Abstract
In the present study, we tested the hypothesis that a mild cerebral tissue injury promotes subsequent glioma invasion via activation of the ADAM17-EGFR-PI3K-Akt pathway. Mild injury was induced by Photodynamic therapy (PDT), which employs tissue-penetrating laser light exposure following systemic administration of a tumor-localizing photosensitizer. Athymic nude mice were treated with sublethal PDT (80J/cm2 with 2mg/kg Photofrin). Hypoxic stress and ADAM17-EGFR-PI3K-Akt were measured using Western blot and immunostaining. Additional groups with/without pro-sublethal PDT were subsequently implanted with U87 glioma tumor cell. Tumor invasion and ADAM17-EGFR-PI3K-Akt pathway in tumor area were measured. After a sublethal dose of PDT, HIF-1α expression was increased by a factor of three in PDT-treated normal brain tissue compared to contralateral control brain tissue. PDT-treated brain tissue exhibited a significant increase in ADAM17, p-EGFR, p-Akt expression compared to non-treated tissue. ADAM17 positive area significantly increased from 1.78% to 10.89%. The percentage of p-EGFR and p-Akt positive cells significantly increased from 9.50% and 14.50% to 21.31% and 32.29%,respectively, PDT treatment significantly increased subsequent implanted U87 glioma cell invasion by 3.68-fold and increased ADAM17, EGFR, p-EGFR, Akt, p-Akt expression by 178%, 43.9%,152.7%, 89.6%,and 164.2%, respectively, compared to control group. Our data showed that a sublethal sensitization of cerebral tissue with PDT significantly increased U87 cell invasion in nude mice, and that glioma cell invasion is highly correlated with activation of the ADAM17-EGFR-PI3K-Akt pathway (r=0.928, 0.775, 0.870, 0.872, and 0.883, respectively), most likely via HIF-1α.
Keywords: Glioma, photodynamic therapy, invasion, ADAM17, EGFR, Akt
Introduction
Malignant gliomas are both highly vascularized and invasive, characterized by high incidence of recurrence and poor prognosis. Photodynamic therapy (PDT) has been clinically developed as an adjuvant local therapy for brain tumors [1]. Preclinical and clinical studies have suggested an improved survival time after PDT and demonstrated the effectiveness of PDT in producing necrosis of solid malignant gliomas [2; 3; 4]. However, to date, the efficacy of PDT treatment of CNS neoplasms remains inconclusive. In addition to an incomplete killing of tumor cells, a reason for the failure of PDT as an effective treatment of glioma may be attributed to PDT-induced tumor invasion and recurrence to and from brain adjacent to tumor (BAT). Treating solid malignancies with PDT involves tissue-penetrating laser light exposure following systemic administration of a tumor-localizing photosensitizer. Tissues surrounding the focally-treated area or BAT tissues are often subjected to a low dose PDT (sublethal dose of PDT). Therefore, therapeutic interventions intended to enhance tumor cell death also promote changes in the tumor or BAT tissue microenvironment.
PDT results in the generation of cytotoxic singlet oxygen, which causes tumor destruction directly through tumor cell damage, microvascular damage and the induction of vascular stasis [5; 6; 7], or through a combination of these mechanisms. The therapy-induced decrease in blood flow leads to strong and persistent tumor tissue hypoxia [6]. Further, because the PDT photochemistry consumes oxygen, oxygen consumption during the therapy can also create rapid and severe transient tissue hypoxia [8; 9; 10].
Our previous data identify that tumor necrosis factor-α converting enzyme (TACE)/a disintegrin and metalloprotease domain 17 (ADAM17) is an important signaling component underlying malignancy of glioma [11]. We have shown that a hypoxia-induced increase in ADAM17 proteolytic activity substantially contributes to invasiveness of brain tumor cells in vitro and this ADAM17-mediated hypoxia-induced tumor invasiveness occurs via activation of EGFR, and subsequently, the PI3K-Akt signaling pathway. Therefore, we investigated the correlation of PDT induced invasion and ADAM17-EGFR signaling to address a possible mechanism by which sublethal PDT contributes to glioma cell invasion in vivo.
Materials and Methods
Animal groups
Sublethal dose of PDT treatment on normal brain: nude mice (n=10) were subjected to a sublethal dose of PDT. Animals were sacrificed 3 days after PDT for histological study (n=5) and Western blot analysis (n=5). Homologous contralateral tissues were used as controls in each animal.
Tumor implantation after PDT cerebral tissue sensitization: 18 nude mice were used in this study. Control mice (n=8) were not exposed to laser and the remaining animals (n=10) received a subtherapeutic dose of 80J/cm2. Three days later, 3x105 U87 human glioma cells were implanted into all the 18 nude mice through a craniectomy.
Light delivery
A semiconductor diode laser system (University Health Network, Toronto, Canada) provided the light (632 nm) for the PDT treatment and optical measurement. The light was coupled into a 400 μm optical fiber with a distal microlens (PDT, Santa Barbara, CA, USA) for a 5-mm diameter uniform spot for superficial irradiation. The power at the distal end of the fiber was adjusted to 260 mW and was measured before and after each treatment with a power meter (Photodyne, Westlake Village, CA, USA) with a 1-inch integrating sphere detector head. The irradiation power proved to be stable in all experiments.
Mouse model for intracranial photodynamic therapy
Athymic nude mice were obtained from the National Cancer Institute (Frederick, MD). PDT treatment of mouse brain was performed as previously described[12]. Briefly, Photofrin (QuadraLogic Technologies, Vancouver, BC, Canada) dissolved in dextrose solution was systemically administered to nude mice at a dose of 2 mg/kg. After 24 h, 10 mice were anesthetized with ketamine (80 mg/kg) and xylazine (13 mg/kg) and fixed in a stereotaxic device, the skull was exposed, and a 5 mm diameter craniotomy was drilled over the right hemisphere 2.5 mm to the midline and 2.0 mm anterior to the bregma. Light at a fluency rate of 260 mW/cm2 was delivered by a diode laser through the craniotomy, to deliver 80J/cm2 optical dose. The craniectomy was covered with a film of polyvinyl chloride glued to the surrounding intact bone and the incision was closed with 4–0 silk suture (Ethicon, Somerville, NJ, USA) after surgery. Control animals (n=5) were submitted to sham surgery, with no exposure to light.
