Abstract
No longer considered to be exclusive to cellular developmental pathways, the Wnt family of secreted cysteine-rich glycosylated proteins has emerged as versatile targets for a variety of conditions that involve cardiovascular disease, aging, cancer, diabetes, neurodegeneration, and inflammation. In particular, modulation of Wnt signaling may fill a critical void for the treatment of disorders that impact upon both cellular survival and cellular longevity. Yet, in some scenarios, Wnt signaling can become the catalyst for disease development or promote cell senescence that can compromise clinical utility. This double edge sword in regards to the role of Wnt and its signaling pathways highlights the critical need to further elucidate the cellular mechanisms governed by Wnt in conjunction with the development of robust pharmacological ligands that may open new avenues for disease treatment. Here we discuss the influence of the Wnt pathway during cell survival, metabolism, and aging in order for one to gain a greater insight for the novel role of Wnt signaling as well as exemplify its unique cellular pathways that influence both normal physiology and disease.
Keywords: Alzheimer’s disease, apoptosis, cardiac, diabetes, stem cells, Wnt
1. Introduction
1.1 The Discovery of the Wnt Pathway
Wnt proteins, derived from the Drosophila Wingless (Wg) and the mouse Int-1 genes, have been shown to play a role in both cell development and cell demise (Chong et al., 2007a; Chong et al., 2007c; Li et al., 2006c; Speese & Budnik, 2007). Wnt proteins are secreted cysteine-rich glycosylated proteins that play a role in a variety of cellular functions that involve embryonic cell proliferation, differentiation, survival, and death (Li et al., 2006c; Patapoutian & Reichardt, 2000; Wodarz & Nusse, 1998). More than eighty target genes of Wnt signaling pathways have been demonstrated in human, mouse, Drosophila, Xenopus, and zebrafish. This representation encompasses several cellular populations, such as neurons, cardiomyocytes, endothelial cells, cancer cells, and pre-adipocytes (Chong & Maiese, 2004; Li et al., 2005). Furthermore, at least nineteen of twenty-four Wnt genes that express Wnt proteins have been identified in the human.
The Wnt pathway was initially identified as a proto-oncogene in mammary tumors that was activated by integration of the mouse mammary virus (Nusse & Varmus, 1982). Since then, components of the Wnt signaling pathway have begin linked to tumorigenesis such as with adenomatous polyposis (Munemitsu et al., 1995), colon carcinoma (Morin et al., 1997), medulloblastoma (Dahmen et al., 2001; Sauvageot et al., 2007), tuberous sclerosis (Jozwiak & Wlodarski, 2006), and lung cancer (Xu et al., 2007). Subsequent work has demonstrated the importance of Wnt-Frizzled (FZD) transduction pathway in controlling the pattern of the body axis as well as the development and maturation of the central nervous system (Augustine et al., 1993; Ikeya et al., 1997), cardiovascular system (Marvin et al., 2001; Naito et al., 2006; Palpant et al., 2007; Singh et al., 2007), and the limbs (Kengaku et al., 1997) (Table 1).
Table 1.
Cellular Expression of the Wnt and the Wnt-FZD Receptor with Biological Response
| Cellular Expression of Wnt | Cellular Expression of Wnt-FZD Receptor | Biological Response |
|---|---|---|
| Neurons | Neurons | Brain development and resistance to injury |
| Astrocytes | Astrocytes | Brain development and protection |
| Progenitor stem cells | Progenitor stem cells | Cellular development and maturation |
| Endothelial cells | Endothelial cells | Angiogenesis |
| Progenitor vascular stem cells | Progenitor vascular stem cells | Angiogenesis and cardiomyogenesis |
| Vascular smooth muscle cells | Vascular smooth muscle cells | Angiogenesis, vascular remodeling, and cytoprotection |
| Progenitor cardiac stem cells | Progenitor cardiac stem cells | Cardiomyogenesis |
| Endocardial cells | Endocardial cells | Endocardial cushion formation |
| Cardiomyocytes | Cardiomyocytes | Cardiac remodeling and cytoprotection |
During embryological development, alternations of the Wnt-FZD pathway can lead to abnormal morphogenesis in animal models (Ikeya et al., 1997; Liu et al., 1999; Stark et al., 1994) and congenital defects in humans (Jordan et al., 2001; Niemann et al., 2004; Rodova et al., 2002). In mature tissues, the Wnt-FZD pathway is involved in the self-renewal of pluripotent embryonic stem cells (Bakre et al., 2007), bone formation (Canalis et al., 2007), and may be responsible for the maintenance of many normal tissues (He et al., 2004; Reya et al., 2003; Ross et al., 2000; Willert et al., 2003) as well as cellular senescence (Liu et al., 2007). Other studies have revealed that dysfunction of the Wnt-FZD pathway can lead to neurodegenerative disorders, such as Alzheimer’s disease (Balaraman et al., 2006; Chong et al., 2007a; Marambaud et al., 2002; Morin et al., 2004; Soriano et al., 2001) and heart failure (Barandon et al., 2003; Barandon et al., 2005; Li et al., 2006c; van de Schans et al., 2007).
1.2 Functional Classes and Receptors of Wnt Proteins
The molecular structural characteristics that all Wnt proteins share with varying degrees of sequence identity involve the 39–46 kDa lipid-modified secreted glycoproteins containing 350–400 amino acids with a highly conserved pattern of 23–24 cysteine residues and several asparagines-linked glycosylation sites (Li et al., 2005, 2006c). Some Wnt proteins also have an additional domain, such as the Drosophila Wg, that contains an 85-amino acid domain near the center of the protein (Nusse & Varmus, 1992). Wnt proteins are generally divided into functional classes based on their ability to induce a secondary body axis in Xenopus embryos and to activate certain signaling cascades that consist of the Wnt1 class and the Wnt5a class. These involve intracellular signaling pathways that are critical for Wnt signal transduction. However, it should be stated that the lines between these pathways are sometimes blurred and not distinct, especially with the reliance upon common pathways that can involve calcium signaling and Dishevelled (DVL), a cytoplasmic multifunctional phosphoprotein (Axelrod et al., 1998; Boutros et al., 1998). The mammalian DVL protein family contains DVL-1, DVL-2, and DVL-3. DVL family members have three conserved domains that include an N-terminal DIX domain named for DVL and Axin, a central PDZ domain termed for Postsynaptic density-95, Discs-large and Zonula occludens-1, and a C-terminal DEP that is named for DVL, Egl-10 and Pleckstrin. DVL is a key transducer of Wnt signaling that acts at the plasma membrane or in the cytoplasm in all three Wnt-FZD signaling pathways. Yet, DVL also acts within the nucleus, since nuclear localization of DVL can be vital for functioning in the Wnt-FZD signaling pathway (Itoh et al., 2005).
One of the Wnt pathways controls target gene transcription through β-catenin, generally referred to as the canonical pathway that involves Wnt1, Wnt3a, and Wnt8 (Figure 1). The members of the Wnt1 class are inducers of a secondary body axis in Xenopus and include Wnt1, Wnt2, Wnt3, Wnt3a, Wnt8, and Wnt8a. Wnt proteins of this class facilitate activation of the FZD transmembrane receptor and the co-receptor lipoprotein related protein 5 and 6 (LRP-5/6). Ultimately, this leads to the activation of the Wnt/β-catenin pathway and DVL.
Figure 1. Intracellular signaling for Wnt is mediated through the canonical and non-canonical pathways.
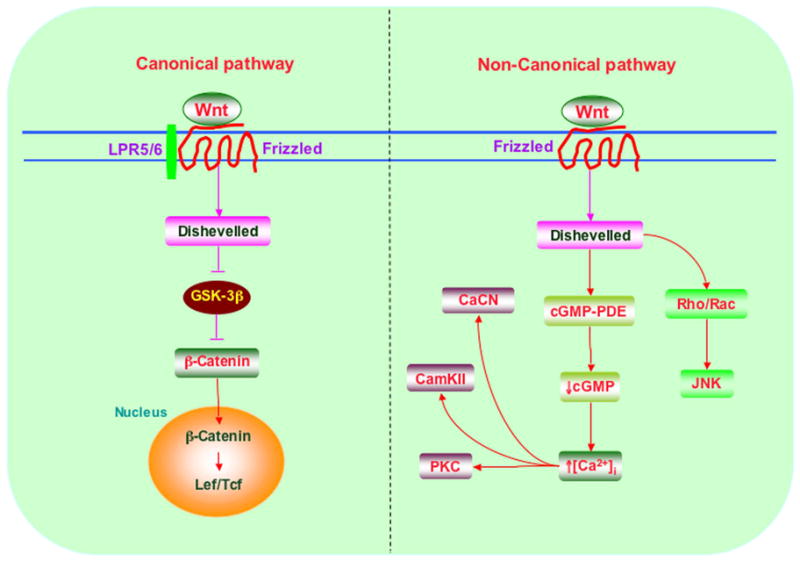
A representative schematic for the canonical and non-canonical pathways is shown. The canonical Wnt signaling pathway is referred to as the Wnt/β-catenin pathway since it can regulate β-catenin. All Wnt signaling pathways are initiated by interaction of Wnt proteins with Frizzled (FZD) receptors. The Wnt signaling pathway will only be activated if the binding of the Wnt protein to the FZD receptor takes place in the presence of the co-receptor LRP-5/6 resulting in the formation of a Wnt-FZD-LRP-5/6 tri-molecular complex. Once Wnt protein binds to the FZD receptor and the co-receptor LRP-5/6, this is followed by recruitment of Dishevelled (DVL). DVL is phosphorylated by casein kinase Iε to form a complex with Frat1 and inhibit glycogen synthase kinase (GSK-3β) activity. The non-canonical or atypical Wnt signaling pathway has two intracellular signaling cascades that consist of the Wnt/Ca2+ pathway and the Wnt/PCP pathway. In the Wnt/Ca2+ pathway, Wnt protein binds to FZD receptors on the cell surface resulting in several cellular processes that involve stimulation of heterotrimeric G proteins, increased intracellular Ca2+ release, decreased cyclic guanosine monophosphate (cGMP) levels, and activation of the two kinases Ca2+-calmodulin-dependent protein kinase II (CamKII) or calcineurin (CaCN) and protein kinase C (PKC). Activation of these pathways can stimulate several transcription factors. In the Wnt/PCP pathway, Wnt proteins bind to FZD receptors on the cell surface followed by activating Rho/Rac small GTPase and Jun N-terminal kinase (JNK) to assist with cytoskeletal organization and gene expression.
Other Wnt pathways pertain to intracellular calcium (Ca2+) release (Figure 1). These involve the non-canonical or Wnt/Ca2+ pathway consisting primarily of Wnt-4, Wnt-5a, and Wnt-11 that functions through non-β-catenin-dependent pathways and also include the planar cell polarity (PCP) pathway (Patapoutian & Reichardt, 2000) and the Wnt-Ca2+-dependent pathways (Patapoutian & Reichardt, 2000; Slusarski et al., 1997). The Wnt5a class cannot induce secondary axis formation in Xenopus and includes the Wnt proteins of Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a, and Wnt11. Wnt proteins in the PCP pathway bind the FZD transmembrane receptor and employ DVL to activate effector pathways through Rho/Rac small GTPase and Jun N-terminal kinase (JNK) (Seifert & Mlodzik, 2007). In the Wnt-Ca2+-dependent pathways, calcium dependent kinases are activated through G-protein signaling that leads to elevations in intracellular Ca2+ either through cGMP or phospholipase activation (Ma & Wang, 2006; Schulte & Bryja, 2007; Slusarski et al., 1997).
The receptors of Wnt proteins consist of at least ten mammalian isoforms of the FZD family. The FZD proteins are named after the first member of the Drosophila tissue polarity gene Frizzled (Adler et al., 1990; Vinson et al., 1989). Members of the FZD protein family are listed as a distinct family of G-protein-coupled receptors (Foord et al., 2005). The FZD proteins have a N-terminal signal peptide, an extracellular domain that contains a 120-amino acids, a cysteine-rich domain followed by a hydrophilic linker region that shows little sequence similarity among family members, a highly conserved seven-transmembrane domain separated by short extracellular and cytoplasmic loops, and a cytoplasmic domain of variable size and little sequence homology among family members (Adler et al., 1990; Vinson et al., 1989; Wodarz & Nusse, 1998). Some Wnt proteins, such as Wnt8, can directly bind with the full-length FZD receptor protein. A single Wnt protein also can bind to a combination of FZD receptor proteins, including homologous members from a different species (Hsieh et al., 1999b). Interestingly, FZD proteins can bind proteins from other protein families, such as R-spondin and Norrin, and the FZD cysteine-rich domain also exists in several other proteins that include the soluble secreted FZD-related proteins (sFRPs) (Mayr et al., 1997; Melkonyan et al., 1997), some receptors of tyrosine kinases (Jennings et al., 1993; Wilson et al., 1993), carboxypeptidase Z (Song & Fricker, 1997), the membrane-bound serine protease Corin (Yan et al., 1999), and an isoform of collagen (Rehn & Pihlajaniemi, 1995). These proteins appear to function as important regulators during Wnt-FZD signaling. For example, the sFRPs have been found to function as antagonists of the Wnt pathway (Hsieh et al., 1999a; Mayr et al., 1997; Melkonyan et al., 1997).
In addition to the FZD protein receptors, other obligate co-receptors also are necessary for canonical Wnt-FZD signaling pathway. A transmembrane protein termed LRP-5/6 from the low-density-lipoprotein receptor family is required (Pinson et al., 2000; Tamai et al., 2000; Wehrli et al., 2000). In the canonical Wnt-FZD pathway, Wnt binds to both the FZD receptor and the co-receptor low-density lipoprotein receptor-related protein 5/6 (LRP-5/6) (Wehrli et al., 2000) resulting in the inhibition of the downstream component glycogen synthase kinase-3β (GSK-3β). Wnt signaling also can be transmitted through the binding of extracellular domain of LRP-5/6 to Axin, a key component in the GSK-3β complex, indicating that the LRP-5/6 receptor is an important part of the Wnt-FZD signaling pathway (Mao et al., 2001; Tolwinski et al., 2003).
