Abstract
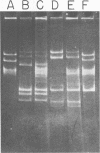
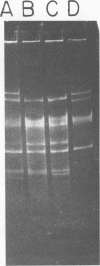
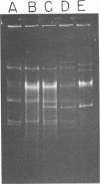
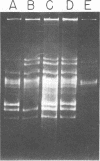
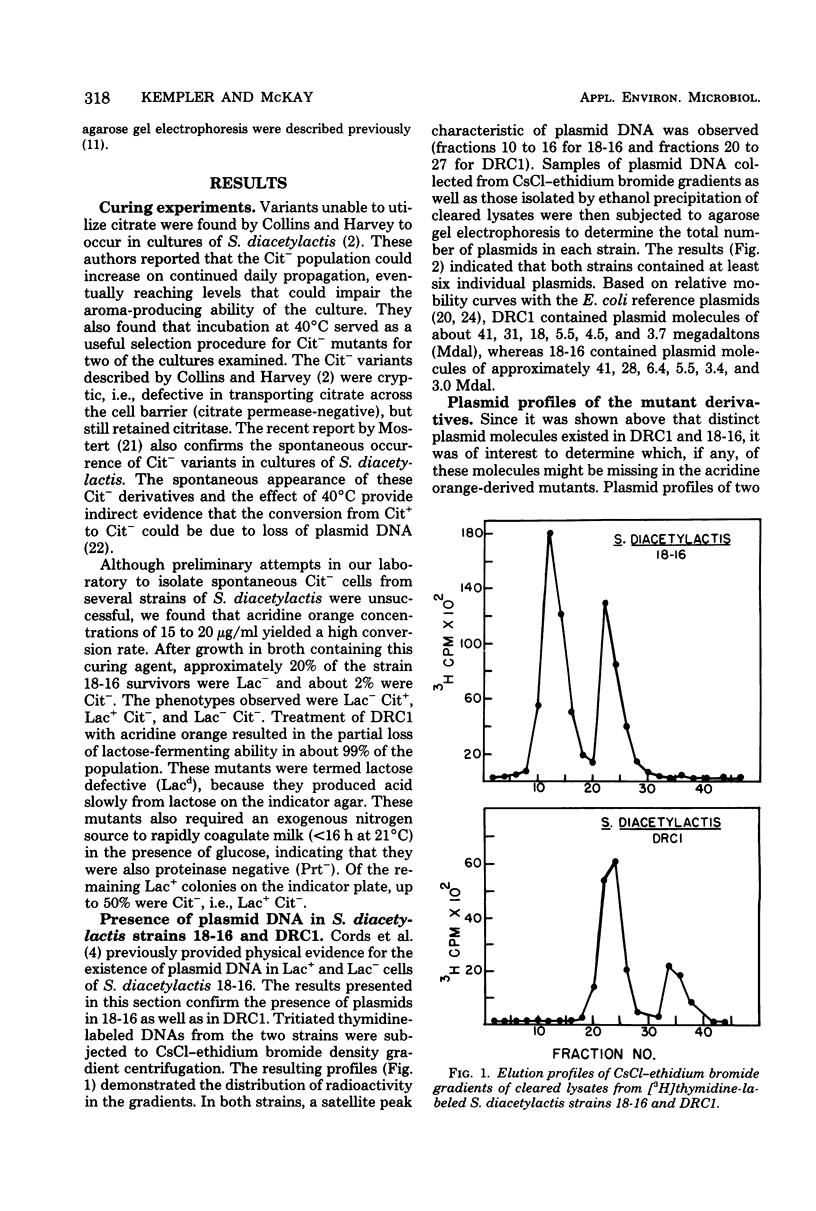
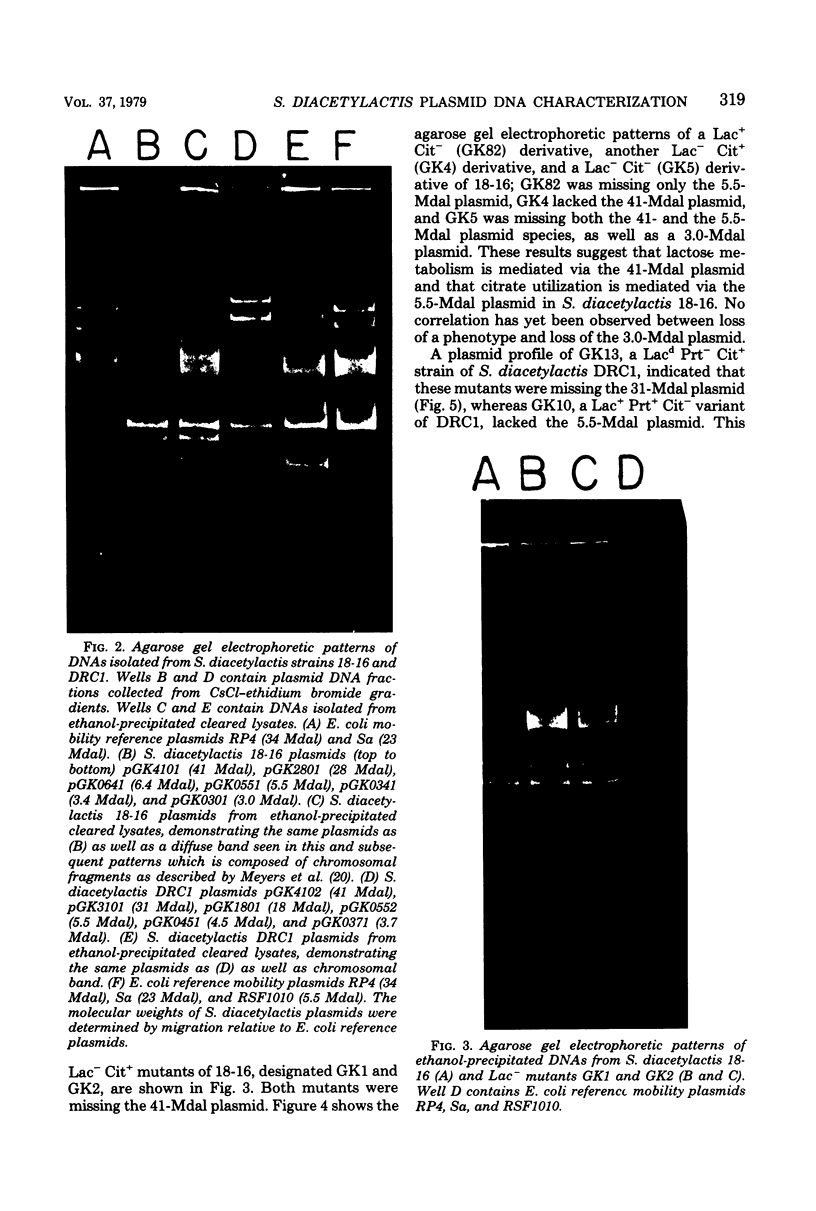
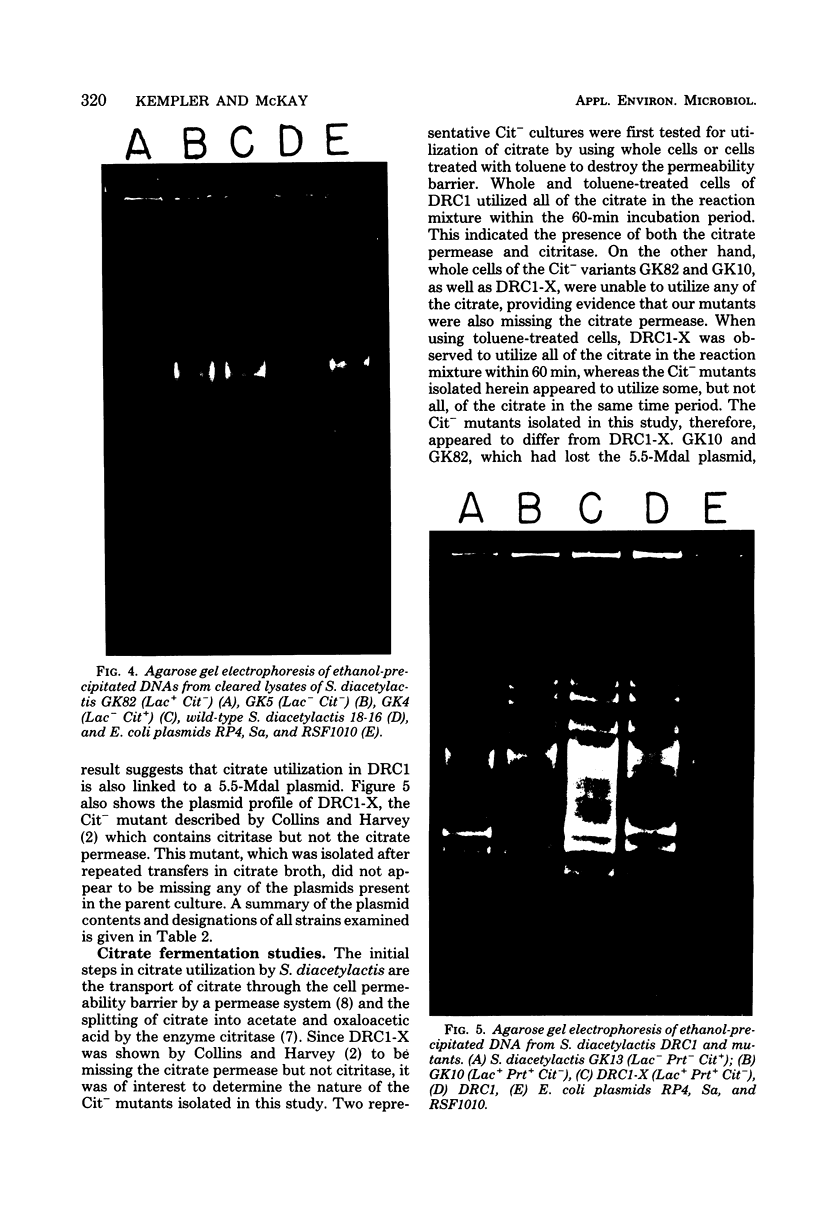
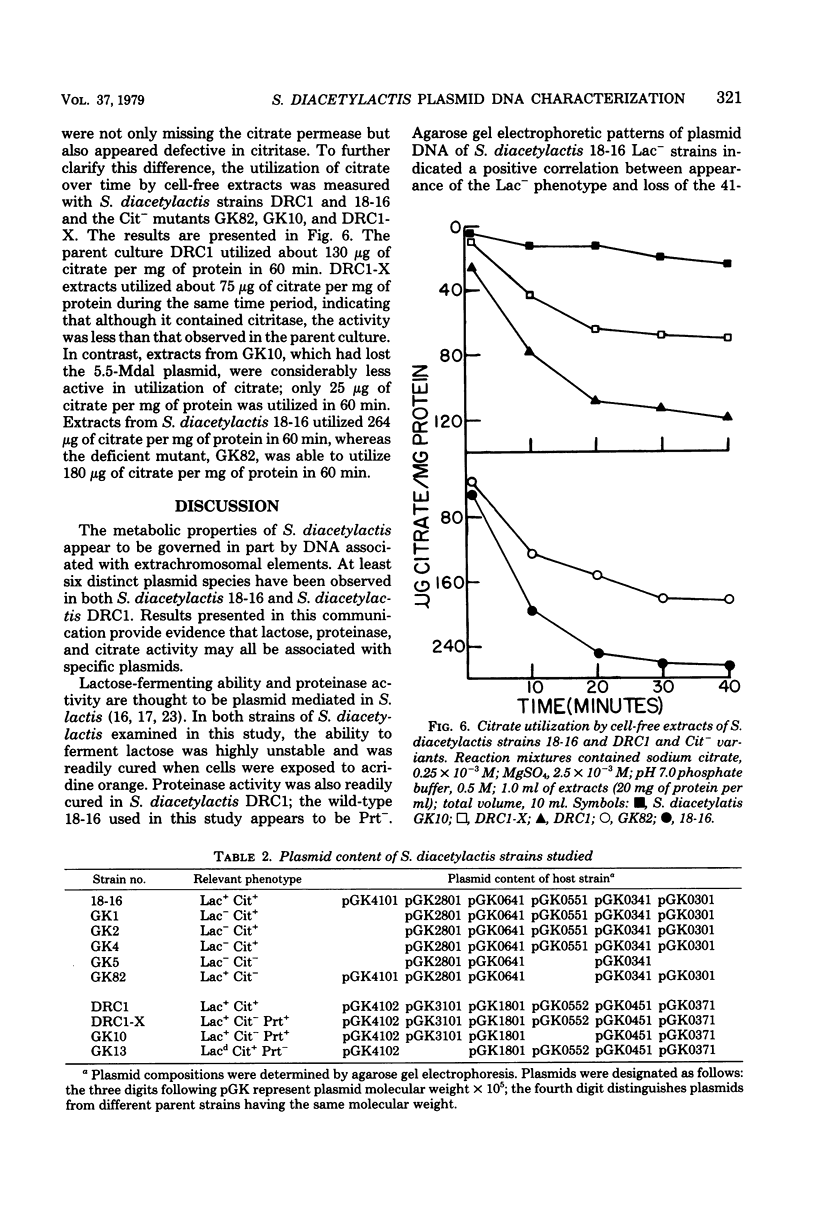
The use of Streptococcus diacetylactis as a flavor producer in dairy fermentations is dependent upon its ability to produce diacetyl from citrate. Treatment of S. diacetylactis strains 18-16 and DRC1 with acridine orange resulted in the conversion of approximately 2% of the DRC1 population and 20% of the 18-16 population to citrate negative, which is indicative of the involvement of plasmid deoxyribonucleic acid (DNA). Growth in the presence of acridine orange also resulted in the appearance of 2% lactose-negative derivatives in S. diacetylactis 18-16 and 99% lactose-defective, proteinase-negative derivatives in S. diacetylactis DRC1. Cesium chloride-ethidium bromide equilibrium density gradients of cleared lysate material from each strain revealed the presence of covalently closed circular DNA. Samples of this covalently closed circular DNA were subjected to