Human U87 glioma cell culture
U87 cells (ATCC, Manassas, VA, USA) were maintained in monolayer culture (37°C, 5% CO2, 95% O2) in minimum essential media (MEM) with Eagle’s salts, supplemented with 10% fetal bovine serum, penicillin and streptomycin (Gibco, Grand Island, NY). Cells were subcultured and used for implantation when they reached an exponential phase of growth. To harvest, cells were incubated with 0.05% trypsin EDTA (0.53 mM, Invitrogen) for 5 min, and then MEM was added to make a single cell suspension. After the suspension was centrifuged at 1000 rpm (280 x g) for 5 min at 4°C, the media was removed and the cells were re-suspended in 1XPBS. Cell viability was determined by trypan blue exclusion (nonviable cells were stained blue). The number of unstained cells was counted using a hemocytometer under a light microscope and then the suspension was further diluted with 1XPBS to a final concentration of 1x108 cells/ml.
U87 cell implantation in athymic nude mice
Athymic nude mice were anesthetized with ketamine (80 mg/kg) and xylazine (13 mg/kg) administered intramuscularly (i.m.). Atropine (0.02 ml) was injected (i.m.) at the time of anesthesia induction. Once the animals were fixed in a stereotaxic device, a 5–6 mm incision was made directly down the midline, the scalp was retracted and the cranium exposed. Using a drill, a 3 mm diameter circular craniectomy was made on the right hemisphere anterior to the coronal suture. A 10μL Hamilton syringe was lowered to a depth of 2.5 mm beneath the dura so as to inject a 5×105 U87 cells in 5 μL volume of cells over a 5 minute interval. The craniectomy was covered with a film of polyvinyl chloride glued to the surrounding intact bone. The incision was closed with 4-0 silk suture (Ethicon, Somerville, NJ, USA).
Western blot analysis
Brain tissues isolated from PDT-treated or contralateral areas were rinsed with 1XPBS and proteins were extracted using Trazol lysis buffer. Equal amounts of protein, as determined by the BCA method (Pierce, Rockford, IL, USA), were loaded on 10% Bis-Tris Gels (Invitrogen) after being denatured. The proteins were then transferred to PVDF membranes (Invitrogen). The membranes were blocked at room temperature for 1 h with 5% BSA in TBS-T (10 mM Tris-HCl, pH 7.6, and 150 mM NaCl, 0.1% Tween-20). Afterward the membranes were incubated with primary antibodies against HIF-1α (Novus Biologicals, Littleton, Co. USA) in 3% BSA overnight at 4°C. The membranes were washed with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies (Bio-Rad Laboratories, Hercules, CA, USA) for 1 h at room temperature. Following washing, the immunoblots were detected using a SuperSignal West Pico Chemiluminescent Substrate kit (Pierce). β-Actin was used as the internal control. The densities of bands were analyzed using the Gelpro 4.5 program (Silver Spring, MD, US).
Histopathology
Anesthetized animals were sacrificed and perfused with 4% paraformaldehyde. Brains were removed, post-fixed and cut into 1-mm-thick blocks which then were processed and embedded in paraffin. Six-μm thick sections were further cut from each of the blocks containing the tumor and were used for hematoxylin and eosin (H&E) staining and other immunohistochemical stainings.
Measurement of tumor invasion
Six-μm thick coronal sections obtained serially at 0.5 mm intervals from each of the blocks containing the tumor were stained with H&E for light microscopic examination and calculation of tumor size. All of the tumors from pre-PDT treatment group and control group were analyzed. H&E-stained sections were scanned into the computer. In tumored brain, dark purple tumor masses are clearly identified. The tumor area (mm2) was measured by tracing the demarcation of the tumor on the computer screen using MCID imaging software (Imaging Research Inc., St. Catharine’s, Ontario, Canada). The section (usually section C or D) with the largest tumor area in each animal was used to count the cell islets around the main tumor mass. The number of cell islets was normalized by tumor area.
Immunohistochemistry and quantification
Antibodies against ADAM17, p-EGFR, EGFR, p-Akt and Akt (Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used to examine the expression in normal and tumor brain. A series of 6-μm thick sections at 50-μm intervals were cut from the block containing the maximum cross-sectional tumor area. After dehydration, sections were boiled in 1% citric acid buffer (pH 6.0) for 10 min, cooled down to room temperature. Subsequently, the sections were incubated with 1% BSA to block the non-specific signals. The sections were incubated with primary antibody overnight at 4°C, followed by incubation with biotinylated secondary antibody for 1 h at room temperature. The 3,3-diaminobenzidine-tetrahydrochloride (DAB; Sigma) was used to routinely detect the immunoreactivity, which can be visualized directly by bright-field light. Four fields of view from PDT-treated and contralateral hemisphere areas in each section were digitized under a 40X objective (Olympus BX40) using a 3-CCD color video camera (Sony DXC-970MD), interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada, Fig. 1A). Eight fields of view from tumor were analyzed under the same procedure Fig. 1B. The quantitative data are presented as the total number of the immunoreactive cells or the positive area within each field.
Fig. 1.
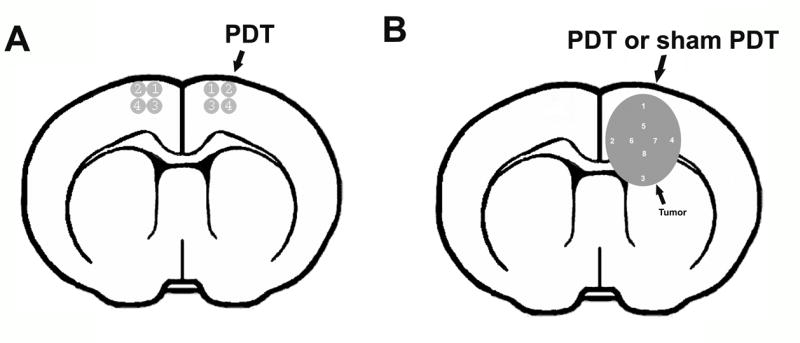
Four high magnification fields from digitized images of the PDT-treated region and corresponding contralateral area were selected for immunohistochemistry analysis (A). Eight high magnification fields from digitized images of tumor implanted in nude mice with/without pro-PDT treatment (B).