Ryk also represents another co-receptor that belongs to one of divergent members of the receptor tyrosine kinase family. Ryk not only can form a complex with FZD proteins such as the co-receptor LRP-5/6 resulting in activation of the canonical Wnt-FZD signaling pathway, but also can regulate the non-canonical Wnt-FZD signaling pathway through FZD independent pathways (Bejsovec, 2005; Lu et al., 2004). The molecular structure of all Ryk genes is characterized by an extracellular domain with homology to Wnt inhibitory factor-1 (WIF1), a single transmembrane-spanning sequence to which Wnt proteins bind (Schneider et al., 1999). In addition, a conserved intracellular PDZ-binding motif exists which links Ryk to downstream molecules of Wnt-FZD signaling pathway, such as DVL (Bejsovec, 2005; Lu et al., 2004). Wnt proteins can bind to the extracellular domain of the Ryk receptor through the intracellular PDZ-binding domain in the Ryk receptor to regulate multiple cellular functions through either the canonical or the non- canonical Wnt-FZD signaling pathway. For example, mammalian Ryk can function as a co-receptor with FZD to bind to Wnt1 and Wnt3a through its WIF domain and interact with DVL via its PDZ domain resulting in the stimulation of neurite outgrowth (Lu et al., 2004).
1.3 The Canonical and Non-Canonical Wnt Pathways
The canonical Wnt signaling pathway is referred to as the Wnt/β-catenin pathway since it can regulate β-catenin protein levels to control the activation of Wnt-responsive target genes (Figure 1). All Wnt signaling pathways are initiated by interaction of Wnt proteins with FZD receptors, but in this pathway, the Wnt signaling pathway will only be activated if the binding of the Wnt protein to the FZD receptor takes place in the presence of the co-receptor LRP-5/6 resulting in the formation of a Wnt-FZD-LRP-5/6 tri-molecular complex (Mao et al., 2001; Pinson et al., 2000; Wehrli et al., 2000). Once Wnt protein binds to the FZD receptor and the co-receptor LRP-5/6, this is followed by recruitment of DVL. DVL is phosphorylated by casein kinase Iε to form a complex with Frat1 and inhibit GSK-3β activity (Kishida et al., 2001; Lee et al., 1999; Papkoff & Aikawa, 1998).
The formation of the Wnt-FZD-LRP-5/6 complex also promotes the LRP-5/6-mediated degradation of Axin (Mao et al., 2001). The inhibition of GSK-3β activity by Wnt with the degradation of Axin blocks the formation of the protein complex consisting of GSK-3β, Axin, and adenomatous polyposis coli (APC) tumor suppressor protein. If the formation of the protein complex of GSK-3β, Axin and APC tumor suppressor protein does not occur, accumulation of free β-catenin results for translocation to the nucleus (Ikeda et al., 1998). Once positioned in the nucleus, the free β-catenin acts as a transcription factor and activates Tcf and Lef by forming nuclear complexes with members of the Tcf/Lef transcription factor family (Ishitani et al., 2003). This leads to the transcription and expression of a variety of Wnt-responsive target genes such as c-Myc (He et al., 1998), cyclin D1 (Shtutman et al., 1999; Tetsu & McCormick, 1999), and Axin 2 (Jho et al., 2002; Lustig et al., 2002). In addition, the complexes of Tcf/Lef and β-catenin may cooperate with factors activated by other signaling pathways to alter cellular remodeling processes.
The canonical Wnt signaling pathway also is activated by several other cellular mechanisms. The shifting of proteins from the cadherin-bound pool to the cytoplasmic pool can increase the amount of available free β-catenin for the activation of target genes. Several receptor tyrosine kinases can phosphorylate tyrosine residues of the β-catenin and cadherin-catenin complex to allow β-catenin to become dissociated from the complex and increase the amount of β-catenin in the cytoplasm for subsequent translocation to the nucleus. Furthermore, surface receptors, such as epidermal growth factor receptor, c-RON, cErbB2, and erythropoietin receptor (EPOR) can then stimulate the canonical Wnt signaling pathway (Bonvini et al., 2001; Danilkovitch-Miagkova et al., 2001; Graham & Asthagiri, 2004; Li et al., 2006b). In addition, both insulin-like growth factor and erythropoietin (EPO) lead to β-catenin stabilization (Li et al., 2006b; Playford et al., 2000). The canonical Wnt signaling pathway also regulates cyclin D1 through the inhibition of GSK-3β and cAMP-responsive element-binding protein (CREB) pathway (D’Amico et al., 2000) as well as protein kinase A and CREB (Chen et al., 2005).
The non-canonical or atypical Wnt signaling pathway has two intracellular signaling cascades that consist of the Wnt/Ca2+ pathway and the Wnt/PCP pathway (Figure 1). In the Wnt/Ca2+ pathway, Wnt protein binds to FZD receptors on the cell surface resulting in several cellular processes that involve stimulation of heterotrimeric G proteins, increased intracellular Ca2+ release, decreased cyclic guanosine monophosphate (cGMP) levels, and activation of the two kinases Ca2+-calmodulin-dependent protein kinase II (CamKII) or calcineurin (CaCN) and protein kinase C (PKC). Activation of these pathways can stimulate nuclear factor (NF)-AT and other transcription factors (Li et al., 2006c; Wang & Malbon, 2003). Thus, the Wnt/Ca2+ pathway is most likely a G-protein dependent signaling pathway (Chong & Maiese, 2004; Maiese et al., 2005a; Wang & Malbon, 2004). In the Wnt/PCP pathway, Wnt proteins bind to FZD receptors on the cell surface followed by activating Rho/Rac small GTPase (Habas et al., 2003) and JNK (Moriguchi et al., 1999) to assist in the subsequent regulation of cytoskeletal organization and gene expression.
Interestingly, several of the downstream proteins identified in the Wnt-FZD signaling pathway can independently function with proteins from other cellular systems. For example, DVL is able to directly regulate JNK activity (Li et al., 1999) and GSK-3β activity (Boutros et al., 1998). Free β-catenin also can form a complex with α-catenin and members of cadherins family to function as a structural adaptor protein linking cadherins to the actin cytoskeleton in cell-cell adhesion processes. Association with cadherins can effectively sequester β-catenin from the cytoplasmic pool that is responsive to Wnt-FZD signaling (Fagotto et al., 1996; Sadot et al., 1998). As a result, the modulation of cadherin expression and function can indirectly regulate the Wnt-FZD signaling pathway through β-catenin. Because both cadherins and Wnt proteins have been demonstrated to regulate aspects of synapse formation, the interaction between these proteins also may play a critical role in the development of nervous system (Hall et al., 2000; Patapoutian & Reichardt, 2000; Tanaka et al., 2000).
2. The Wnt Pathway in Development and Metabolism
2.1 The Wnt Pathway, Stem Cells, and Development
The Wnt-FZD signaling pathway forms a critical component for the development of the brain, spinal cord, and the cardiovascular system (Table 1). Wnt can regulate the course of progenitor cells in various regions of the nervous system (Bronner-Fraser, 2004; Hirabayashi et al., 2004; Lee et al., 2004; Muroyama et al., 2004). Wnt pathway components that involve β-catenin and GSK-3β can control hippocampal and subventricular zone progenitor cell proliferation and differentiation (Adachi et al., 2007; Wexler et al., 2007). Modulation of β-catenin in the Wnt pathway also may be involved in the control of the orphan nuclear receptor Nurr1 that is vital for the development and maintenance of midbrain dopaminergic neurons (Kitagawa et al., 2007). The Wnt pathway, such as through Wnt1, also controls neuronal proliferation in the caudal midbrian region (Panhuysen et al., 2004). Even during periods of injury, such as during ischemia, Wnt signaling has recently been associated with neural stem cell proliferation (Wang et al., 2007b). However, abnormal Wnt signaling can lead to progenitor cell proliferation, but ultimately may not influence cell survival or differentiation (Chevallier et al., 2005).
In regards to the cardiovascular system, Wnt signaling has recently been shown to mediate the development of early blood and endothelial cells from human embryonic stem cells (Woll et al., 2007) (Table 1). Activation of the Wnt pathway through Wnt3a promotes the development of multipotential mesendodermal progenitor cells to eventually differentiate along endothelial, cardiac, and vascular smooth muscle lineages (Bakre et al., 2007). Wnt2 also has been found to influence progenitor cells to differentiate along endothelial and cardiac lineages (Wang et al., 2007a). Furthermore, canonical Wnt signaling fosters the expression of mammalian cardiac progenitors (Kwon et al., 2007) as well as right ventricular growth (Ai et al., 2007).
During cardiac development, Wnt9a has been found to stimulate β-catenin-responsive transcription in avian cardiac atrioventricular endocardial cushions and lead to cell proliferation (Person et al., 2005). It also has been shown that conditional targeting of APC, a protein that can down-regulate intracellular levels of β-catenin in the neural crest, yields apoptosis of cardiac neural crest cells, resulting in cardiac anomalies at birth (Hasegawa et al., 2002). The Wnt/β-catenin also assists with the aggregation of cardiomyocytes during development (Toyofuku et al., 2000). Recent work that has generated conditional β-catenin-deletion mutant animals in the proepicardium demonstrate that the epicardial β-catenin pathway is required for the development of the subepicardial space and the differentiation of epicardium-derived mesenchymal cells into coronary smooth muscle cells (Zamora et al., 2007). These studies suggest that Wnt signaling and β-catenin are required for proper development of cardiac tissue. Several studies also provide support for the ability of the Wnt system through GSK-3β to regulate cardiac development and hypertophy (Hardt & Sadoshima, 2002). In addition, the BMP pathway appears to be vital for the specification of the first heart field, but that Wnt/β-catenin signaling regulates the second heart-field (Klaus et al., 2007). Other studies that examine β-catenin expression in the avian mesonephros, a transitory embryonic kidney that is used in the study of vascular development and degeneration, have shown that degenerating mesonephros and glomerular capillary tufts had significantly depressed β-catenin expression. These observations are in contrast to viable cells with prominent β-catenin expression, suggesting that β-catenin expression was linked to remodeling of the vascular system (Nacher et al., 2005).
Timing of events, temporal location, and non-canonical pathways also appear to be notable factors to mention. Inhibition of Wnt signaling can promote cardiac formation in the anterior lateral mesoderm, but active Wnt signaling in the posterior lateral mesoderm is required for blood development (Marvin et al., 2001). Activation of the Wnt/β-catenin pathway in the early phase during embryoid body (EB) formation enhances embryonic stem cell differentiation into cardiomyocytes, but activation of this pathway in the late phase after EB formation inhibits cardiomyocyte differentiation and enhances the expression of hematopoietic/vascular marker genes through suppression of BMP (Naito et al., 2006). Non-canonical Wnt pathways and antagonists of the Wnt pathway also play a role with cardiac development, such as with stromal vascular cells derived from the stromal vascular fraction of adipose tissue (Palpant et al., 2007) and embryonic stem cell differentiation into cardiomyocytes (Singh et al., 2007). In addition, Wnt11 can promote cardiomyogenic differentiation of human circulating endothelial progenitor cells through activating the non-canonical protein kinase C (PKC)-dependent signaling pathway (Koyanagi et al., 2005).
A number of studies have demonstrated that several components of the Wnt-FZD signaling pathway also can oversee cell proliferation and migration during development in the nervous system. In Xenopus embryos, the Wnt-FZD signaling pathway has been shown to activate neuronal development through inhibition of bone morphogenetic protein (BMP) 4 expression (Baker et al., 1999). BMP 5 also is involved in dorsal pattern specification, which may play an important role in dorsal-ventral patterning of the developing brain which is ultimately under control of the Wnt-FZD pathway (Ellies et al., 2000; Golden et al., 1999). BMPs through Wnt signaling also can increase the number of tyrosine hydroxylase-positive locus coeruleus neurons (Holm et al., 2006). BMP and a Wnt-BMP signaling loop can regulate cell proliferation, migration, and axonal guidance of neurons in the developing nervous system (Chizhikov & Millen, 2005; Yeo & Gautier, 2004). It also appears that blockade of Wnt8 function, as shown by over-expression of a dominant negative Wnt8, can inhibit the expression of neural crest markers, suggesting that Wnt signaling pathway also is necessary for neural crest induction (LaBonne & Bronner-Fraser, 1998; Lewis et al., 2004). Other studies that employ gain-and loss-of-function for Wnt signaling have demonstrated that the Wnt-FZD signaling pathway plays a critical role either in neural crest induction or in the specification of the neural crest competence territory (Bastidas et al., 2004). Wnt genes, genes encoding FZD- Wnt receptors, or secreted FZD-related proteins and Tcf/Lef-1 transcription factors, also are expressed in postnatal mouse cerebral cortex, indicating that Wnt signaling represents a major cortical input during embryonic brain development (Shimogori et al., 2004). Furthermore, in models of C. elegans, the Wnt-FZD pathway may regulate the subcellular positioning of presynaptic terminals to determine synaptic connections in the nervous system (Klassen & Shen, 2007). Interestingly, the Wnt pathway may control only specific elements of development, such as cell adhesion, but not retinal neurogenesis (Fu et al., 2006).
Additional work demonstrates that autoregulation of the canonical Wnt/β-catenin signaling pathway can control midbrain development through the expression of transcription factor Tcf-4 isoforms and Wnt2b (Kunz et al., 2004). In addition, Wnt signaling can foster midbrain differentiation into dopaminergic neurons through GSK-3β modulation (Castelo-Branco et al., 2004). Lef1/Tcf proteins also regulate the generation of dentate gyrus granule cells and the development of the hippocampus (Galceran et al., 2000). Mouse embryos homozygous for a Lef1-lacZ fusion gene, which encodes a protein that not only is deficient in DNA binding, but also interferes with β-catenin-mediated transcriptional activation by other Lef1/Tcf proteins, are absent of the hippocampal structure. Other work demonstrates roles for DVL, Rac, and JNK signaling pathways during neuronal development. Wnt7b and DVL can activate Rac and JNK signaling pathways to promote dendritic branching in cultured hippocampal neurons, since application of dominant-negative Rac, administration of dominant-negative JNK, or inhibition of JNK activity can inhibit DVL -mediated dendritic growth (Rosso et al., 2005).