agarose gel electrophoresis to determine the plasmid composition of each strain. S. diacetylactis 18-16 was found to possess six plasmids, of approximately 41, 28, 6.4, 5.5, 3.4, and 3.0 megadaltons (Mdal). S. diacetylactis DRC1 contained six plasmids, of approximately 41, 31, 18, 5.5, 4.5, and 3.7 Mdal. Variants of S. diacetylactis 18-16 which failed to produce acetoin plus diacetyl from citrate (citrate negative) were missing a 5.5-Mdal plasmid. Lactose-negative mutants of the same strain were devoid of a 41-Mdal plasmid. Lactose-defective, proteinase-negative mutants of S. diacetylactis DRC1 were missing a 31-Mdal plasmid. The citrate-negative mutants of S. diacetylactis DRC1 isolated in this study did not possess a 5.5-Mdal plasmid. Thus, we have evidence that there is a correlation between the ability to utilize citrate and the presence of a 5.5-Mdal plasmid. A relationship was also noted between lactose fermentation and proteinase activity and plasmid DNA in S. diacetylactis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Plasmids, loss of lactose metabolism, and appearance of partial and full lactose-fermenting revertants in Streptococcus cremoris B1. J Bacteriol. 1977 Jan;129(1):367–377. doi: 10.1128/jb.129.1.367-377.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L. Characterization of lactose-fermenting revertants from lactose-negative Streptococcus lactis C2 mutants. J Bacteriol. 1974 Sep;119(3):830–839. doi: 10.1128/jb.119.3.830-839.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cords B. R., McKay L. L., Guerry P. Extrachromosomal elements in group N streptococci. J Bacteriol. 1974 Mar;117(3):1149–1152. doi: 10.1128/jb.117.3.1149-1152.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EATON N. R. New press for disruption of microorganisms. J Bacteriol. 1962 Jun;83:1359–1360. doi: 10.1128/jb.83.6.1359-1360.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. Citrate transport system of Streptococcus diacetilactis. J Bacteriol. 1962 May;83:1005–1009. doi: 10.1128/jb.83.5.1005-1009.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARVEY R. J., COLLINS E. B. Role of citritase in acetoin formation by Streptococcus diacetilactis and Leuconostoc citrovorum. J Bacteriol. 1961 Dec;82:954–959. doi: 10.1128/jb.82.6.954-959.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNETEMAN A. Enrichment and isolation of Streptococcus citrophilus van Beynum et Pette. Antonie Van Leeuwenhoek. 1952;18(4):275–290. doi: 10.1007/BF02538616. [DOI] [PubMed] [Google Scholar]
- Klaenhammer T. R., McKay L. L., Baldwin K. A. Improved lysis of group N streptococci for isolation and rapid characterization of plasmid deoxyribonucleic acid. Appl Environ Microbiol. 1978 Mar;35(3):592–600. doi: 10.1128/aem.35.3.592-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A. Simultaneous loss of proteinase- and lactose-utilizing enzyme activities in Streptococcus lactis and reversal of loss by transduction. Appl Microbiol. 1974 Sep;28(3):342–346. doi: 10.1128/am.28.3.342-346.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce L. E., Skipper N. A., Jarvis B. D. Proteinase activity in slow lactic acid-producing variants of Streptococcus lactis. Appl Microbiol. 1974 May;27(5):933–937. doi: 10.1128/am.27.5.933-937.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubens C., Heffron F., Falkow S. Transposition of a plasmid deoxyribonucleic acid sequence that mediates ampicillin resistance: independence from host rec functions and orientation of insertion. J Bacteriol. 1976 Oct;128(1):425–434. doi: 10.1128/jb.128.1.425-434.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]