Statistical Analysis
Data are presented as mean ± SD. Statistical significance was analyzed by a two-tailed Student’s t-test. P values of less than 0.05 are considered statistically significant.
Results
Histopathological study with H&E staining in normal brain treated with sublethal PDT
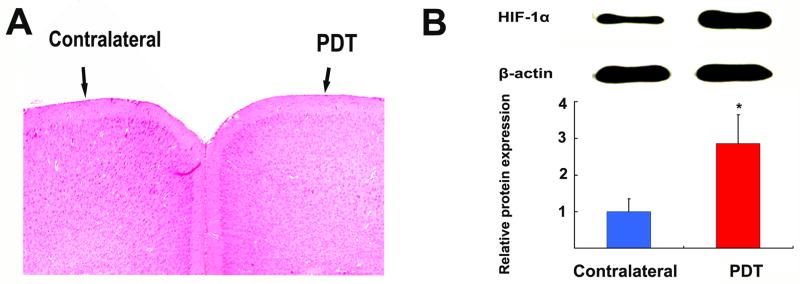
In clinical brain tumor treatment, a light dose of >200J/cm2 is currently used. However, tissue surrounding the focally-treated area or BAT is subjected to unavoidable low doses of PDT (sublethal dose of PDT). To test the changes of normal brain microenvironment after sublethal PDT treatment, 80J/cm2 was selected to treat “not-bearing tumor mice” in accordance with our previous experiments [13]. Five nude mice were sacrificed 3 days after sublethal PDT treatment, and coronal sections were stained with H&E for light microscopic examination. There was no significant tissue damage or architectural changes in PDT-treated area compared to contralateral hemisphere in all 5 animals (Fig. 2A). This observation was consistent with our previous study, where 70% of normal rat brains treated with this dose (2mg/kg Photofrin, 260mW/cm2, for a total dose of 80J/cm2) showed no visible change in cellular architecture after 3 days [13]. However, we cannot exclude the possibility that such changes could occur at later time-points.
Fig. 2.
Animals were sacrificed 3 days after sublethal PDT treatment with 80J/cm2. H&E examination revealed no significant tissue damage and architecture changes in the PDT-treated area compared to the contralateral hemisphere in all 5 animals (A). A representative Western blot analysis of HIF-1α expression in PDT-treated local area compared to contralateral tissue. PDT induced a 3-fold increase of HIF-1α expression (B).
Sublethal PDT induced hypoxic stress to normal brain in nude mice
HIF-1α is one of the key regulators that orchestrate cellular responses to hypoxia. To measure the HIF-1α expression, five non-tumor-bearing nude mice were sacrificed 3 days after PDT treatment. Brains were removed and tissue from the PDT-treated area and corresponding contralateral tissue were dissected. A representative Western blot analysis displayed an increase of HIF-1α expression in the PDT-treated local area compared to contralateral tissue (Fig 2.B). β-Actin was used as the internal control. The densitometry analysis of the bands showed that HIF-1α expression in PDT-treated tissue was 3 times higher than that in contralateral brain tissue. (Fig 2.B)
HIF-1α is one of the master regulators that orchestrate cellular responses to hypoxia. These results suggested that hypoxic stress is induced in the tissues subjected to sublethal PDT.
Expression of ADAM17, EGFR, pEGFR, Akt, and p-Akt in normal brain after sublethal PDT treatment
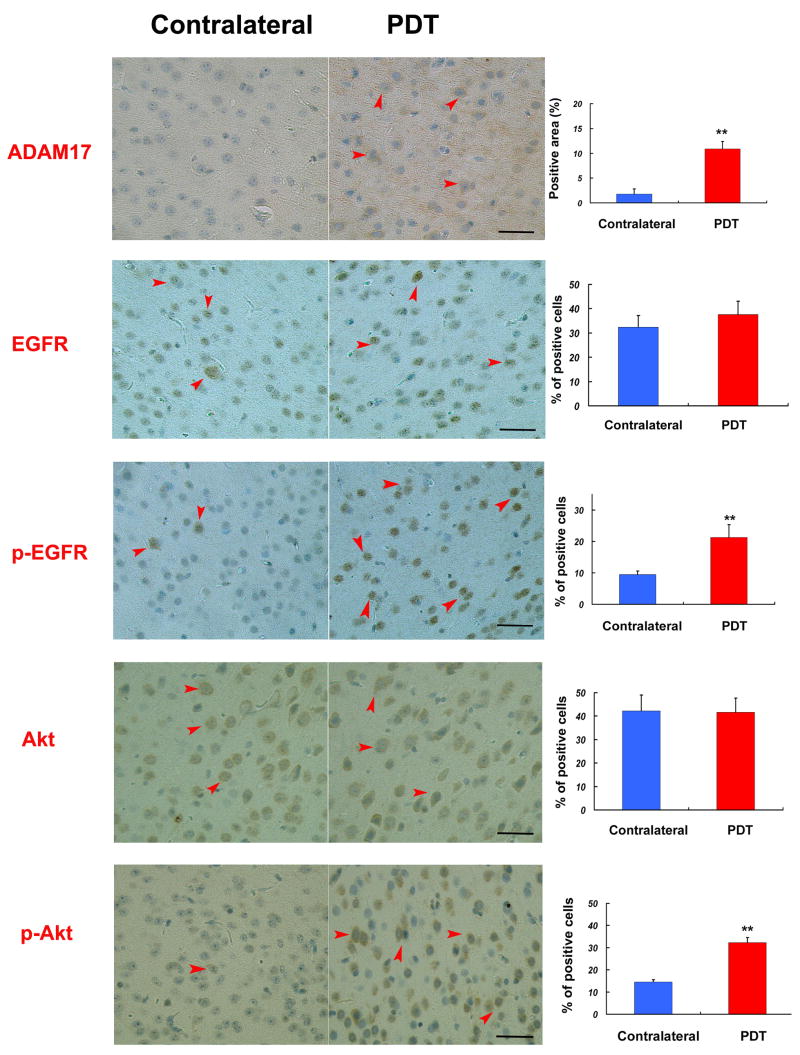
HIF-1α is a potent activator of angiogenesis and invasion through its up-regulation of target genes critical for these functions. Our previous studies have shown that hypoxia increases U87 cell invasion through ADAM17-induced EGFR-PI-3K-Akt pathway activation [11]. To test if sublethal PDT, through enhanced hypoxia stress, induces the same signaling pathway in normal animal brain, five nude mice were sacrificed 3 days after PDT treatment. ADAM17, EGFR, pEGFR, Akt, and p-Akt expression were examined by immunohistochemistry. For quantitative studies, four high magnification fields from digitized images of the PDT-treated region and corresponding contra-lateral area were used as shown in Fig. 1C. The positive ADAM17-immunoreactive area within the area of PDT treatment and contralateral area were analyzed and the data are presented as percentage of the immunoreactive area. The number of EGFR, p-EGFR, Akt and pAkt immunoreactive cells within the area of PDT treatment and contralateral area were counted in each section. The data are presented as percentage of the immunoreactive cells. PDT significantly increased ADAM17 positive area (10.89%) compared to the contralateral area (1.78%, Fig 3 A,B,C p<0.01); Compared to the contralateral area, p-EGFR and p-Akt positive cells were increased from 9.50% and 14.50% to 21.31% and 32.29% in the PDT-treated area, respectively (Fig 3, p<0.01). There were no significant changes in the expression of total EGFR and Akt in the area of PDT treatment compared to contralateral tissue (Fig. 3).