It is important to note that during development of the neuronal and cardiovascular systems, the modulation of apoptotic pathways by Wnt signaling is a critical component for the regulation of cell and tissue growth. Wnt signaling can either facilitate or prevent apoptosis depending upon the environmental stimuli. For example, Wnt proteins can regulate apoptosis within rhombomeres 3 and 5 in the developing hindbrain and in limb buds during vertebrate limb development to control growth of the hindbrain and limbs (Ellies et al., 2000; Grotewold & Ruther, 2002a, 2002b). In addition, sFRPs that function as antagonists of the Wnt-FZD pathway can also modulate apoptosis during development. For example, there exists a negative relationship between the expression of sFRP2 and the occurrence of apoptosis in rhombomeres 3 and 5. The over-expression of sFRP2 in the rhombencephalic neural crest can prevent the apoptosis of premigratory neural crest cells from rhombomeres 3 and 5 by inhibiting the expression of Wnt1 and BMP 4. In contrast, depleting sFRP2 function or over-expressing Wnt1 in rhombomeres results in apoptosis (Ellies et al., 2000). sFRP2 also can function to inhibit Wnt3a expression during cardiomyogenesis as a feedback mechanism (Deb et al., 2007) and regulate myocardial tissue repair following ischemic injury (Mirotsou et al., 2007). The Wnt-β-catenin signaling pathway also is involved in the regulation of apoptotic cell loss during development and can prevent apoptosis through the regulation of β-catenin and Tcf/Lef. In β-catenin mutant embryos, the removal of β-catenin can lead to apoptotic loss of the hindbrain, the melanocyte lineage, neural crest cells, sensory neurons, and dorsal root ganglia (Brault et al., 2001; Hari et al., 2002). Over-expression of exogenous Wnt1 results in the protection of cells against c-Myc induced apoptosis through induction of β-catenin, cyclooxygenase-2, and Wnt1 induced secreted protein (WISP-1) (You et al., 2002).
2.2 The Wnt Pathway and Metabolic Disease
A body of recent work suggests that the Wnt signaling pathway has a significant role in cellular metabolism, especially in disorders that involve diabetes mellitus (DM). DM is a significant health concern for both young and older populations (Maiese et al., 2007a; Maiese et al., 2007c). Approximately 16 million individuals in the United States and more than 165 million individuals worldwide suffer from DM. By the year 2030, it is predicted that more than 360 million individuals will be afflicted with DM and its debilitating conditions (Wild et al., 2004). Type 2 DM represents at least 80 percent of all diabetics and is dramatically increasing in incidence as a result of changes in human behavior and increased body mass index (Laakso, 2001). Type 1 insulin-dependent diabetes mellitus accounts for only 5–10 percent of all diabetics (Maiese et al., 2007c), but is increasing in adolescent minority groups (Dabelea et al., 2007). Yet, the incidence of undiagnosed diabetes, impaired glucose tolerance, and fluctuations in serum glucose in the young raises further concerns (Jacobson et al., 2007). Individuals with impaired glucose tolerance have a greater than two times the risk for the development of diabetic complications than individuals with normal glucose tolerance (Harris & Eastman, 2000).
In regards to the vascular and nervous systems, patients with DM can develop severe neurological and vascular disease (Donahoe et al., 2007) that can lead to an increased risk for cognitive decline (Chong et al., 2005d; Li et al., 2006a; Schnaider Beeri et al., 2004). Disease of the nervous system can become the most debilitating complications for DM and affect sensitive cognitive regions of the brain, such as the hippocampus that modulates memory function, resulting in significant functional impairment and dementia (Awad et al., 2004). DM also has been found to increase the risk for vascular dementia in elderly subjects (Schnaider Beeri et al., 2004; Xu et al., 2004) as well as potentially alter the course of Alzheimer’s disease. Although some studies have found that diabetic patients may have less neuritic plaques and neurofibrillary tangles than non-diabetic patients (Beeri et al., 2005), contrasting work suggests a modest adjusted relative risk of Alzheimer’s disease in patients with diabetes as compared with those without diabetes to be 1.3 (Luchsinger et al., 2001). Furthermore, costs to care for cognitive impairments resulting from diabetes that can mimic Alzheimer’s disease can approach $100 billion a year (Maiese & Chong, 2004; McCormick et al., 2001; Mendiondo et al., 2001).
Interestingly, the development of insulin resistance and the complications of DM in the nervous and vascular systems can be the result of cellular oxidative stress (Maiese et al., 2007a; Maiese et al., 2007c). In patients with DM, elevated levels of ceruloplasmin are suggestive of increased reactive oxygen species (Memisogullari & Bakan, 2004) and acute glucose fluctuations may promote oxidative stress (Monnier et al., 2006). Hyperglycemia can lead to increased production of reactive oxygen species in endothelial cells, liver and pancreatic β-cells (Ceriello et al., 1996; Ihara et al., 1999; Ling et al., 2003; Yano et al., 2004). Prolonged duration of hyperglycemia is not necessary to lead to oxidative stress injury, since even short periods of hyperglycemia, generate reactive oxygen species, such as in vascular cells (Yano et al., 2004). Recent clinical correlates support these experimental studies to show that acute glucose swings in addition to chronic hyperglycemia can trigger oxidative stress mechanisms during type 2 DM, illustrating the importance for therapeutic interventions during acute and sustained hyperglycemic episodes (Monnier et al., 2006).
The preservation of cellular energy reserves is dependent upon the maintenance of mitochondrial integrity during DM (Newsholme et al., 2007). For example, chronic exposure to elevated levels of free fatty acids can increase reactive oxygen species production in cells and has been shown to lead to mitochondrial DNA damage and impaired pancreatic β-cell function (Rachek et al., 2006). In patients with type 2 DM, skeletal muscle mitochondria have been described to be smaller than those in control subjects (Kelley et al., 2002). Furthermore, a decrease in the levels of mitochondrial proteins and mitochondrial DNA in adipocytes has been correlated with the development of type 2 DM (Choo et al., 2006). Insulin resistance in the elderly also has been associated with elevation in fat accumulation and altered mitochondrial oxidative and phosphorylation activity (Petersen et al., 2003; Pospisilik et al., 2007).
Current studies suggests that abnormalities in the Wnt signaling pathways, such as with transcription factor 7-like 2 gene, may impart increased risk for type 2 DM in some populations (Grant et al., 2006; Lehman et al., 2007; Scott et al., 2006) as well as have increased association with obesity (Guo et al., 2006). Additional work has described the expression of Wnt5b in adipose tissue, the pancreas, and the liver in diabetic patients, suggesting a potential regulation of adipose cell function (Kanazawa et al., 2004). Clinical observations in patients with coronary artery disease and the combined metabolic syndrome with hypertension, hyperlipidemia, and DM have observed impaired Wnt signaling through a missense mutation in LRP-6 (Mani et al., 2007) (Table 2). Experimental studies in mice with hyperglycemia through a high fat diet also demonstrate increased expression of some Wnt family members, such as Wnt3a and Wnt7a (Al-Aly et al., 2007). In addition, intact Wnt family members may offer glucose tolerance and increased insulin sensitivity (Wright et al., 2007) as well as protect glomerular mesangial cells from elevated glucose induced apoptosis (Lin et al., 2006). Animals that over-expressed Wnt10b and were placed on a high-fat diet had a reduction in bodyweight, hyperinsulinemia, triglyceride plasma levels, and improved glucose homeostasis (Aslanidi et al., 2007).
Table 2.
Wnt Signaling Pathway in the Diseases of the Nervous and Cardiovascular Systems
| Clinical or Biological Presentation | Wnt Signaling Components | Outcome | References |
|---|---|---|---|
| Alzheimer’s disease | Wnt signaling down-regulated by presenilin 1
β-amyloid production increased LRP-6 variants, possible neurofibrillary changes |
β-catenin degradation increased
GSK-3β activity increased and β-catenin activity decreased; apoptotic neuronal injury increased; microglial activation increased |
Soriano et al., 2001
Salins et al., 2007 Chong et al., 2007a De Ferrari et al., 2007 Jackson et al., 2002 |
| Retinitis pigmentosa | Wnt inhibitory protein Fzd-related protein-2 secretion increased | Increased loss of photoreceptors | Jones et al., 2000 |
| Angiogenesis and inflammatory cell control | Wnt1 expression increased
Wnt1 and β-catenin expression increased Frz A expression increased Fzd 4 increased; CAMKII and PKC activated DVL expression and β-catenin increased Wnt1 activation |
HUVEC proliferation inhibited; HUVEC morphology altered
EC and VSMC proliferation increased Vessel density increased Retinal angiogenesis increased Neovascularization increased Reduction in inflammatory cell activation/proliferation |
Cheng et al., 2003
Wright et al., 1999 Barandon et al., 2003; Dufourcq et al., 2002 Robitaille et al., 2002 Blankesteijn et al., 2000 Chong et al., 2007a |
| Arterial injury | β-catenin accumulation increased
FrzA expression increased |
VSMC survival increased and apoptosis decreased
Proliferating vascular ECs decreased |
Wang et al., 2002
Duplaa et al., 1999 |
| Cardiac injury/Pressure overload | β-catenin expression increased DVL-1 decreased
Increased FZD gene expression |
Infarct size, leukocyte infiltration, and apoptosis decreased; capillary density increased
Cardiac apoptosis decreased; cardiomyocyte adherence enhanced; cardiac function improved Decreased pressure induced cardiac hypertrophy |
Qu et al., 2007
van de Schans et al., 2007 Barandon et al., 2003 Ligon et al., 2001 |
| Diabetes mellitus | Wnt signaling increased with hyperglycemia; trophic factor enhanced Wnt1 expression
Missense mutations LRP-6 |
Vascular/renal cell early and late apoptotic programs decreased
Glucose intolerance |
Chong et al., 2007c
Lin et al., 2006 Manni et al., 2007 |
Abbreviations: CAMKII, calcium/calmodulin-dependent protein kinase II; EC, DVL, disheveled; endothelial cell; Fzd, Frizzled; GSK-3β, glycogen synthase kinase-3β; HUVEC, human umbilical vein endothelial cell; LRP, low-density lipoprotein receptor-related protein; VSMC, vascular smooth muscle cell.
These clinical and experimental observations for the Wnt pathway in conditions associated with hyperglycemia and DM suggest a potential protective cellular mechanism for Wnt. Recent in vitro studies demonstrate that the Wnt1 protein is necessary and sufficient to provide cellular protection during elevated glucose exposure (Chong et al., 2007c). Administration of exogenous Wnt1 protein can significantly prevent apoptotic endothelial cell (EC) injury during elevated glucose exposure. Interestingly, this protection by Wnt1 can be regulated by the growth factor and cytokine erythropoietin (EPO) (Maiese et al., 2004, 2005b; Nangaku & Fliser, 2007). Through the Wnt pathway, EPO may offer an attractive therapy to maintain proper cellular metabolism and mitochondrial membrane potential during conditions of oxidative stress and DM. In cell culture and animal studies, EPO is cytoprotective during elevated glucose (Chong et al., 2007c) and can block apoptotic DNA degradation during elevated glucose similar to other models of oxidative stress in cardiac and vascular cell models (Avasarala & Konduru, 2005; Chong et al., 2002b, 2003a; Chong & Maiese, 2007a; Moon et al., 2006). EPO can at times enhance tissue function (Ben-Dor et al., 2007) and is closely related to the maintenance of mitochondrial membrane potential (ΔΨm ) (Li et al., 2004a). Loss of ΔΨm through the opening of the mitochondrial permeability transition pore represents a significant determinant for cell injury and the subsequent induction of apoptosis (Leuner et al., 2007; Maiese & Chong, 2004). EPO has the capacity to prevent the depolarization of the mitochondrial membrane that also affects the release of cytochrome c (Chong et al., 2002b; Chong et al., 2003d; Miki et al., 2006). With the Wnt pathway, EPO maintains the expression of Wnt1 during elevated glucose exposure and prevents loss of Wnt1 expression that would normally occur in the absence of EPO during elevated glucose. In addition, blockade of Wnt1 with a Wnt1 antibody can neutralize the protective capacity of EPO, illustrating that Wnt1 is a critical component in the cytoprotection of EPO during elevated glucose exposure (Chong et al., 2007c).
3. The Wnt Pathway in Neurodegeneration, Vascular Disease, and Cardiac Dysfunction
3.1 Wnt and Disease of the Nervous System
The Wnt pathway can influence both acute and chronic disease processes of the nervous system. For example, experimental models of behavior suggest that the ability to tolerate stressful environments may be associated with the expression of Wnt2 (Krishnan et al., 2007). Other work suggests that loss of Wnt signaling may contribute to retinal neurodegeneration, since retinal degeneration with the progressive loss of photoreceptors during retinitis pigmentosa has been associated with increased secretion of the Wnt inhibitory protein FZD-related protein-2 (Jones et al., 2000). Studies have also demonstrated that a mutation in the membrane-type FZD-related protein gene may be involved in retinal photoreceptor degeneration (Kameya et al., 2002).
In relation to cognitive function, Wnt has been demonstrated in the brains of individuals affected by neuropsychiatric disorders (Miyaoka et al., 1999). In models of frontotemporal dementia, the Wnt pathway can be up-regulated early during the onset of the disease (Wiedau-Pazos et al., 2007). It is conceivable that this increased expression of Wnt may improve memory function similar to work that has demonstrated improved cognition with agents such as lithium chloride that enhance Wnt activity have shown (De Ferrari et al., 2003). Genetic analysis also suggests that LRP-6 variants of the Wnt pathway may be associated with late onset Alzheimer’s disease (De Ferrari et al., 2007) and that up-regulation of the Wnt pathway such as with agents as cannabidiol may provide alternative treatments for Alzheimer’s disease (Esposito et al., 2006a) (Table 2). Although it is unclear whether Wnt expression has a direct role in the development of neuropsychiatric or dementia disorders, the Wnt pathway has been tied to several of the pathological components of Alzheimer’s disease (Li et al., 2005, 2006c). The production of β-amyloid (Aβ) peptide aggregates composed of a 39–42 amino acid peptides and the accumulation of intracellular neurofibrillary tangles are considered to be two critical pathological mechanisms that lead to Alzheimer’s disease. In Drosophila models of neurodegeneration, Wnt can lead to neurofibrillary pathology (Jackson et al., 2002).
