L-glutamine stimulates intestinal cell proliferation and activates mitogen-activated protein kinases. Am J Physiol. 1997;272:G943–953. doi: 10.1152/ajpgi.1997.272.5.G943. [DOI] [PubMed] [Google Scholar]
- 28.Roth E, Oehler R, Manhart N, Exner R, Wessner B, Strasser E, Spittler A. Regulative potential of glutamine--relation to glutathione metabolism. Nutrition. 2002;18:217–221. doi: 10.1016/s0899-9007(01)00797-3. [DOI] [PubMed] [Google Scholar]
- 29.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. The Journal of biological chemistry. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 30.Ruemmele FM, Seidman EG, Lentze MJ. Regulation of intestinal epithelial cell apoptosis and the pathogenesis of inflammatory bowel disorders. J Pediatr Gastroenterol Nutr. 2002;34:254–260. doi: 10.1097/00005176-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 31.SAS/STAT 91 User's Guide . SAS Institute Inc.; Cary, NC: 2004. [Google Scholar]
- 32.Seger R, Krebs EG. The MAPK signaling cascade. Faseb J. 1995;9:726–735. [PubMed] [Google Scholar]
- 33.Sheng H, Shao J, Dixon DA, Williams CS, Prescott SM, DuBois RN, Beauchamp RD. Transforming growth factor-beta1 enhances Ha-ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mRNA. The Journal of biological chemistry. 2000;275:6628–6635. doi: 10.1074/jbc.275.9.6628. [DOI] [PubMed] [Google Scholar]
- 34.Sheng H, Shao J, DuBois RN. Akt/PKB activity is required for Ha-Ras-mediated transformation of intestinal epithelial cells. The Journal of biological chemistry. 2001;276:14498–14504. doi: 10.1074/jbc.M010093200. [DOI] [PubMed] [Google Scholar]
- 35.Sheng H, Shao J, Townsend CM, Jr., Evers BM. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt E. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in swiss 3T3 cells. The Journal of biological chemistry. 2004;279:16883–16893. doi: 10.1074/jbc.M313225200. [DOI] [PubMed] [Google Scholar]
- 37.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 38.Song J, Li J, Lulla A, Evers BM, Chung DH. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-delta pathway activation. Am J Physiol Cell Physiol. 2006;290:C1469–1476. doi: 10.1152/ajpcell.00486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Storz P, Doppler H, Johannes FJ, Toker A. Tyrosine phosphorylation of protein kinase D in the pleckstrin homology domain leads to activation. The Journal of biological chemistry. 2003;278:17969–17976. doi: 10.1074/jbc.M213224200. [DOI] [PubMed] [Google Scholar]
- 40.Storz P, Doppler H, Toker A. Protein kinase Cdelta selectively regulates protein kinase D-dependent activation of NF-kappaB in oxidative stress signaling. Molecular and cellular biology. 2004;24:2614–2626. doi: 10.1128/MCB.24.7.2614-2626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tappenden KA. Mechanisms of enteral nutrient-enhanced intestinal adaptation. Gastroenterology. 2006;130:S93–99. doi: 10.1053/j.gastro.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 42.Ueda J, Saito H, Watanabe H, Evers BM. Novel and quantitative DNA dot-blotting method for assessment of in vivo proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288:G842–847. doi: 10.1152/ajpgi.00463.2004. [DOI] [PubMed] [Google Scholar]
- 43.Vanhaesebroeck B, Waterfield MD. Signaling by distinct classes of phosphoinositide 3-kinases. Exp Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 44.Wang Q, Wang X, Hernandez A, Hellmich MR, Gatalica Z, Evers BM. Regulation of TRAIL expression by the phosphatidylinositol 3-kinase/Akt/GSK-3 pathway in human colon cancer cells. The Journal of biological chemistry. 2002;277:36602–36610. doi: 10.1074/jbc.M206306200. [DOI] [PubMed] [Google Scholar]
- 45.Yan F, John SK, Polk DB. Kinase suppressor of Ras determines survival of intestinal epithelial cells exposed to tumor necrosis factor. Cancer Res. 2001;61:8668–8675. [PubMed] [Google Scholar]
- 46.Zhou Y, Wang Q, Evers BM, Chung DH. Signal transduction pathways involved in oxidative stress-induced intestinal epithelial cell apoptosis. Pediatr Res. 2005;58:1192–1197. doi: 10.1203/01.pdr.0000185133.65966.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) RIE-1 cells were transfected with PKD siRNA (100 nM) or non-targeting control (NTC) siRNA (100 nM) 24 h prior to experiments. Cells were then maintained in the presence or absence of glutamine (1 mmol/L) for an additional 24 h; DNA fragmentation ELISA was then performed (* = p < 0.05 vs. control {Gln-deprived, NTC transfection}; † = p < 0.05 vs. Gln treated, NTC transfection). (B) RIE-1 cells were transfected with either PKD siRNA or non-targeting control (NTC) 24 h prior to experiments. Following transfection, cells were maintained in the presence or absence of glutamine for an additional 24 h. Western blots were performed to detect phosphorylated PKD(916) (p-PKD(916)) and total PKD; β-actin was used as a loading control.