Fig. 3.
ADAM17, EGFR, p-EGFR, Akt, p-Akt expression in normal brain. The positive ADAM17-immunoreactive area within the area of PDT treatment and contralateral were analyzed by MCID. 80J/cm2 PDT significantly increased ADAM17 positive area (10.89%) compared to contralateral (1.78%) The number of EGFR, P-EGFR, Akt, pAkt–immunoreactive cells were counted in each section. There were no significant changes in the expression of total EGFR or Akt in the area of PDT treatment and contralateral tissue. p-EGFR and p-Akt positive cells percentage increased from 9.50% and 14.50% to 21.31% and 32.29%, respectively, in the PDT-treated area compared to the contralateral. tissue. Bar is 50μm. **,P<0.01, n=5
Activation of EGFR-Akt has been implicated to be one of the consequences of ADAM17 activation induced by hypoxia. These results suggest sublethal dose of PDT induced EGFR-Akt pathway activation may be mediated by hypoxia induced ADAM17.
Infiltrative changes of tumors implanted in PDT sensitized brain
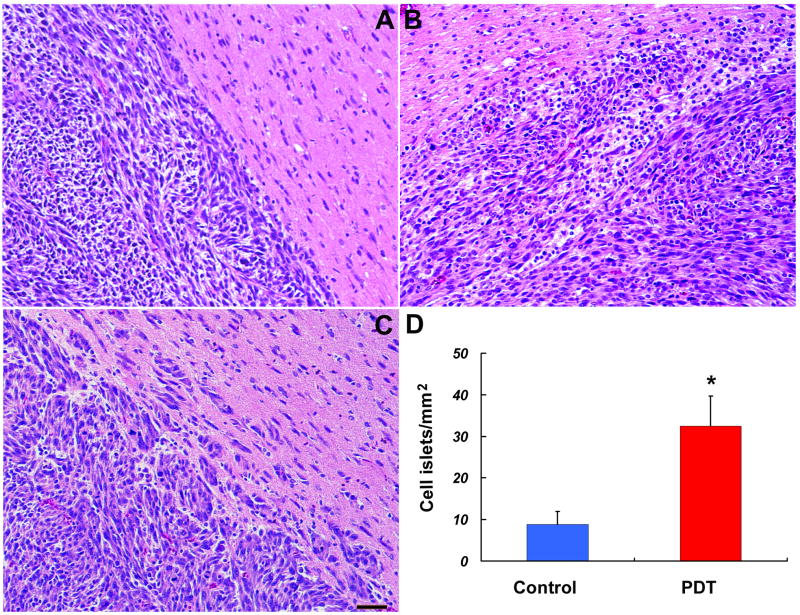
To test how cerebral tissue microenvironment modified by PDT affects U87 glioma invasion, Photofrin was administered to 18 nude mice. Twenty-four hours later a craniotomy was made to expose a defined brain region to a diode laser. Control mice (n=8) were not exposed to laser and the remaining animals (n=10) received a subtherapeutic dose of 80J/cm2. Three days later, 3x105 U87 human glioma cells were implanted through the same craniotomy. The animals were sacrificed three weeks later, brains were removed and fixed. Sequential coronal sections were obtained and tumor volumes for each animal were calculated. Calculation of the tumor volume revealed that the tumors in the group pretreated with PDT were significantly larger than those in the sham PDT (control) group (data previously reported [13]), In this study, our purpose was to test the infiltrative character of tumors implanted in normal brain, or in the brain pretreated with subtherapeutic PDT. Under the light microscope, there was no hemorrhage or tumor necrosis observed in all groups (Fig. 4). We observed the shape and margins of the tumors. The tumor mass implanted in brain with or without sublethal dose of PDT treatment has a clearly-defined appearance. Tumors from animals treated with sham PDT, were round in shape, and exhibited well-delineated margins with very limited signs of local infiltration (Fig. 4A). Tumors from animals of PDT sensitized group displayed an irregular shape and indefinite margins (Fig. 4B), with many more tumor cell islets (Fig. 4C) invading the adjacent normal brain parenchyma than that from animals without PDT treatment. The section with the largest tumor area in each animal was selected to count number of tumor cell islets outside the tumor mass. The number of tumor cell islets above 6 cells was counted under the microscope and normalized by tumor area. Sublethal PDT significantly increased tumor cell islets by 3.68-fold (Fig. 4D). These results suggest that sensitization of cerebral tissue in nude mice with photodynamic therapy promotes glioma cell invasion
Fig. 4.