The proteolytic processing of amyloid precursor protein (APP) during Alzheimer’s disease has been closely linked to the Wnt pathway through presenilin 1 (PS1) and DVL. APP cleaves β-amyloid into products that include a 40-residue peptide and a 42-residue peptide (Aβ1-42). However, it is Aβ1-42 that is considered to be the β-amyloid product that most directly leads to Alzheimer’s disease and apoptotic injury (Chong et al., 2005e; Maiese & Chong, 2004). Wnt may regulate APP isoform expression (Morin et al., 2004). In addition, PS1 is required for the processing of APP and has been shown to down-regulate Wnt signaling and interact with β-catenin to promote its turnover (Soriano et al., 2001). Using a familial Alzheimer’s disease-associated PS-1 mutant, PS-1 (L286V), other studies have shown that pharmacological inhibition of T cell factor/β-catenin/cAMP-response element-binding protein (CREB)-binding protein (CBP)-mediated transcription restores normal neurite outgrowth, illustrating the integration of the Wnt in the PS pathway (Teo et al., 2005). DVL also can regulate the α-secretase cleavage of APP through protein kinase C/mitogen-activated protein kinase dependent pathways, increasing production of soluble APP (Mudher et al., 2001). Furthermore, over-expression of mouse DVL-1 and –2 inhibits GSK-3β mediated phosphorylation of tau protein and may thus prevent formation of neurofibrillary tangles during Alzheimer’s disease (Wagner et al., 1997).
Current studies also have examined the ability of Wnt to directly reduce β-amyloid toxicity. Investigations with lovastatin as an extension of prior clinical work that suggests that a lower prevalence of Alzheimer’s disease can occur during statin administration show that this agent can reduce β-amyloid production and cellular injury through increased β-catenin activity and the inhibition of GSK-3β (Salins et al., 2007). In addition, the pathways controlled by Wnt1 appear to be critical for neuronal protection during β-amyloid exposure against genomic DNA degradation, membrane PS exposure, and microglial activation (Chong et al., 2007a). The neuroprotective attributes of Wnt1 against β-amyloid are lost during gene silencing of Wnt1 protein expression. More importantly, Wnt 1 protection is dependent upon protein kinase B (Akt) activity and the inhibition of GSK-3β with the cellular translocation β-catenin to the nucleus (Chong et al., 2007a) (Figure 2).
Figure 2. Wnt1 promotes nuclear translocation of β-catenin during amyloid-beta.
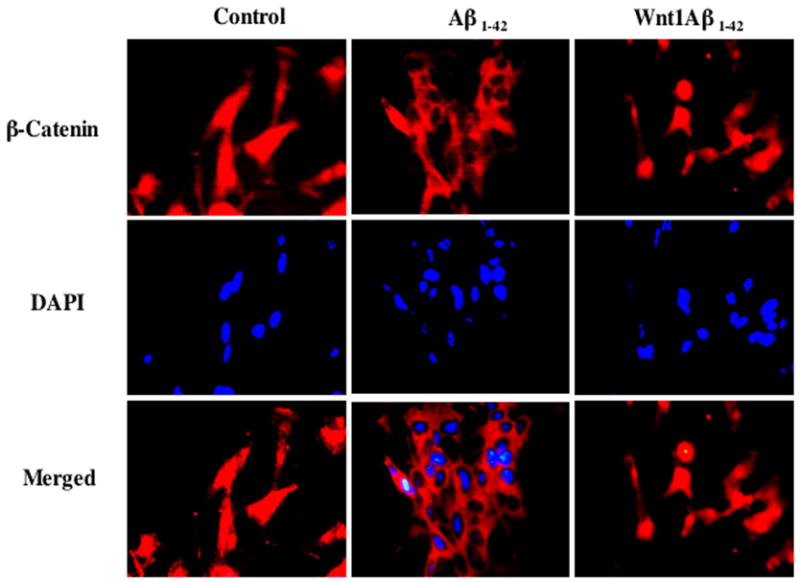
(Aβ) exposure. Immunofluorescence staining for β-catenin was performed at 12 hours following Aβ1-42 (20 μM) application in SH-SY5Y cells by using rabbit anti-β-catenin antibody followed by Texas-red labeled anti-rabbit second antibody. The nucleus was counterstained with DAPI. Representative pictures of staining for β-catenin, DAPI, and merged images are illustrated. In control untreated cells, β-catenin is expressed in both nucleus and cytoplasm. Aβ1-42 application resulted in predominant distribution of β-catenin in the cytoplasm. However, the expression of β-catenin was mainly observed in nucleus in Wnt1 over-expressing SH-SY5Y cells. In merged images, cells with Aβ1-42 exposure show decreased β-catenin staining (blue) in the nucleus while Wnt1 over-expression reveals strong β-catenin staining in the nucleus (red).
3.2 Wnt, Vascular Integrity, and Immunity
In many respects, the ability of Wnt to foster new vascular growth is complimentary to the ability of Wnt to afford protection in the nervous system. Several Wnt family members can control endothelial cell development (Woll et al., 2007), proliferation, and migration (Cheng et al., 2008). New capillary formation from pre-existing vessels into an avascular area is a process known as angiogenesis (Chong et al., 2002a). Angiogenesis is present during embryogenesis, during menstruation, and during pathological processes that involve wound healing, chronic inflammation, and tumor growth (Risau, 1997). In several vascular cell populations, Wnt signaling plays a significant part in the modulation of new vessel formation. Several Wnt ligands, such as Wnt2, Wnt5a, Wnt7a, and Wnt10b, are expressed endogenously in ECs and vascular smooth muscle cells. Wnt receptors that involve Fzd1, Fzd2, Fzd3, and Fzd5, as well as cysteine-rich 61 that contains Wnt-induced secreted proteins-1, 2 and 3 are also expressed in these cell populations for Wnt to exert a direct biological effect (Brigstock, 2002; Li et al., 2006c). For example, mice deficient in Wnt2 and Fzd5 display vascular abnormalities that include defective placental vasculature as well as embryonic lethal mutations (Ishikawa et al., 2001). Antagonism of the Wnt system, such as through sFRP expression also blocks endothelial cell proliferation (Ezan et al., 2004). In contrast, over-expression of Wnt1 can lead to the proliferation of cultured primary ECs, increase the free pool of β-catenin, and activate transcription through Tcf/Lef, suggesting that the Wnt-FZD signaling pathway is closely involved in the proliferation of ECs (Wright et al., 1999). Yet, Wnt1 signaling also can have a regulatory role, such as during the proliferation of umbilical vein ECs, to block further growth through mechanisms that involve cell-cell contact (Cheng et al., 2003). Studies with mice homozygous for the deletion of the Wnt receptor ligand Fzd5 that can synergize with Wnt2, Wnt5a, and Wnt10b lead to embryos that die in utero approximately 10 days post coitum as a result of defects in yolk sac angiogenesis, supporting a critical role for Wnt during embryo vascular development (Ishikawa et al., 2001). In contrast, mice heterozygotes were found to be viable, fertile and appear normal. In disorders that involve failure of peripheral retinal vascularization, mutations in Fzd4, a gene encoding the Wnt receptor FZD-4, are believed to account for the vascular failure. Studies have shown that injection of only wildtype Fzd4, but not mutated Fzd4, into Xenopus embryos can activate CamKII and PKC, components of the Wnt/Ca2+ signaling pathway, to control retinal angiogenesis (Robitaille et al., 2002), supporting that Wnt- FZD signaling pathway broadly controls vascular development and function in a number of organ systems. In addition, FrzA, as a member of secreted FZD-related protein, can increase migration and tube formation of ECs to result in enlarged, longer, and mature of vessels, further supporting the necessity of the Wnt- FZD signaling pathway during development of the vasculature (Dufourcq et al., 2002) (Table 2).
In addition to vascular development, the Wnt pathway participates in inflammatory cell control and the regulation of vascular injury. In fact, the ability of the Wnt pathway to modulate inflammatory cell activity can ultimately impact upon cell survival and longevity since activated immune cells can lead to the phagocytic removal of both neurons and vascular cells (Chong et al., 2005a; Chong et al., 2004a; Kang et al., 2003b). During inflammation, microglial cells require the activation of intracellular cytoprotective pathways (Chong et al., 2007b; Li et al., 2006b) to proliferate and remove injured cells (Li et al., 2005; Mallat et al., 2005). Subsequently, microglia can form a barrier for the removal of foreign microorganisms from the central nervous system and promote tissue repair during neuronal and vascular cell injury (Chong et al., 2007b; Dringen, 2005). Yet, microglia also may lead to cellular damage through the generation of reactive oxygen species (Maiese & Chong, 2004; Sankarapandi et al., 1998) and through the production of cytokines (Benzing et al., 1999; Mehlhorn et al., 2000). During injury to cells, activation of microglia can parallel the induction of cellular apoptosis and correlate well with the severity of an ischemic insult (Chong et al., 2004a; Kang et al., 2003a, 2003b). Furthermore, microglial activation has been associated with several neurodegenerative disorders, such as Alzheimer’s disease with the co-localization of microglia and amyloid plaque development (Sheng et al., 1997).
Given the significant impact that inflammatory cells may play during oxidative stress and cell injury, it becomes essential to understand the role of Wnt during inflammatory cell activation. Early work has shown that Wnt Transcripts for Wnt 4, 7b, 10b and 13 were up-regulated in macrophages during human colorectal cancer (Smith et al., 1999). Subsequent work has suggested that Wnt family members, such as Wnt7b in macrophages, may be responsible for the apoptotic cell death of adjacent vascular cells (Lobov et al., 2005) and that inflammatory cells require components of the Wnt signaling pathway to maintain cellular integrity (Chong et al., 2007b). Interestingly, the canonical Wnt pathway can increase monocyte adhesion to ECs (Lee et al., 2006a) and control transendothleial migration of monocytes (Tickenbrock et al., 2006). Wnt also can influence matrix metalloproteinase (MMP) 2 and MMP9 expression to support T cell migration through basement membranes (Wu et al., 2007a). Wnt1 has recently been shown to possess a unique capacity to not only prevent early apoptotic membrane PS exposure, but also directly modulate inflammatory microglial activation and proliferation (Chong et al., 2007a) that can lead to cellular engulfment and removal (Chong et al., 2005a).
Investigations that employ vascular cell injury models also support a direct cytoprotective role for the Wnt signaling pathway that may be independent from inflammatory cell modulation. In a rat aorta balloon injury model, the FZD receptor (rFrz) genes, rFrz1 and rFrz 2, have been shown to be transiently down-regulated as early as one hour following balloon injury. Yet, Frzb-1, a secreted protein that acts as an antagonist of Wnt signaling, can be increased and appears to coincide with the arrest of aortic smooth muscle cell proliferation (Mao et al., 2000). Similarly, the secreted protein FrzA can be elevated in ECs during traumatic manipulation and subsequently block the proliferation of ECs (Duplaa et al., 1999). In regards to apoptotic vascular injury, it has been shown that within eight hours following EC shear stress, expression of β-catenin is significantly increased at the cell-cell junctions of ECs (Noria et al., 1999). In addition, transfection of a degradation-resistant β-catenin into rat vascular smooth muscle cells can prevent apoptotic vascular cell injury. Furthermore, use of a dominant negative Tcf-4 transgene lacking the β-catenin binding domain, Tcf4 (N31), can eliminate cytoprotection from the Wnt pathway (Wang et al., 2002). Similar to arterial vessels, the Wnt signaling pathway also plays a role during venous injuries. In a crush injury vein model, Wnt5a can have increased expression that may be beneficial to overcome antagonists of the Wnt pathway (Price et al., 2004). In brain ECs, endogenous activation of Wnt1 also may offer a minimum level of protection during elevated glucose exposure, since application of a Wnt1 antibody results in a slight increase in EC injury. Furthermore, administration of exogenous Wnt1 protein significantly increases EC survival and prevents apoptotic EC degeneration during elevated glucose exposure. More importantly, administration of a Wnt1 antibody in the presence of exogenous Wnt1 application can neutralize the protective capacity of Wnt1, illustrating that Wnt1 is an important component in the cytoprotection of ECs during elevated glucose exposure (Chong et al., 2007c) (Table 2).
3.3 Wnt and Cardiac Dysfunction
During the complicated process of wound healing in the heart, changes in components of the Wnt- FZD signaling pathway, such as Wnt (Barandon et al., 2003), FZD (van Gijn et al., 2001), DVL-1 (Chen et al., 2004), GSK-3β (El Jamali et al., 2004), β-catenin (Bergmann et al., 2004), and sFRP (Barandon et al., 2003) can occur. For example, during myoblast proliferation and migration following myocardial infarction, elevated expression of FZD genes including FZD 1, 2, 5, 6, 7, 8, and 10 have been identified during heart remodeling. The over-expression of some FZD genes may be associated with reduction in infarct size and the prevention of cardiac rupture to improve cardiac function (Barandon et al., 2003). It is important to note that different members of the Wnt family may contribute to distinct events during cardiac injury. Both Wnt8a and Wnt10b are up-regulated following myocardial infarction while Wnt7b is down-regulated to undetectable levels during this period (Barandon et al., 2003). Additional work also has demonstrated that β-catenin is translocated from the plasma membrane to the cytoplasm of ECs following the expression of DVL during the phase of the neovascularization after myocardial infarction (Blankesteijn et al., 2000). Furthermore, over-expression of antagonists of the Wnt/FZD pathway can reverse the benefit of treatments such as ischemic preconditioning and minimize β-catenin and Akt activity (Barandon et al., 2005). Interestingly, mice heterozygous for the loss of β-catenin (homozygous mice die at birth) have no structural or functional abnormalities, but suffer from significantly decreased heart weight and heart weight/body weight ratio during transverse aortic constriction (Qu et al., 2007). These data suggest that the Wnt system and its components that involve β-catenin can prevent cardiomyocyte injury and preserve potential myocardial function (Table 2).
Furthermore, the expression of another Wnt component, DVL-1, appears to have a vital role during cardiac injury. The expression of DVL-1 mRNA and cytoplasmic DVL-1 protein are significantly enhanced within days in myofibroblasts, vascular ECs, and smooth muscle cells of newly formed and pre-existing blood vessels in the region of a myocardial infarction (Chen et al., 2004). In addition, mice without DVL-1 demonstrate a benefit from pressure overload cardiac hypertrophy that is mediated through Akt and GSK-3β (van de Schans et al., 2007).
In addition to FZD, DVL-1, and several Wnt genes, other members of the Wnt signaling cascade also appear to have relevant roles during myocardial injury. Expression of sFRP3 and sFRP4 in failing human ventricular myocardium is increased when compared to donor hearts. This increased expression of sFRP is associated with the expression of the pro-apoptotic Fas proteins, but inversely linked to the expression of the anti-apoptotic protein Bcl-xL (Schumann et al., 2000). Other studies illustrate that cardiac rupture following myocardial infarction is correlated with decreased levels of β-catenin in cardiomyocytes (Blankesteijn et al., 1999). The lack of β-catenin in the adherence junctions of cardiomyocytes may lead to impaired structural integrity of the heart, since β-catenin may play a vital role in a structural adaptor protein linking cadherins to the actin cytoskeleton in cell- cell adhesion (Ligon et al., 2001). Yet, the role of β-catenin during cardiac infarction is not entirely clear, since cytosolic β-catenin exists in vascular ECs and smooth muscle cells that reside in the area of myocardial infarction (Barandon et al., 2003). Pathological cardiac hypertrophy also may be dependent upon modulation of the Wnt pathway. In volume-overloaded rabbit hearts, cardiac hypertrophy is accompanied by suppressed mRNA expression of β-catenin (Itoh et al., 2002). The suppressed levels of β-catenin may be a result of increased GSK-3β activity. Other work has demonstrated that inhibition of GSK-3β by Akt through phosphorylation of a serine residue at position 9 can play an important role in the development of cardiac hypertrophy (Haq et al., 2000; Hardt & Sadoshima, 2002) especially during pressure overload (van de Schans et al., 2007).
4. Wnt Governs Vital Cytoprotetective Pathways
4.1 Wnt, Oxidative Stress, and Apoptosis
Wnt signaling controls a variety of signal transduction pathways for cytoprotection that can involve protein kinase B, caspases, forkhead transcription factors, GSK-3β, and nuclear factor κB. Intimately associated with Wnt signaling pathways that control cellular survival and longevity are the injury mechanisms associated with apoptosis (Figure 3). Oxidative stress occurs as a result of the development of reactive oxygen species that consist of oxygen free radicals and additional chemical entities.
Figure 3. Wnt signaling is cytoprotective through the modulation of multiple cellular pathways.
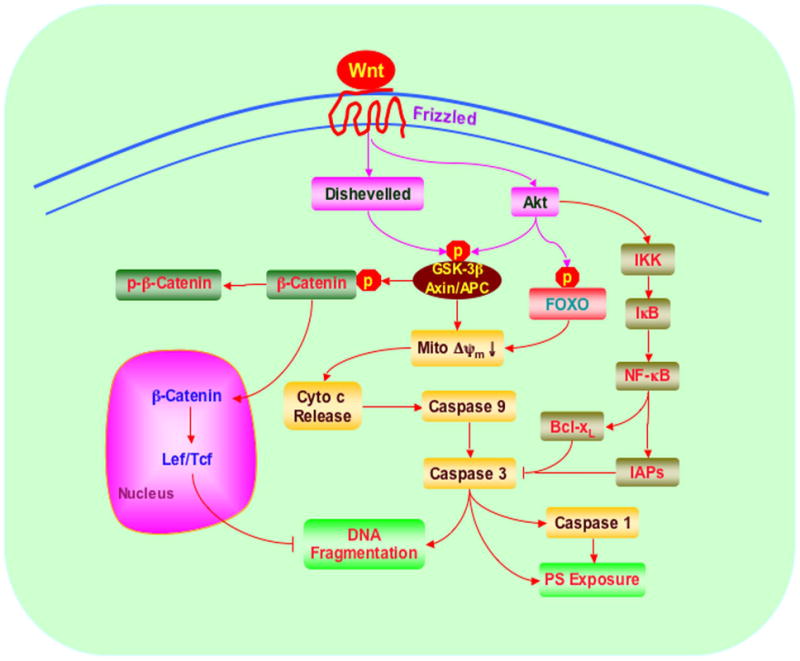
Wnt signaling begins with Frizzled (FZD) receptors resulting in the activation of Dishevelled (DVL) followed by the inhibition of glycogen synthase kinase (GSK-3β), Axin, and adenomatous polyposis coli (APC) tumor suppressor protein complex. The suppressed GSK-3β, Axin and APC complex prevents phosphorylation (p) of β-catenin and leads to the accumulation of β-catenin. β-catenin enters into cellular nucleus and contributes to the formation of Lef/Tcf lymphocyte enhancer factor/T cell factor (Lef/Tcf) and β-catenin complex that may cooperate with factors activated by other signaling pathways resulting in cellular proliferation, differentiation, survival and apoptosis through the induction of target nuclear gene transcription. Interconnected pathways with Wnt involve IκB kinase (IKK), IκB, inhibitors of apoptotic protein (IAPs), the forkhead family members (FOXO), glycogen synthase kinase-3β (GSK-3β), nuclear factor-κB (NF-κB), mitochondrial membrane potential (ΔΨm), cytochrome c, (Cyto-c), and caspases. Ultimately these pathways converge upon early apoptotic injury with phosphatidylserine (PS) exposure and later apoptotic DNA degradation.
Oxygen consumption in organisms, or at least the rate of oxygen consumption in organisms, has intrigued a host of investigators and may have had some of its original origins with the work of Pearl. Pearl proposed that increased exposure to oxygen through an increased metabolic rate could lead to a shortened life span (Pearl, 1928). Subsequent work by multiple investigators has furthered this hypothesis by demonstrating that increased metabolic rates could be detrimental to animals in an elevated oxygen environment (Muller et al., 2007). When one examines more current work, oxygen free radicals and mitochondrial DNA mutations have become associated with oxidative stress injury, aging mechanisms, and accumulated toxicity for an organism (Yui & Matsuura, 2006).
Oxygen free radicals can be generated in elevated quantities during the reduction of oxygen and subsequently lead to cell injury and apoptosis. Reactive oxygen species can involve superoxide free radicals, hydrogen peroxide, singlet oxygen, nitric oxide (NO), and peroxynitrite (Chong et al., 2005d). Most species are produced at low levels during normal physiological conditions and are scavenged by endogenous antioxidant systems that include superoxide dismutase (SOD), glutathione peroxidase, catalase, and small molecule substances such as vitamins C and E. Other closely linked pathways to oxidative stress may be tempered by different vitamins, such as vitamin D3 (Regulska et al., 2007) and the amide form of niacin or vitamin B3, nicotinamide (Chlopicki et al., 2007; Chong et al., 2002c; Feng et al., 2006; Hara et al., 2007; Ieraci & Herrera, 2006; Lin et al., 2000; Maiese & Chong, 2003).
Oxidative stress leads to the destruction of multiple cell types that include neuronal and vascular cells (Chong et al., 2006a; De Felice et al., 2007; Lin & Maiese, 2001). More importantly, it has recently been shown that genes involved in the apoptotic process are replicated early during processes that involve cell replication and transcription, suggesting a much broader role for these genes than originally anticipated (Cohen et al., 2007). Apoptotic induced oxidative stress in conjunction with processes of mitochondrial dysfunction can contribute to a variety of disease states such as diabetes, ischemia, general cognitive loss, Alzheimer’s disease, and trauma (Chong et al., 2005d, 2005e; Harris et al., 2007; Leuner et al., 2007; Okouchi et al., 2007). Oxidative stress can lead to apoptosis in a variety of cell types that involve neurons, ECs, cardiomyocytes, and smooth muscle cells through multiple cellular pathways (Chong et al., 2004a; Chong et al., 2007b; Harris et al., 2007; Kang et al., 2003b; Karunakaran et al., 2007; Verdaguer et al., 2007).
Externalization of membrane phosphatidylserine (PS) externalization is an early event during cell apoptosis (Maiese et al., 2000; Mari et al., 2004) and can become a signal for the phagocytosis of cells (Chong et al., 2005a; Li et al., 2006b; Lin & Maiese, 2001). The loss of membrane phospholipid asymmetry leads to the externalization of membrane PS residues and assists microglia to target cells for phagocytosis (Chong et al., 2003c; Kang et al., 2003a, 2003b; Maiese & Chong, 2003; Mallat et al., 2005). This process occurs with the expression of the phosphatidylserine receptor (PSR) on microglia during oxidative stress (Li et al., 2006a, 2006c), since blockade of PSR function in microglia prevents the activation of microglia (Chong et al., 2003b; Kang et al., 2003a). As an example, externalization of membrane PS residues occur in neurons during anoxia (Maiese, 2001; Maiese & Boccone, 1995; Vincent & Maiese, 1999a), nitric oxide exposure (Chong et al., 2003e; Maiese et al., 1997), and during the administration of agents that induce the production of reactive oxygen species, such as 6-hydroxydopamine (Salinas et al., 2003). Membrane PS externalization on platelets also has been associated with clot formation in the vascular system (Leytin et al., 2006).
The cleavage of genomic DNA into fragments (Maiese et al., 1999; Maiese & Vincent, 2000a, 2000b) is considered to be a later event during apoptotic injury (Chong et al., 2004b). Several enzymes responsible for DNA degradation have been differentiated and include the acidic, cation independent endonuclease (DNase II), cyclophilins, and the 97 kDa magnesium - dependent endonuclease (Chong et al., 2005d; Chong & Maiese, 2007b). Three separate endonuclease activities are present in neurons that include a constitutive acidic cation-independent endonuclease, a constitutive calcium/magnesium-dependent endonuclease, and an inducible magnesium dependent endonuclease (Vincent & Maiese, 1999b; Vincent et al., 1999a).
During oxidative stress, mitochondrial membrane transition pore permeability also is increased (Chong et al., 2003a; Di Lisa et al., 2001; Kang et al., 2003b; Lin et al., 2000), a significant loss of mitochondrial NAD+ stores occurs, and further generation of superoxide radicals leads to cell injury (Chong et al., 2005f; Maiese & Chong, 2003). In addition, mitochondria are a significant source of superoxide radicals that are associated with oxidative stress (Chong et al., 2005d; Maiese & Chong, 2004). Blockade of the electron transfer chain at the flavin mononucleotide group of complex I or at the ubiquinone site of complex III results in the active generation of free radicals which can impair mitochondrial electron transport and enhance free radical production (Chong & Maiese, 2007b; Li et al., 2006a). Furthermore, mutations in the mitochondrial genome have been associated with the potential development of a host of disorders, such as hypertension, hypercholesterolemia, and hypomagnesemia (Li et al., 2004b; Wilson et al., 2004). Reactive oxygen species also may lead to cellular acidosis and subsequent mitochondrial failure (Chong et al., 2005e). Disorders, such as hypoxia (Roberts & Chih, 1997), diabetes (Cardella, 2005; Kratzsch et al., 2006), and excessive free radical production (Ito et al., 1997; Vincent et al., 1999a, 1999b) can result in the disturbance of intracellular pH. When one considers the potential of Wnt pathways to foster cell development and survival, Wnt signaling may offer new therapeutic avenues against oxidative stress that leads to both early and late apoptotic stages in several disorders.
4.2 Wnt, Akt, and Caspases
One attractive pathway that may work in concert with Wnt to maintain cell survival and block inflammatory cell activation involves protein kinase B, or Akt (Chong et al., 2007a; Chong et al., 2007c; Li et al., 2006c; Speese & Budnik, 2007). Phosphorylation of Akt in conjunction leads to its activation and protects cells against genomic DNA degradation and membrane PS exposure (Chong et al., 2003a, 2003b; 2003d). Up-regulation of Akt activity during multiple injury paradigms, such as vascular and cardiomyocyte ischemia (Miki et al., 2006; Parsa et al., 2003), free radical exposure (Chong et al., 2003b; Matsuzaki et al., 1999), N-methyl-D-aspartate toxicity (Dzietko et al., 2004), hypoxia (Chong et al., 2002b; Zhang et al., 2007), β-amyloid toxicity (Chong et al., 2005c; Du et al., 2004; Nakagami et al., 2002), heat exposure (Shein et al., 2007), DNA damage (Chong et al., 2002b, 2004a; Henry et al., 2001; Kang et al., 2003a), and oxidative stress (Chong et al., 2004a; Kang et al., 2003a, 2003b) increases cell survival. Cytoprotection through Akt also can involve control of inflammatory cell activation (Chong et al., 2003a; Kang et al., 2003a, 2003b), transcription factor regulation (Chong & Maiese, 2007a), maintenance of mitochondrial membrane potential (ΔΨm ), prevention of cytochrome c release (Chong et al., 2003a, 2003b; Chong et al., 2003d), and blockade of caspase activity (Chong et al., 2002b, 2003a, 2003b).
Evidence for the dependence of Wnt on the Akt pathway can be drawn from a variety of cell populations (Chong et al., 2005b). For example, neuronal cell differentiation that is dependent upon Wnt signaling and trophic factor induction is blocked during the repression of Akt activity (Fukumoto et al., 2001), differentiation of cardiomyocytes does not proceed without Akt activation, (Naito et al., 2005) Wnt has been shown in preadipocytes to increase Akt phosphorylation (Longo et al., 2002), and the Wnt-induced secreted protein in a fibroblast cell line uses Akt to block apoptotic death (Su et al., 2002). sFRP2, which can modulate Wnt signaling, also employs Akt for cardiac tissue repair (Mirotsou et al., 2007). Reduction in tissue injury during pressure overload cardiac hypertrophy also is linked to Akt activation (van de Schans et al., 2007) and the benefits of cardiac ischemic preconditioning appear to rely upon Akt (Barandon et al., 2005). In the neuronal system, Wnt over-expression can independently increase the phosphorylation and the activation of Akt to promote neuronal protection. Inhibition of the phosphatidylinositol 3-kinase (PI 3-K) pathway or gene silencing of Akt expression prevents Wnt from blocking apoptotic injury and microglial activation (Chong et al., 2007a).
Modulation of Akt activity ultimately controls the apoptotic pathways of the caspase family. Caspases are a family of cysteine proteases that are synthesized as inactive zymogens which are proteolytically cleaved into subunits at the onset of apoptosis (Li et al., 2006a; Maiese et al., 2005a; Okouchi et al., 2007). The apoptotic-associated caspases include initiator caspases, such as caspase 2, 8, 9, and 10, that activate downstream effector caspases, resulting in an amplification of cascade activity. The initiator caspases consist of long N-terminal prodomains that contain caspase recruitment domains (CARDs) in caspase 2 and caspase 9 or death effector domains (DEDs) in caspase 8 and caspase 10 (Hofmann et al., 1997). The effector caspases consist of caspase 3, 6, and 7 that function to directly cleave crucial cellular protein substrates to result in cell destruction.