Light microscope examination found no hemorrhage or tumor necrosis in the all groups. Tumors from animals treated with sham PDT, were round in shape, and exhibited well-delineated margins with very limited signs of local infiltrations (A). Tumors from animals treated with pro-PDT displayed an irregular shape and indefinite margins (B), with many more tumor cell islets (C, D) invaded to the adjacent normal brain parenchyma. **,P<0.01. Control group: n=8, Pro-PDT treated group: n=10
ADAM17, EGFR, pEGFR, Akt, and p-Akt expression in tumor implanted in pro-PDT-treated and Non-pro-PDT-treated brain
ADAM17 plays a significant role in tumor invasion under hypoxic conditions[11]. EGFR is an important mediator responsible for the invasiveness of malignant gliomas.[14; 15; 16] EGFR ligand binding results in receptor self dimerization, autophosphorylation and subsequent activation of downstream PI3K/Akt signaling pathways, which contribute to the malignant phenotype [17; 18; 19]. We have previously demonstrated that ADAM17 activates the EGFR-Akt pathway under hypoxic conditions in vitro [11]. Therefore, we tested whether ADAM17 and this key signal transduction pathway are altered by sublethal dose of PDT in U87 tumors implanted in nude mice.
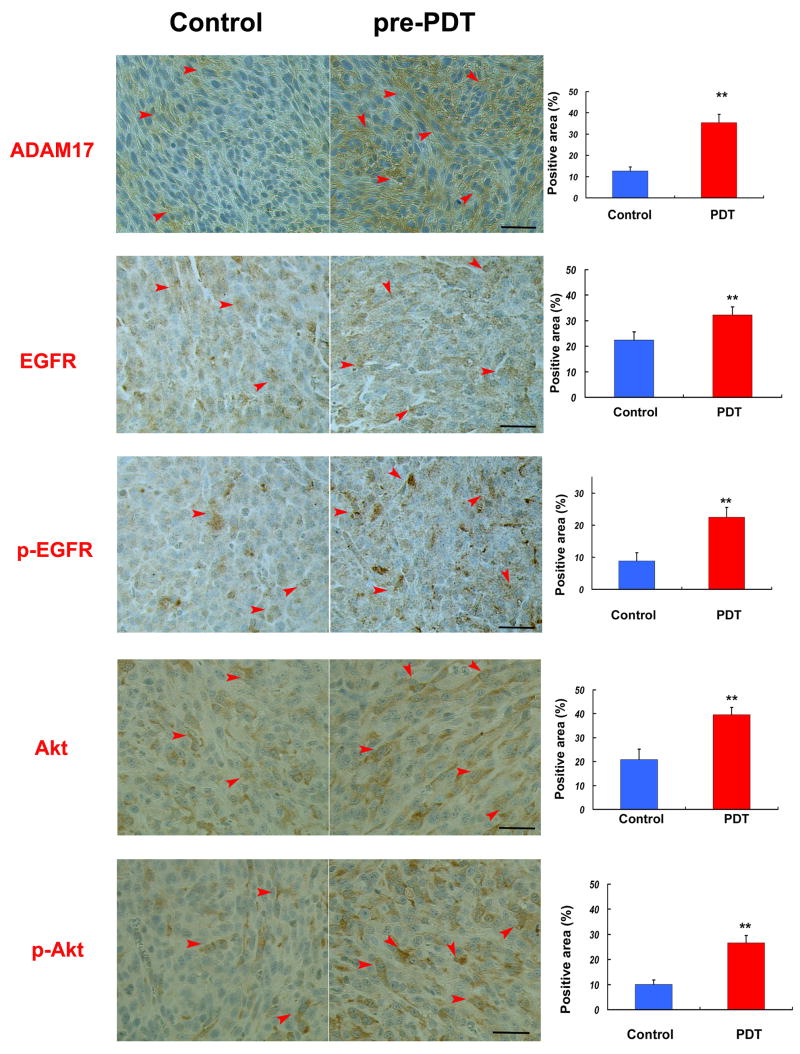
The animals were subjected to PDT or sham PDT and then, 3 days later, implanted with U87 cells. The animals were sacrificed three weeks after tumor implantation, brains were removed, fixed and coronal sections were analyzed by immunohistochemistry. Eight fields of view from tumor were analyzed (Fig. 1B). Expression of ADAM17, EGFR, pEGFR, Akt, and p-Akt in tumor was analyzed and the data are presented as percentage of the immunoreactive area in the field. Expression of ADAM17 in PDT sensitized treatment group was significantly increased compared to the sham PDT group by 2.78-fold (Fig5). Expression of EGFR, p-EGFR, Akt, p-Akt (Fig. 5) in tumors of PDT sensitized treatment group was significantly increased by 43.9%, 152.7%, 89.6%,and 164.2%, respectively, compared to control group. Further correlation analysis showed that expression of ADAM17, EGFR, pEGFR, Akt, and p-Akt are highly correlated to infiltrative cell islets around tumor mass with r values 0.928, 0.775, 0.870, 0.872, and 0.883, respectively.
Fig. 5.
ADAM17, EGFR, p-EGFR, Akt, p-Akt expression in tumors implanted in nude mice. The positive ADAM17, EGFR, pEGFR, Akt and p-Akt-immunoreactive area within tumors implanted in nude mice with or without pro-PDT treatment were analyzed. Expression of ADAM17, EGFR, pEGFR, Akt and p-Akt in Pro-PDT treatment group are significantly increased compare to sham PDT group (control) by 178%, 43.9%, 152.7%, 89.6% and 164.2%. Bar is 50μm. **, P<0.01. Control group: n=8, Pro-PDT treated group: n=10
These data indicate microenvironment changes induced by hypoxic stress after sublethal PDT sensitization enhanced the ADAM17-EGFR-Akt pathway in tumor, thereby, facilitating proliferation and infiltration of U87 brain tumor.
Discussion
In the current study, we demonstrated that a sublethal dose of PDT to normal brain alters the brain microenvironment, which induces hypoxia-related ADAM17 and activates its downstream EGFR-Akt signaling pathway. Tumor invasion was enhanced in PDT sensitized brain, which activated the ADAM17-EGFR-PI3K-Akt pathway activation. Our current findings indicate that microenvironment changes caused by sublethal dose of PDT facilitate tumor growth and invasiveness through, or partially through, the activation of the ADAM17-EGFR-PI3K-Akt signal pathway.
PDT causes low levels of oxygen (hypoxia) in the localy treated area[5; 6; 7]. Hypoxia initiates cellular invasive processes which occur under both physiologic and pathologic conditions, e.g. human blastocyst implantation and tumor invasion and metastasis [20]. PDT also indirectly induces other changes in the microenvironment, such as the activation of immune and inflammatory factors including VEGF and metalloproteinases [21; 22; 23].
In our current study, the increased expression of HIF-1α in nude mouse brain demonstrated that PDT induced hypoxic stress in normal brain even after a dose without significant tissue damage and architecture changes. Clinical and experimental data have provided evidence for an association between the hypoxic tumor microenvironment and metastatic disease through different signaling pathways [22; 24; 25]. Sub-lethal doses of PDT result in oxidative stress and induction of hypoxia responsive genes that are involved in cell survival and angiogenesis [13]. Tumor growth and metastasis are dependent upon infiltration, a phenotype which also correlates with a relative resistance to therapy, including the up-regulation of genes conferring resistance to apoptosis. Hypoxia induces transcriptional activation of genes that alter cellular metabolism and promote proliferation and invasion [25; 26; 27; 28; 29]. Indeed, hypoxic tumors have been reported to have a predilection for tissue invasion [30]. Therefore, hypoxia is a significant aspect in the treatment-response of glioma. In glioma, HIF-1α is a potent activator of angiogenesis and invasion through its up-regulation of target genes critical for these functions [29; 31]. We found that sublethal dose of PDT facilitates U87 tumor invasion which is characterized by an irregular tumor shape and indefinite margins, with many more invading tumor cell islets in the adjacent normal brain parenchyma. These results indicate that PDT induced-hypoxia may contribute to tumor invasion in nude mice. Increase of hypoxic stress by sublethal dose of PDT in normal brain indicates that the unavoidable lower dose of PDT to the brain adjacent to tumor (BAT) area during PDT treatment in brain tumor enhances hypoxia in nontumoral tissue, which subsequently facilitates tumor recurrence.
TACE/ADAM17 is the primary secretase responsible for releasing the soluble form of tumor necrosis factor-α (TNF-α) from the plasma membrane [32]. However, the expression of ADAM17 is ubiquitous [33] and not only limited to TNF-α-producing cells. ADAM17 also plays a pivotal role in development through the processing of numerous growth factors and growth factor receptors [34; 35; 36; 37]. A member of the ADAM family of transmembrane, multidomain zinc metalloproteinases [33; 38], ADAM17 has protease activity against multiple substrates including TNF-α, transforming growth factor-α (TGF-α), TNF receptor, interleukin-6, and L-selectin [34; 35; 36; 37; 39]. Owing to the physiological importance of ADAM17-mediated shedding of vital regulatory proteins, the mechanism of ADAM17 activation has been investigated. An increase of ADAM17 activity generally occurs when cells are exposed to cell activators such as phorbol esters, ionophores, and growth factors [40; 41; 42]. ADAM17 is involved in the proteolysis of collagen IV of the extracellular matrix (ECM) and, also in the cell surface release of several integral proteins [32; 43], suggesting that ADAM17 may influence the invasive activity of cells including brain tumor.
EGFR is commonly amplified and rearranged in glioblastoma multiforme leading to over-expression of wild type and mutant EGFRs. Expression of wild-type EGFR ligands, such as transforming growth factor-alpha (TGFα) or heparin-binding EGF (HB-EGF), is also often increased in gliomas resulting in an autocrine loop that contributes to the growth autonomy of glioma cells [44]. ADAM17 is a primary sheddase for multiple EGFR pro-ligands such as HB-EGF and TGFα [45; 46; 47]. EGFR signaling is a critical factor in glioma progression [48].
In our previous study, we have proved that a hypoxia-induced increase in ADAM17 proteolytic activity substantially contributes to invasiveness of brain tumor cells in vitro and this ADAM17-mediated hypoxia-induced tumor invasiveness is through activation of EGFR, and subsequently, the PI3K/Akt signal transduction pathway. Our current data showed sub-lethal dose of PDT induced ADAM17 expression in normal brain and then activated EGFR/Akt pathway. These microenvironment changes induced the activation of the same pathway in tumor tissues implanted subsequently to the focally-PDT treated area.
Our current data demonstrated that PDT enhanced glioma cell invasion is correlated with activation of the ADAM17-EGFR-PI3K-Akt pathway. We have previously shown that the ADAM17 and EGFR-PI3K-Akt pathway induces glioma invasion under hypoxic condition in vitro, and inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness[11]. Therefore, the current findings suggest that stimulation of ADAM17-induced EGFR-PI3K-Akt activity, most likely through HIF-1α activation, is an important signal transduction pathway for tumor invasion in PDT sensitized cerebral tissue.
The prognosis for patients with malignant gliomas has not significantly changed in recent years. Despite radiation, surgical debulking, and cytotoxic chemotherapy, median survival has changed little and is still measured in weeks [49]. Malignant gliomas are both highly vascularized and invasive, characterized by high incidence of recurrence and poor prognosis. PDT has been clinically developed as an adjuvant local therapy for brain tumors [1]. However, the efficacy of PDT treatment of CNS neoplasms remains inconclusive. A reason for the failure of PDT as an effective treatment of glioma may be due to PDT-induced activation of cellular signal pathway, which enhances tumor proliferation, invasion, and recurrence from the BAT tissue after sublethal dose of PDT. Radiation and surgical debulking are the other two main therapeutic strategies on glioma treatment. They may also induce the similar reaction to the BAT cerebral tissue due to hypoxia[50] and thermal injury caused by the cautery system during operative procedures[12].
Our data support the hypothesis that hypoxia induced activation of ADAM17-EGFR-PI3K-Akt pathway may promote tumor proliferation and invasion after traditional therapy in vivo. One possible strategy to overcome this shortcoming is to combine drugs treatment, which will counteract ADAM17-EGFR-PI3K-Akt pathway activation under hypoxic conditions, with traditional therapy, such as PDT and radiation.