The caspases 1 and 3 have each been linked to the apoptotic pathways of genomic DNA cleavage and cellular membrane PS exposure (Chong et al., 2003a; Chong et al., 2003d; Takahashi et al., 1999). These caspases, in addition to caspase 8 and 9, are also tied to the direct activation and proliferation of microglia (Chong et al., 2003b; Kang et al., 2003a, 2003b). Caspase 1 is believed to be principally responsible for the externalization of membrane PS residues in several cell systems that can subsequently activate microglial phagocytosis (Chong et al., 2005a; Chong et al., 2004a; Kang et al., 2003b). Furthermore, caspase 9 is activated through a process that involves the cytochrome c -apoptotic protease-activating factor-1 (Apaf-1) complex (Chong et al., 2003a; Chong et al., 2005d; Kang et al., 2003a). In addition, caspase 8 serves as an upstream initiator of executioner caspases, such as caspase 3, and also leads to the mitochondrial release of cytochrome c (Engels et al., 2000; Stegh et al., 2002). Following caspase 8 and caspase 9 activation, caspase 3 directly leads to genomic DNA degradation.
In reference to the Wnt pathway and caspase activity, loss of Wnt4 and Wnt5a expression during elevated glucose in mesangial cells results in elevated caspase 3 activity and apoptotic cell death (Lin et al., 2006). Furthermore, reduction in caspase 9 activity by the Wnt pathway (Chen et al., 2001) and the prevention of cytochrome c release with caspase pathway activation (You et al., 2002) has been attributed to the resistance of cells to cancer therapeutics designed to induce apoptosis. Other members of the Wnt pathway that are mutated in disease, such as the APC gene during colonic polyp progression, can suppress caspase 3, 7, and 9 activities to prevent apoptotic demise of colonic polyps (Chen et al., 2003). In patients with Alzheimer’s disease, observation of Wnt pathway impairment and the increased phosphorylation of beta-catenin that can lead to its degradation has been suggested as a possible mechanism of pathology that is associated with elevated caspase 3 activity (Ghanevati & Miller, 2005).
4.3 Wnt and Forkhead Transcription Factors
As a central regulatory protein, Akt controls the activity and function of a number of robust pathways such as the mammalian forkhead transcription factor family that oversees processes that can involve cell metabolism, hormone modulation, and apoptosis (Cuesta et al., 2007; Maiese et al., 2007a; Maiese et al., 2007b). The mammalian forkhead transcription factor family preferentially bind to the core consensus DNA sequence 5′-TTGTTTAG-3′, the forkhead response element (Chong et al., 2005d; Chong et al., 2004c; Wijchers et al., 2006). The first member of this family was the Drosophila melanogaster gene Fork head. Since this time, greater than 100 forkhead genes and 19 human subgroups are known to exist that extend from FOXA to FOXS (Maiese et al., 2007b; Wijchers et al., 2006). The forkhead box (FOX) family of genes is characterized by a conserved forkhead domain commonly noted as a “forkhead box” or a “winged helix” as a result of the butterfly-like appearance on X-ray crystallography (Clark et al., 1993) and nuclear magnetic resonance (Jin et al., 1998). All Fox proteins contain the 100-amino acid winged helix domain, but it should be noted that not all winged helix domains are Fox proteins (Larson et al., 2007).
FOXO3a is one member of the forkhead family of transcription factors that exemplifies the ability to function as a versatile target for a number of disorders (Maiese et al., 2007b). Akt controls the “pro-apoptotic” forkhead transcription factor FOXO3a by inhibiting the nuclear translocation of FOXO3a that would normally activate the transcription of apoptotic nuclear genes (Chong & Maiese, 2007a). As a result, control of FOXO3a is considered to be a viable therapeutic target for agents such as metabotropic glutamate receptors (Chong et al., 2006b) and NAD+ precursors (Chong et al., 2004c; Li et al., 2006a, 2006b) to increase cell survival. In addition, FOXO3a interfaces with several pathways that regulate cellular lifespan (Lehtinen et al., 2006) and function to control neoplastic growth (Li et al., 2007).
If one then considers the role of forkhead transcription factors in the Wnt pathway, early work has shown that FoxD3 is activated by the Wnt pathway and inhibited by BMP signaling to control neural plate development (Pohl & Knochel, 2001). Other forkhead family members also rely upon the Wnt pathway for cellular proliferation and function. FoxI1 activates the Wnt/β-catenin pathway to increase extracellular proteoglycans, promote gastrointestinal cell proliferation (Perreault et al., 2001), and possibly foster carcinogenesis (Perreault et al., 2005). Yet, the Wnt pathway also utilizes forkhead members to regulate cellular function and can activate FoxN1 for regulatory control of thymic function (Balciunaite et al., 2002). In other examples of cell development, Wnt signaling has been shown to rely upon Foxf1 and Foxf2 during intestinal maturation in murine models (Ormestad et al., 2006). In addition, Foxa2 may be a significant component during in early anterior-posterior axis polarization (Kimura-Yoshida et al., 2007). Interestingly, β-catenin that is believed to function independently from other Wnt signaling pathways has been shown to bond to FOXO and enhance its transcriptional activity (Essers et al., 2005).
4.4 Wnt, GSK-3β, and NF-κB
Both Wnt1 and Akt1 also share a common pathway through the phosphorylation of GSK-3β (Chong & Maiese, 2007b). DVL is phosphorylated by casein kinase Iε to form a complex with Frat1 and inhibit GSK-3β activity. Cell injury during GSK-3β activation may occur through the phosphorylation of β-catenin that can lead to its ubiquitination and subsequent degradation. As a result, Wnt “anti-apoptotic” pathways can be dismantled in the absence of β-catenin and cannot support cell survival or block apoptosis (Chen et al., 2001; Li et al., 2006c; Terry et al., 2006; You et al., 2004). Modulation of GSK-3β activity can regulate progenitor cell proliferation and differentiation (Adachi et al., 2007; Wexler et al., 2007), promote midbrain differentiation (Castelo-Branco et al., 2004), control cardiac hypertophy (Haq et al., 2000; Hardt & Sadoshima, 2002), and increase cell survival during oxidative stress, such as during neurofibrillary pathology (Wagner et al., 1997), amyloid toxicity (Chong et al., 2007a; Salins et al., 2007), and cardiac injury (van de Schans et al., 2007). GSK-3β activity also can influence inflammatory cell survival and activation (Chong et al., 2007a; Chong et al., 2007c; Li et al., 2006b). As a result, GSK-3β is considered to be an important treatment strategy for several degenerative disorders (Chong et al., 2005d, 2007b; Rowe et al., 2007; Wu et al., 2007b). For example, inactivation of GSK-3β by small molecule inhibitors or RNA interference prevents toxicity from high concentrations of glucose and increases rat beta cell replication, suggesting a possible target of GSK-3β for pancreatic beta cell regeneration in DM (Mussmann et al., 2007). Clinical applications for GSK-3β are especially attractive when considered in concert with trophic factors such as EPO. For example, both the potential benefits of EPO to improve cardiovascular function in diabetic patients (Silverberg et al., 2006; Silverberg et al., 2001) and the positive effects of exercise to improve glycemic control during DM (Maiorana et al., 2002) appear to rely upon the inhibition of GSK-3β activity. EPO blocks GSK-3β activity (Chong et al., 2005d, 2007b; Rowe et al., 2007; Wu et al., 2007b) and combined with exercise may offer synergistic benefits, since physical exercise also has been shown to phosphorylate and inhibit GSK-3β activity (Howlett et al., 2006).
Protection through Wnt signaling also may require the activation of nuclear factor-κB (NF-κB). NF-κB proteins are composed of several homo- and heterodimer proteins that can bind to common DNA elements. It is the phosphorylation of IκB proteins by the IκB kinase (IKK) and their subsequent degradation that lead to the release of NF-κB for its translocation to the nucleus to initiate gene transcription (Hayden & Ghosh, 2004). Dependent upon Akt controlled pathways, the transactivation domain of the p65 subunit of NF-κB is activated by IKK and the IKKα catalytic subunit to lead to the induction of protective anti-apoptotic pathways (Chong et al., 2005b). NF-κB itself can be cytoprotective and lead to the induction of several anti-apoptotic genes, such as inhibitors of apoptotic proteins (IAPs), that can specifically inhibit caspases 3, 7, and 9 (Chong et al., 2005c; Li et al., 2006b; Spandou et al., 2006). Increased expression of NF-κB during injury models can occur in inflammatory microglial cells (Chong et al., 2005c, 2007b; Guo & Bhat, 2006) and in neurons (Sanz et al., 2002). NF-κB represents a critical pathway that is responsible for the maintenance of Bcl-xL expression (Chen et al., 2000; Chong et al., 2005e) and the protection of cells against oxidative stress (Chong et al., 2005c). Although NF-κB has not consistently been found to be beneficial in all cell systems (Esposito et al., 2006b; Jacobsen et al., 2006) and sometimes may not be cytoprotective (Nurmi et al., 2006; Xu et al., 2005), cytoprotection through Wnt signaling in some scenarios has been associated with expression of NF-κB. In neuronal cell lines, over-expression of Wnt1 protects cells from serum deprivation and is accompanied by NF-κB activation (Bournat et al., 2000). In addition, sFRP2 appears to yield an anti-apoptotic effect in mammary cancer cells through NF-kappaB activation that may in addition require JNK suppression (Lee et al., 2006b).
5. Perspectives and Considerations for Wnt Signaling
As our appreciation for the expansive pathways governed by the Wnt pathway continue to unfold, both basic research as well as future clinical studies should provide direction for the potential therapeutic applications of Wnt. The scope of the Wnt pathway is exceedingly broad and is involved in the development of the brain, spinal cord, and the extension of numerous sub-populations of sensory and motor neurons. Furthermore, Wnt signaling regulates the processes of vascular remolding, cardiac development, and cardiac hypertrophy. Integration of the nervous and cardiovascular systems through Wnt occurs at several cellular levels that relate to oxidative stress, apoptotic injury, and inflammatory cell activation eliciting excitement for the development of treatment strategies that range from the repair of infarcted cardiac tissue to the restoration of cognitive loss (Figure 3).
Unfortunately as with all therapeutic modalities, a “double edge sword” exists in regards to the benefits and risks of any treatment strategy. Although Wnt signaling is a critical component for the regulation of cell and tissue growth in the neuronal and vascular systems, Wnt can either facilitate or prevent apoptosis depending upon the environmental stimuli. This knowledge in regards to Wnt is of particular concern, since recent studies have shown that Wnt signaling can suppress apoptotic pathways during neoplastic growth and limit the effectiveness of treatments directed to control or eliminate tumor growth. In addition, in other studies that involve DM, neuronal disorders, or cardiac disease, it is not entirely evident whether mutations in genes of the Wnt pathway or alterations in protein expression of the Wnt pathway components during these disorders confer protective or detrimental effects. Therefore, it becomes imperative if the “protectionist” Wnt pathway is to offer us the ability to live well and “age gracefully” to clearly focus upon the multiple cellular mechanisms controlled by Wnt. Only with this conviction in the forefront of objectives can we hope to offer patients robust and safe treatment regimens for a variety of disorders that can involve neurodegeneration, cardiac insufficiency and diabetes.
Acknowledgments
This research was supported by the following grants (KM): American Diabetes Association, American Heart Association (National), Bugher Foundation Award, Janssen Neuroscience Award, LEARN Foundation Award, MI Life Sciences Challenge Award, Nelson Foundation Award, NIH NIEHS (P30 ES06639), and NIH NINDS/NIA.