Acknowledgments
This work was supported by National Institutes of Health grants PO1 CA043892 and RO1 CA100486. The authors thank Cynthia Roberts for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Henderson BW, Dougherty TJ. How does photodynamic therapy work? Photochem Photobiol. 1992;55:145–57. doi: 10.1111/j.1751-1097.1992.tb04222.x. [DOI] [PubMed] [Google Scholar]
- 2.Chopp M, Dereski MO, Madigan L, Jiang F, Logie B. Sensitivity of 9L gliosarcomas to photodynamic therapy. Radiat Res. 1996;146:461–5. [PubMed] [Google Scholar]
- 3.Muller P, Wilson B. Photodynamic therapy of brain tumours--post-operative “field fractionation”. J Photochem Photobiol B. 1991;9:117–9. doi: 10.1016/1011-1344(91)80009-7. [DOI] [PubMed] [Google Scholar]
- 4.Jiang F, Lilge L, Belcuig M, Singh G, Grenier J, Li Y, Chopp M. Photodynamic therapy using Photofrin in combination with buthionine sulfoximine (BSO) to treat 9L gliosarcoma in rat brain. Lasers Surg Med. 1998;23:161–6. doi: 10.1002/(sici)1096-9101(1998)23:3<161::aid-lsm5>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 5.Fingar VH, Kik PK, Haydon PS, Cerrito PB, Tseng M, Abang E, Wieman TJ. Analysis of acute vascular damage after photodynamic therapy using benzoporphyrin derivative (BPD) Br J Cancer. 1999;79:1702–8. doi: 10.1038/sj.bjc.6690271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Geel IP, Oppelaar H, Rijken PF, Bernsen HJ, Hagemeier NE, van der Kogel AJ, Hodgkiss RJ, Stewart FA. Vascular perfusion and hypoxic areas in RIF-1 tumours after photodynamic therapy. Br J Cancer. 1996;73:288–93. doi: 10.1038/bjc.1996.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engbrecht BW, Menon C, Kachur AV, Hahn SM, Fraker DL. Photofrin-mediated photodynamic therapy induces vascular occlusion and apoptosis in a human sarcoma xenograft model. Cancer Res. 1999;59:4334–42. [PubMed] [Google Scholar]
- 8.Mitra S, Cassar SE, Niles DJ, Puskas JA, Frelinger JG, Foster TH. Photodynamic therapy mediates the oxygen-independent activation of hypoxia-inducible factor 1alpha. Mol Cancer Ther. 2006;5:3268–74. doi: 10.1158/1535-7163.MCT-06-0421. [DOI] [PubMed] [Google Scholar]
- 9.Foster TH, Murant RS, Bryant RG, Knox RS, Gibson SL, Hilf R. Oxygen consumption and diffusion effects in photodynamic therapy. Radiat Res. 1991;126:296–303. doi: 10.2307/3577919. [DOI] [PubMed] [Google Scholar]
- 10.Busch TM, Hahn SM, Evans SM, Koch CJ. Depletion of tumor oxygenation during photodynamic therapy: detection by the hypoxia marker EF3 [2-(2-nitroimidazol-1[H]-yl)-N-(3,3,3-trifluoropropyl)acetamide ] Cancer Res. 2000;60:2636–42. [PubMed] [Google Scholar]
- 11.Zheng X, Jiang F, Katakowski M, Kalkanis SN, Hong X, Zhang X, Zhang ZG, Yang H, Chopp M. Inhibition of ADAM17 reduces hypoxia-induced brain tumor cell invasiveness. Cancer Sci. 2007;98:674–84. doi: 10.1111/j.1349-7006.2007.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Jiang F, Kalkanis SN, Yang H, Zhang Z, Katakowski M, Hong X, Zheng X, Chopp M. Combination of surgical resection and photodynamic therapy of 9L gliosarcoma in the nude rat. Photochem Photobiol. 2006;82:1704–11. doi: 10.1562/2006-06-16-RA-934. [DOI] [PubMed] [Google Scholar]
- 13.deCarvalho AC, Zhang X, Roberts C, Jiang F, Kalkanis SN, Hong X, Lu M, Chopp M. Subclinical photodynamic therapy treatment modifies the brain microenvironment and promotes glioma growth. Glia. 2007;55:1053–60. doi: 10.1002/glia.20525. [DOI] [PubMed] [Google Scholar]
- 14.Tsatas D, Kanagasundaram V, Kaye A, Novak U. EGF receptor modifies cellular responses to hyaluronan in glioblastoma cell lines. J Clin Neurosci. 2002;9:282–8. doi: 10.1054/jocn.2001.1063. [DOI] [PubMed] [Google Scholar]
- 15.Van Meter TE, Broaddus WC, Rooprai HK, Pilkington GJ, Fillmore HL. Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor-mediated signaling in gliomas. Neuro Oncol. 2004;6:188–99. doi: 10.1215/S1152851703000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laerum OD, Nygaar SJ, Steine S, Mork SJ, Engebraaten O, Peraud A, Kleihues P, Ohgaki H. Invasiveness in vitro and biological markers in human primary glioblastomas. J Neurooncol. 2001;54:1–8. doi: 10.1023/a:1012565503958. [DOI] [PubMed] [Google Scholar]
- 17.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–43. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 18.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 19.Lal A, Glazer CA, Martinson HM, Friedman HS, Archer GE, Sampson JH, Riggins GJ. Mutant epidermal growth factor receptor up-regulates molecular effectors of tumor invasion. Cancer Res. 2002;62:3335–9. [PubMed] [Google Scholar]
- 20.Canning MT, Postovit LM, Clarke SH, Graham CH. Oxygen-mediated regulation of gelatinase and tissue inhibitor of metalloproteinases-1 expression by invasive cells. Exp Cell Res. 2001;267:88–94. doi: 10.1006/excr.2001.5243. [DOI] [PubMed] [Google Scholar]
- 21.Korbelik M. PDT-associated host response and its role in the therapy outcome. Lasers Surg Med. 2006;38:500–8. doi: 10.1002/lsm.20337. [DOI] [PubMed] [Google Scholar]
- 22.Gomer CJ, Ferrario A, Luna M, Rucker N, Wong S. Photodynamic therapy: combined modality approaches targeting the tumor microenvironment. Lasers Surg Med. 2006;38:516–21. doi: 10.1002/lsm.20339. [DOI] [PubMed] [Google Scholar]
- 23.Ferrario A, Chantrain CF, von Tiehl K, Buckley S, Rucker N, Shalinsky DR, Shimada H, DeClerck YA, Gomer CJ. The matrix metalloproteinase inhibitor prinomastat enhances photodynamic therapy responsiveness in a mouse tumor model. Cancer Res. 2004;64:2328–32. doi: 10.1158/0008-5472.can-04-0071. [DOI] [PubMed] [Google Scholar]
- 24.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–50. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- 25.Munoz-Najar UM, Neurath KM, Vumbaca F, Claffey KP. Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene. 2006;25:2379–92. doi: 10.1038/sj.onc.1209273. [DOI] [PubMed] [Google Scholar]
- 26.Yoon SO, Shin S, Mercurio AM. Hypoxia stimulates carcinoma invasion by stabilizing microtubules and promoting the Rab11 trafficking of the alpha6beta4 integrin. Cancer Res. 2005;65:2761–9. doi: 10.1158/0008-5472.CAN-04-4122. [DOI] [PubMed] [Google Scholar]
- 27.Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza GL, Ellis LM. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946–56. doi: 10.1093/jnci/djh168. [DOI] [PubMed] [Google Scholar]
- 28.Graham CH, Forsdike J, Fitzgerald CJ, Macdonald-Goodfellow S. Hypoxia-mediated stimulation of carcinoma cell invasiveness via upregulation of urokinase receptor expression. Int J Cancer. 1999;80:617–23. doi: 10.1002/(sici)1097-0215(19990209)80:4<617::aid-ijc22>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 29.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1alpha in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88:2606–18. [PubMed] [Google Scholar]
- 30.Buchler P, Reber HA, Lavey RS, Tomlinson J, Buchler MW, Friess H, Hines OJ. Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model. J Surg Res. 2004;120:295–303. doi: 10.1016/j.jss.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-oncol. 2005;7:134–53. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black RA. Tumor necrosis factor-alpha converting enzyme. Int J Biochem Cell Biol. 2002;34:1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 33.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 34.Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–7. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 35.Zatovicova M, Sedlakova O, Svastova E, Ohradanova A, Ciampor F, Arribas J, Pastorek J, Pastorekova S. Ectodomain shedding of the hypoxia-induced carbonic anhydrase IX is a metalloprotease-dependent process regulated by TACE/ADAM17. Br J Cancer. 2005;93:1267–76. doi: 10.1038/sj.bjc.6602861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowe PD, Walter BN, Mohler KM, Otten-Evans C, Black RA, Ware CF. A metalloprotease inhibitor blocks shedding of the 80-kD TNF receptor and TNF processing in T lymphocytes. J Exp Med. 1995;181:1205–10. doi: 10.1084/jem.181.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mullberg J, Durie FH, Otten-Evans C, Alderson MR, Rose-John S, Cosman D, Black RA, Mohler KM. A metalloprotease inhibitor blocks shedding of the IL-6 receptor and the p60 TNF receptor. J Immunol. 1995;155:5198–205. [PubMed] [Google Scholar]
- 38.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Becherer JD, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733–6. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 39.Bennett TA, Lynam EB, Sklar LA, Rogelj S. Hydroxamate-based metalloprotease inhibitor blocks shedding of L-selectin adhesion molecule from leukocytes: functional consequences for neutrophil aggregation. J Immunol. 1996;156:3093–7. [PubMed] [Google Scholar]
- 40.Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J. 1997;321(Pt 2):265–79. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan H, Derynck R. Ectodomain shedding of TGF-alpha and other transmembrane proteins is induced by receptor tyrosine kinase activation and MAP kinase signaling cascades. Embo J. 1999;18:6962–72. doi: 10.1093/emboj/18.24.6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan H, Turck CW, Derynck R. Characterization of growth factor-induced serine phosphorylation of tumor necrosis factor-alpha converting enzyme and of an alternatively translated polypeptide. J Biol Chem. 2003;278:18617–27. doi: 10.1074/jbc.M300331200. [DOI] [PubMed] [Google Scholar]
- 43.Bauvois B. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene. 2004;23:317–29. doi: 10.1038/sj.onc.1207124. [DOI] [PubMed] [Google Scholar]
- 44.Ramnarain DB, Park S, Lee DY, Hatanpaa KJ, Scoggin SO, Otu H, Libermann TA, Raisanen JM, Ashfaq R, Wong ET, Wu J, Elliott R, Habib AA. Differential gene expression analysis reveals generation of an autocrine loop by a mutant epidermal growth factor receptor in glioma cells. Cancer Res. 2006;66:867–74. doi: 10.1158/0008-5472.CAN-05-2753. [DOI] [PubMed] [Google Scholar]
- 45.Lee DC, Sunnarborg SW, Hinkle CL, Myers TJ, Stevenson MY, Russell WE, Castner BJ, Gerhart MJ, Paxton RJ, Black RA, Chang A, Jackson LF. TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann N Y Acad Sci. 2003;995:22–38. doi: 10.1111/j.1749-6632.2003.tb03207.x. [DOI] [PubMed] [Google Scholar]
- 46.Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004;64:1943–50. doi: 10.1158/0008-5472.can-03-3720. [DOI] [PubMed] [Google Scholar]
- 47.Ruhe JE, Streit S, Hart S, Ullrich A. EGFR signaling leads to downregulation of PTP-LAR via TACE-mediated proteolytic processing. Cell Signal. 2006;18:1515–27. doi: 10.1016/j.cellsig.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Andersson U, Guo D, Malmer B, Bergenheim AT, Brannstrom T, Hedman H, Henriksson R. Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol (Berl) 2004;108:135–42. doi: 10.1007/s00401-004-0875-6. [DOI] [PubMed] [Google Scholar]
- 49.Mikkelsen T. Cytostatic Agents in the Management of Malignant Gliomas. Cancer Control. 1998;5:150–162. doi: 10.1177/107327489800500206. [DOI] [PubMed] [Google Scholar]
- 50.Brown JM. Tumor hypoxia in cancer therapy. Methods Enzymol. 2007;435:297–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]