Abbreviations
- Aβ
β-amyloid
- APC
adenomatous polyposis coli
- Akt
protein kinase B
- Apaf-1
apoptotic protease-activating factor
- BMP
bone morphogenetic protein
- CaCN
calcineurin
- Ca2+
calcium
- CamKII
Ca2+-calmodulin-dependent protein kinase II
- CARD
caspase recruitment domain
- cGMP
cyclic guanosine monophosphate
- DED
death effector domain
- DM
diabetes mellitus
- DVL
Dishevelled
- EC
endothelial cell
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- FOX
Forkhead box
- FZD
Frizzled
- GSK-3β
glycogen synthase kinase-β
- IAP
inhibitor of apoptosis protein
- IKK
IκB kinase
- JNK
Jun N-terminal kinase
- LRP
low-density lipoprotein receptor-related protein
- ΔΨm
mitochondrial membrane potential
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- PI 3-K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PS
phosphatidylserine
- PSR
phosphatidylserine receptor
- SOD
superoxide dismutase
- Wg
Drosophila Wingless
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, Okano H, Sawamoto K. beta-Catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25(11):2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Adler PN, Vinson C, Park WJ, Conover S, Klein L. Molecular structure of frizzled, a Drosophila tissue polarity gene. Genetics. 1990;126(2):401–416. doi: 10.1093/genetics/126.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104(22):9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- Aslanidi G, Kroutov V, Philipsberg G, Lamb K, Campbell-Thompson M, Walter GA, Kurenov S, Ignacio Aguirre J, Keller P, Hankenson K, Macdougald OA, Zolotukhin S. Ectopic expression of Wnt10b decreases adiposity and improves glucose homeostasis in obese rats. Am J Physiol Endocrinol Metab. 2007;293(3):E726–736. doi: 10.1152/ajpendo.00248.2007. [DOI] [PubMed] [Google Scholar]
- Augustine K, Liu ET, Sadler TW. Antisense attenuation of Wnt-1 and Wnt-3a expression in whole embryo culture reveals roles for these genes in craniofacial, spinal cord, and cardiac morphogenesis. Dev Genet. 1993;14(6):500–520. doi: 10.1002/dvg.1020140611. [DOI] [PubMed] [Google Scholar]
- Avasarala JR, Konduru SS. Recombinant erythropoietin down-regulates IL-6 and CXCR4 genes in TNF-alpha-treated primary cultures of human microvascular endothelial cells: implications for multiple sclerosis. J Mol Neurosci. 2005;25(2):183–189. doi: 10.1385/JMN:25:2:183. [DOI] [PubMed] [Google Scholar]
- Awad N, Gagnon M, Messier C. The relationship between impaired glucose tolerance, type 2 diabetes, and cognitive function. J Clin Exp Neuropsychol. 2004;26(8):1044–1080. doi: 10.1080/13803390490514875. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, Miller JR, Shulman JM, Moon RT, Perrimon N. Differential recruitment of Dishevelled provides signaling specificity in the planar cell polarity and Wingless signaling pathways. Genes Dev. 1998;12(16):2610–2622. doi: 10.1101/gad.12.16.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JC, Beddington RS, Harland RM. Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development. Genes Dev. 1999;13(23):3149–3159. doi: 10.1101/gad.13.23.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakre MM, Hoi A, Mong JC, Koh YY, Wong KY, Stanton LW. Generation of multipotential mesendodermal progenitors from mouse embryonic stem cells via sustained Wnt pathway activation. J Biol Chem. 2007;282(43):31703–31712. doi: 10.1074/jbc.M704287200. [DOI] [PubMed] [Google Scholar]
- Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer’s disease: pathophysiological and therapeutic significance. Cell Mol Life Sci. 2006;63(11):1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Hollander GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3(11):1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- Barandon L, Couffinhal T, Ezan J, Dufourcq P, Costet P, Alzieu P, Leroux L, Moreau C, Dare D, Duplaa C. Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation. 2003;108(18):2282–2289. doi: 10.1161/01.CIR.0000093186.22847.4C. [DOI] [PubMed] [Google Scholar]
- Barandon L, Dufourcq P, Costet P, Moreau C, Allieres C, Daret D, Dos Santos P, Daniel Lamaziere JM, Couffinhal T, Duplaa C. Involvement of FrzA/sFRP-1 and the Wnt/frizzled pathway in ischemic preconditioning. Circ Res. 2005;96(12):1299–1306. doi: 10.1161/01.RES.0000171895.06914.2c. [DOI] [PubMed] [Google Scholar]
- Bastidas F, De Calisto J, Mayor R. Identification of neural crest competence territory: role of Wnt signaling. Dev Dyn. 2004;229(1):109–117. doi: 10.1002/dvdy.10486. [DOI] [PubMed] [Google Scholar]
- Beeri MS, Silverman JM, Davis KL, Marin D, Grossman HZ, Schmeidler J, Purohit DP, Perl DP, Davidson M, Mohs RC, Haroutunian V. Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci Med Sci. 2005;60(4):471–475. doi: 10.1093/gerona/60.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejsovec A. Wnt pathway activation: new relations and locations. Cell. 2005;120(1):11–14. doi: 10.1016/j.cell.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Ben-Dor I, Hardy B, Fuchs S, Kaganovsky E, Kadmon E, Sagie A, Coleman R, Mansur M, Politi B, Fraser A, Harell D, Okon E, Battler A, Haim M. Repeated low-dose of erythropoietin is associated with improved left ventricular function in rat acute myocardial infarction model. Cardiovasc Drugs Ther. 2007;21(5):339–346. doi: 10.1007/s10557-007-6049-8. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20(6):581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Bergmann MW, Rechner C, Freund C, Baurand A, El Jamali A, Dietz R. Statins inhibit reoxygenation-induced cardiomyocyte apoptosis: role for glycogen synthase kinase 3beta and transcription factor beta-catenin. J Mol Cell Cardiol. 2004;37(3):681–690. doi: 10.1016/j.yjmcc.2004.05.025. [DOI] [PubMed] [Google Scholar]
- Blankesteijn WM, Gijn MEv, Daemen MJAP, Wynshaw-Boris Smits JFM, Pratt RE. Alterations in the cadherin–catenin complex of dishevelled-1 knockout mice lead to infarct rupture after myocardial infarction. Circulation. 1999;100(suppl):156. [Google Scholar]
- Blankesteijn WM, van Gijn ME, Essers-Janssen YP, Daemen MJ, Smits JF. Beta-catenin, an inducer of uncontrolled cell proliferation and migration in malignancies, is localized in the cytoplasm of vascular endothelium during neovascularization after myocardial infarction. Am J Pathol. 2000;157(3):877–883. doi: 10.1016/s0002-9440(10)64601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvini P, An WG, Rosolen A, Nguyen P, Trepel J, Garcia de Herreros A, Dunach M, Neckers LM. Geldanamycin abrogates ErbB2 association with proteasome-resistant beta-catenin in melanoma cells, increases beta-catenin-E-cadherin association, and decreases beta-catenin-sensitive transcription. Cancer Res. 2001;61(4):1671–1677. [PubMed] [Google Scholar]
- Bournat JC, Brown AM, Soler AP. Wnt-1 dependent activation of the survival factor NF-kappaB in PC12 cells. J Neurosci Res. 2000;61(1):21–32. doi: 10.1002/1097-4547(20000701)61:1<21::AID-JNR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt DI, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94(1):109–118. doi: 10.1016/s0092-8674(00)81226-x. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128(8):1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brigstock DR. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-rich 61 (CYR61) Angiogenesis. 2002;5(3):153–165. doi: 10.1023/a:1023823803510. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Development. Making sense of the sensory lineage. Science. 2004;303(5660):966–968. doi: 10.1126/science.1094732. [DOI] [PubMed] [Google Scholar]
- Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357(9):905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- Cardella F. Insulin therapy during diabetic ketoacidosis in children. Acta Biomed. 2005;76(Suppl 3):49–54. [PubMed] [Google Scholar]
- Castelo-Branco G, Rawal N, Arenas E. GSK-3beta inhibition/beta-catenin stabilization in ventral midbrain precursors increases differentiation into dopamine neurons. J Cell Sci. 2004;117(Pt 24):5731–5737. doi: 10.1242/jcs.01505. [DOI] [PubMed] [Google Scholar]
- Ceriello A, dello Russo P, Amstad P, Cerutti P. High glucose induces antioxidant enzymes in human endothelial cells in culture. Evidence linking hyperglycemia and oxidative stress. Diabetes. 1996;45(4):471–477. doi: 10.2337/diab.45.4.471. [DOI] [PubMed] [Google Scholar]
- Chen AE, Ginty DD, Fan CM. Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature. 2005;433(7023):317–322. doi: 10.1038/nature03126. [DOI] [PubMed] [Google Scholar]
- Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L) Mol Cell Biol. 2000;20(8):2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wu Q, Guo F, Xia B, Zuo J. Expression of Dishevelled-1 in wound healing after acute myocardial infarction: possible involvement in myofibroblast proliferation and migration. J Cell Mol Med. 2004;8(2):257–264. doi: 10.1111/j.1582-4934.2004.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152(1):87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Yang I, Irby R, Shain KH, Wang HG, Quackenbush J, Coppola D, Cheng JQ, Yeatman TJ. Regulation of caspase expression and apoptosis by adenomatous polyposis coli. Cancer Res. 2003;63(15):4368–4374. [PubMed] [Google Scholar]
- Cheng CW, Smith SK, Charnock-Jones DS. Wnt-1 signaling inhibits human umbilical vein endothelial cell proliferation and alters cell morphology. Exp Cell Res. 2003;291(2):415–425. doi: 10.1016/j.yexcr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2008;365(2):285–290. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- Chevallier NL, Soriano S, Kang DE, Masliah E, Hu G, Koo EH. Perturbed neurogenesis in the adult hippocampus associated with presenilin-1 A246E mutation. Am J Pathol. 2005;167(1):151–159. doi: 10.1016/S0002-9440(10)62962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277(2):287–295. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Chlopicki S, Swies J, Mogielnicki A, Buczko W, Bartus M, Lomnicka M, Adamus J, Gebicki J. 1-Methylnicotinamide (MNA), a primary metabolite of nicotinamide, exerts anti-thrombotic activity mediated by a cyclooxygenase-2/prostacyclin pathway. Br J Pharmacol. 2007;152(2):230–239. doi: 10.1038/sj.bjp.0707383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang J, Li F, Maiese K. mGluRI Targets Microglial Activation and Selectively Prevents Neuronal Cell Engulfment Through Akt and Caspase Dependent Pathways. Curr Neurovasc Res. 2005a;2(3):197–211. doi: 10.2174/1567202054368317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Angiogenesis and plasticity: role of erythropoietin in vascular systems. J Hematother Stem Cell Res. 2002a;11(6):863–871. doi: 10.1089/152581602321080529. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation. 2002b;106(23):2973–2979. doi: 10.1161/01.cir.0000039103.58920.1f. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Apaf-1, Bcl-xL, Cytochrome c, and Caspase-9 Form the Critical Elements for Cerebral Vascular Protection by Erythropoietin. J Cereb Blood Flow Metab. 2003a;23(3):320–330. doi: 10.1097/01.WCB.0000050061.57184.AE. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Erythropoietin fosters both intrinsic and extrinsic neuronal protection through modulation of microglia, Akt1, Bad, and caspase-mediated pathways. Br J Pharmacol. 2003b;138(6):1107–1118. doi: 10.1038/sj.bjp.0705161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Metabotropic glutamate receptors promote neuronal and vascular plasticity through novel intracellular pathways. Histol Histopathol. 2003c;18(1):173–189. doi: 10.14670/HH-18.173. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Akt1 drives endothelial cell membrane asymmetry and microglial activation through Bcl-x(L) and caspase 1, 3, and 9. Exp Cell Res. 2004a;296(2):196–207. doi: 10.1016/j.yexcr.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxid Redox Signal. 2004b;6(2):277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005b;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Erythropoietin requires NF-kappaB and its nuclear translocation to prevent early and late apoptotic neuronal injury during beta-amyloid toxicity. Curr Neurovasc Res. 2005c;2(5):387–399. doi: 10.2174/156720205774962683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005d;75(3):207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Stress in the brain: novel cellular mechanisms of injury linked to Alzheimer’s disease. Brain Res Brain Res Rev. 2005e;49(1):1–21. doi: 10.1016/j.brainresrev.2004.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Attempted Cell Cycle Induction in Post-Mitotic Neurons Occurs in Early and Late Apoptotic Programs Through Rb, E2F1, and Caspase 3. Curr Neurovasc Res. 2006a;3(1):25–39. doi: 10.2174/156720206775541741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Group I Metabotropic Receptor Neuroprotection Requires Akt and Its Substrates that Govern FOXO3a, Bim, and beta-Catenin During Oxidative Stress. Curr Neurovasc Res. 2006b;3(2):107–117. doi: 10.2174/156720206776875830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Cellular demise and inflammatory microglial activation during beta-amyloid toxicity are governed by Wnt1 and canonical signaling pathways. Cell Signal. 2007a;19(6):1150–1162. doi: 10.1016/j.cellsig.2006.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. The pro-survival pathways of mTOR and protein kinase B target glycogen synthase kinase-3beta and nuclear factor-kappaB to foster endogenous microglial cell protection. Int J Mol Med. 2007b;19(2):263–272. [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. Erythropoietin prevents early and late neuronal demise through modulation of Akt1 and induction of caspase 1, 3, and 8. J Neurosci Res. 2003d;71(5):659–669. doi: 10.1002/jnr.10528. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Kang JQ, Maiese K. The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cell Mol Neurobiol. 2003e;23(4–5):561–578. doi: 10.1023/A:1025158314016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Li F, Maiese K. The sirtuin inhibitor nicotinamide enhances neuronal cell survival during acute anoxic injury through Akt, Bad, PARP, and mitochondrial associated “anti-apoptotic” pathways. Curr Neurovasc Res. 2005f;2(4):271–285. doi: 10.2174/156720205774322584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. Nicotinamide Modulates Mitochondrial Membrane Potential and Cysteine Protease Activity during Cerebral Vascular Endothelial Cell Injury. J Vasc Res. 2002c;39(2):131–147. doi: 10.1159/000057762. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Lin SH, Maiese K. The NAD+ precursor nicotinamide governs neuronal survival during oxidative stress through protein kinase B coupled to FOXO3a and mitochondrial membrane potential. J Cereb Blood Flow Metab. 2004c;24(7):728–743. doi: 10.1097/01.WCB.0000122746.72175.0E. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Targeting WNT, protein kinase B, and mitochondrial membrane integrity to foster cellular survival in the nervous system. Histol Histopathol. 2004;19(2):495–504. doi: 10.14670/hh-19.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. Erythropoietin involves the phosphatidylinositol 3-kinase pathway, 14-3-3 protein and FOXO3a nuclear trafficking to preserve endothelial cell integrity. Br J Pharmacol. 2007a;150(7):839–850. doi: 10.1038/sj.bjp.0707161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Maiese K. The Src homology 2 domain tyrosine phosphatases SHP-1 and SHP-2: diversified control of cell growth, inflammation, and injury. Histol Histopathol. 2007b;22(11):1251–1267. doi: 10.14670/hh-22.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Maiese K. Vascular injury during elevated glucose can be mitigated by erythropoietin and Wnt signaling. Curr Neurovasc Res. 2007c;4(3):194–204. doi: 10.2174/156720207781387150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia. 2006;49(4):784–791. doi: 10.1007/s00125-006-0170-2. [DOI] [PubMed] [Google Scholar]
- Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364(6436):412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Cordeiro-Stone M, Kaufman DG. Early replication and the apoptotic pathway. J Cell Physiol. 2007;213(2):434–439. doi: 10.1002/jcp.21156. [DOI] [PubMed] [Google Scholar]
- Cuesta I, Zaret KS, Santisteban P. The forkhead factor FoxE1 binds to the thyroperoxidase promoter during thyroid cell differentiation and modifies compacted chromatin structure. Mol Cell Biol. 2007;27(20):7302–7314. doi: 10.1128/MCB.00758-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico M, Hulit J, Amanatullah DF, Zafonte BT, Albanese C, Bouzahzah B, Fu M, Augenlicht LH, Donehower LA, Takemaru K, Moon RT, Davis R, Lisanti MP, Shtutman M, Zhurinsky J, Ben-Ze’ev A, Troussard AA, Dedhar S, Pestell RG. The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3beta and cAMP-responsive element-binding protein-dependent pathways. J Biol Chem. 2000;275(42):32649–32657. doi: 10.1074/jbc.M000643200. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Bell RA, D’Agostino RB, Jr, Imperatore G, Johansen JM, Linder B, Liu LL, Loots B, Marcovina S, Mayer-Davis EJ, Pettitt DJ, Waitzfelder B. Incidence of diabetes in youth in the United States. Jama. 2007;297(24):2716–2724. doi: 10.1001/jama.297.24.2716. [DOI] [PubMed] [Google Scholar]
- Dahmen RP, Koch A, Denkhaus D, Tonn JC, Sorensen N, Berthold F, Behrens J, Birchmeier W, Wiestler OD, Pietsch T. Deletions of AXIN1, a component of the WNT/wingless pathway, in sporadic medulloblastomas. Cancer Res. 2001;61(19):7039–7043. [PubMed] [Google Scholar]
- Danilkovitch-Miagkova A, Miagkov A, Skeel A, Nakaigawa N, Zbar B, Leonard EJ. Oncogenic mutants of RON and MET receptor tyrosine kinases cause activation of the beta-catenin pathway. Mol Cell Biol. 2001;21(17):5857–5868. doi: 10.1128/MCB.21.17.5857-5868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry. 2003;8(2):195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, Myers A, Saez K, Henriquez JP, Zhao A, Wollmer MA, Nitsch RM, Hock C, Morris CM, Hardy J, Moon RT. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104(22):9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb A, Davis BH, Guo J, Ni A, Huang J, Zhang Z, Mu H, Dzau VJ. SFRP2 regulates cardiomyogenic differentiation by inhibiting a positive transcriptional auto-feedback loop of Wnt3a. Stem Cells. 2007 doi: 10.1634/stemcells.2007-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Menabo R, Canton M, Barile M, Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in postischemic reperfusion of the heart. J Biol Chem. 2001;276(4):2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–775. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal. 2005;7(9–10):1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- Du B, Ohmichi M, Takahashi K, Kawagoe J, Ohshima C, Igarashi H, Mori-Abe A, Saitoh M, Ohta T, Ohishi A, Doshida M, Tezuka N, Takahashi T, Kurachi H. Both estrogen and raloxifene protect against beta-amyloid-induced neurotoxicity in estrogen receptor alpha-transfected PC12 cells by activation of telomerase activity via Akt cascade. J Endocrinol. 2004;183(3):605–615. doi: 10.1677/joe.1.05775. [DOI] [PubMed] [Google Scholar]
- Dufourcq P, Couffinhal T, Ezan J, Barandon L, Moreau C, Daret D, Duplaa C. FrzA, a secreted frizzled related protein, induced angiogenic response. Circulation. 2002;106(24):3097–3103. doi: 10.1161/01.cir.0000039342.85015.5c. [DOI] [PubMed] [Google Scholar]
- Duplaa C, Jaspard B, Moreau C, D’Amore PA. Identification and cloning of a secreted protein related to the cysteine-rich domain of frizzled. Evidence for a role in endothelial cell growth control. Circ Res. 1999;84(12):1433–1445. doi: 10.1161/01.res.84.12.1433. [DOI] [PubMed] [Google Scholar]
- Dzietko M, Felderhoff-Mueser U, Sifringer M, Krutz B, Bittigau P, Thor F, Heumann R, Buhrer C, Ikonomidou C, Hansen HH. Erythropoietin protects the developing brain against N-methyl-D-aspartate receptor antagonist neurotoxicity. Neurobiol Dis. 2004;15(2):177–187. doi: 10.1016/j.nbd.2003.10.006. [DOI] [PubMed] [Google Scholar]
- El Jamali A, Freund C, Rechner C, Scheidereit C, Dietz R, Bergmann MW. Reoxygenation after severe hypoxia induces cardiomyocyte hypertrophy in vitro: activation of CREB downstream of GSK3beta. Faseb J. 2004;18(10):1096–1098. doi: 10.1096/fj.03-1054fje. [DOI] [PubMed] [Google Scholar]
- Ellies DL, Church V, Francis-West P, Lumsden A. The WNT antagonist cSFRP2 modulates programmed cell death in the developing hindbrain. Development. 2000;127(24):5285–5295. doi: 10.1242/dev.127.24.5285. [DOI] [PubMed] [Google Scholar]
- Engels IH, Stepczynska A, Stroh C, Lauber K, Berg C, Schwenzer R, Wajant H, Janicke RU, Porter AG, Belka C, Gregor M, Schulze-Osthoff K, Wesselborg S. Caspase-8/FLICE functions as an executioner caspase in anticancer drug-induced apoptosis. Oncogene. 2000;19(40):4563–4573. doi: 10.1038/sj.onc.1203824. [DOI] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Carnuccio R, Izzo AA, Iuvone T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J Mol Med. 2006a;84(3):253–258. doi: 10.1007/s00109-005-0025-1. [DOI] [PubMed] [Google Scholar]
- Esposito G, De Filippis D, Maiuri MC, De Stefano D, Carnuccio R, Iuvone T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci Lett. 2006b;399(1–2):91–95. doi: 10.1016/j.neulet.2006.01.047. [DOI] [PubMed] [Google Scholar]
- Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science. 2005;308(5725):1181–1184. doi: 10.1126/science.1109083. [DOI] [PubMed] [Google Scholar]
- Ezan J, Leroux L, Barandon L, Dufourcq P, Jaspard B, Moreau C, Allieres C, Daret D, Couffinhal T, Duplaa C. FrzA/sFRP-1, a secreted antagonist of the Wnt-Frizzled pathway, controls vascular cell proliferation in vitro and in vivo. Cardiovasc Res. 2004;63(4):731–738. doi: 10.1016/j.cardiores.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of beta-catenin during axis formation in Xenopus. J Cell Biol. 1996;132(6):1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Paul IA, LeBlanc MH. Nicotinamide reduces hypoxic ischemic brain injury in the newborn rat. Brain Res Bull. 2006;69(2):117–122. doi: 10.1016/j.brainresbull.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foord SM, Bonner TI, Neubig RR, Rosser EM, Pin JP, Davenport AP, Spedding M, Harmar AJ. International Union of Pharmacology. XLVI. G protein-coupled receptor list. Pharmacol Rev. 2005;57(2):279–288. doi: 10.1124/pr.57.2.5. [DOI] [PubMed] [Google Scholar]
- Fu X, Sun H, Klein WH, Mu X. Beta-catenin is essential for lamination but not neurogenesis in mouse retinal development. Dev Biol. 2006;299(2):424–437. doi: 10.1016/j.ydbio.2006.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, Perrella MA, Lee ME. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276(20):17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- Galceran J, Miyashita-Lin EM, Devaney E, Rubenstein JL, Grosschedl R. Hippocampus development and generation of dentate gyrus granule cells is regulated by LEF1. Development. 2000;127(3):469–482. doi: 10.1242/dev.127.3.469. [DOI] [PubMed] [Google Scholar]
- Ghanevati M, Miller CA. Phospho-beta-catenin accumulation in Alzheimer’s disease and in aggresomes attributable to proteasome dysfunction. J Mol Neurosci. 2005;25(1):79–94. doi: 10.1385/JMN:25:1:079. [DOI] [PubMed] [Google Scholar]
- Golden JA, Bracilovic A, McFadden KA, Beesley JS, Rubenstein JL, Grinspan JB. Ectopic bone morphogenetic proteins 5 and 4 in the chicken forebrain lead to cyclopia and holoprosencephaly. Proc Natl Acad Sci U S A. 1999;96(5):2439–2444. doi: 10.1073/pnas.96.5.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham NA, Asthagiri AR. Epidermal growth factor-mediated T-cell factor/lymphoid enhancer factor transcriptional activity is essential but not sufficient for cell cycle progression in nontransformed mammary epithelial cells. J Biol Chem. 2004;279(22):23517–23524. doi: 10.1074/jbc.M314055200. [DOI] [PubMed] [Google Scholar]
- Grant SF, Thorleifsson G, Reynisdottir I, Benediktsson R, Manolescu A, Sainz J, Helgason A, Stefansson H, Emilsson V, Helgadottir A, Styrkarsdottir U, Magnusson KP, Walters GB, Palsdottir E, Jonsdottir T, Gudmundsdottir T, Gylfason A, Saemundsdottir J, Wilensky RL, Reilly MP, Rader DJ, Bagger Y, Christiansen C, Gudnason V, Sigurdsson G, Thorsteinsdottir U, Gulcher JR, Kong A, Stefansson K. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38(3):320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Ruther U. Bmp, Fgf and Wnt signalling in programmed cell death and chondrogenesis during vertebrate limb development: the role of Dickkopf-1. Int J Dev Biol. 2002a;46(7):943–947. [PubMed] [Google Scholar]
- Grotewold L, Ruther U. The Wnt antagonist Dickkopf-1 is regulated by Bmp signaling and c-Jun and modulates programmed cell death. Embo J. 2002b;21(5):966–975. doi: 10.1093/emboj/21.5.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo G, Bhat NR. Hypoxia/Reoxygenation Differentially Modulates NF-kappaB Activation and iNOS Expression in Astrocytes and Microglia. Antioxid Redox Signal. 2006;8(5–6):911–918. doi: 10.1089/ars.2006.8.911. [DOI] [PubMed] [Google Scholar]
- Guo YF, Xiong DH, Shen H, Zhao LJ, Xiao P, Guo Y, Wang W, Yang TL, Recker RR, Deng HW. Polymorphisms of the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with obesity phenotypes in a large family-based association study. J Med Genet. 2006;43(10):798–803. doi: 10.1136/jmg.2006.041715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17(2):295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100(5):525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol. 2000;151(1):117–130. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara N, Yamada K, Shibata T, Osago H, Hashimoto T, Tsuchiya M. Elevation of cellular NAD levels by nicotinic acid and involvement of nicotinic acid phosphoribosyltransferase in human cells. J Biol Chem. 2007;282(34):24574–24582. doi: 10.1074/jbc.M610357200. [DOI] [PubMed] [Google Scholar]
- Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90(10):1055–1063. doi: 10.1161/01.res.0000018952.70505.f1. [DOI] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of beta-catenin in neural crest development. J Cell Biol. 2002;159(5):867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MI, Eastman RC. Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev. 2000;16(4):230–236. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr122>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ. A genetic association analysis of cognitive ability and cognitive ageing using 325 markers for 109 genes associated with oxidative stress or cognition. BMC Genet. 2007;8:43. doi: 10.1186/1471-2156-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S, Sato T, Akazawa H, Okada H, Maeno A, Ito M, Sugitani Y, Shibata H, Miyazaki Ji J, Katsuki M, Yamauchi Y, Yamamura Ki K, Katamine S, Noda T. Apoptosis in neural crest cells by functional loss of APC tumor suppressor gene. Proc Natl Acad Sci U S A. 2002;99(1):297–302. doi: 10.1073/pnas.012264999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- He XC, Zhang J, Tong WG, Tawfik O, Ross J, Scoville DH, Tian Q, Zeng X, He X, Wiedemann LM, Mishina Y, Li L. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36(10):1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- Henry MK, Lynch JT, Eapen AK, Quelle FW. DNA damage-induced cell-cycle arrest of hematopoietic cells is overridden by activation of the PI-3 kinase/Akt signaling pathway. Blood. 2001;98(3):834–841. doi: 10.1182/blood.v98.3.834. [DOI] [PubMed] [Google Scholar]
- Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, Gotoh Y. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 2004;131(12):2791–2801. doi: 10.1242/dev.01165. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Bucher P, Tschopp J. The CARD domain: a new apoptotic signalling motif. Trends Biochem Sci. 1997;22(5):155–156. doi: 10.1016/s0968-0004(97)01043-8. [DOI] [PubMed] [Google Scholar]
- Holm PC, Rodriguez FJ, Kele J, Castelo-Branco G, Kitajewski J, Arenas E. BMPs, FGF8 and Wnts regulate the differentiation of locus coeruleus noradrenergic neuronal precursors. J Neurochem. 2006;99(1):343–352. doi: 10.1111/j.1471-4159.2006.04039.x. [DOI] [PubMed] [Google Scholar]
- Howlett KF, Sakamoto K, Yu H, Goodyear LJ, Hargreaves M. Insulin-stimulated insulin receptor substrate-2-associated phosphatidylinositol 3-kinase activity is enhanced in human skeletal muscle after exercise. Metabolism. 2006;55(8):1046–1052. doi: 10.1016/j.metabol.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Kodjabachian L, Rebbert ML, Rattner A, Smallwood PM, Samos CH, Nusse R, Dawid IB, Nathans J. A new secreted protein that binds to Wnt proteins and inhibits their activities. Nature. 1999a;398(6726):431–436. doi: 10.1038/18899. [DOI] [PubMed] [Google Scholar]
- Hsieh JC, Rattner A, Smallwood PM, Nathans J. Biochemical characterization of Wnt-frizzled interactions using a soluble, biologically active vertebrate Wnt protein. Proc Natl Acad Sci U S A. 1999b;96(7):3546–3551. doi: 10.1073/pnas.96.7.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Nicotinamide Protects against Ethanol-Induced Apoptotic Neurodegeneration in the Developing Mouse Brain. PLoS Med. 2006;3(4):e101. doi: 10.1371/journal.pmed.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Toyokuni S, Uchida K, Odaka H, Tanaka T, Ikeda H, Hiai H, Seino Y, Yamada Y. Hyperglycemia causes oxidative stress in pancreatic beta-cells of GK rats, a model of type 2 diabetes. Diabetes. 1999;48(4):927–932. doi: 10.2337/diabetes.48.4.927. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3beta and beta-catenin and promotes GSK-3beta-dependent phosphorylation of beta-catenin. Embo J. 1998;17(5):1371–1384. doi: 10.1093/emboj/17.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389(6654):966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Tamai Y, Zorn AM, Yoshida H, Seldin MF, Nishikawa S, Taketo MM. Mouse Wnt receptor gene Fzd5 is essential for yolk sac and placental angiogenesis. Development. 2001;128(1):25–33. doi: 10.1242/dev.128.1.25. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling. Mol Cell Biol. 2003;23(4):1379–1389. doi: 10.1128/MCB.23.4.1379-1389.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito N, Bartunek J, Spitzer KW, Lorell BH. Effects of the nitric oxide donor sodium nitroprusside on intracellular pH and contraction in hypertrophied myocytes. Circulation. 1997;95(9):2303–2311. doi: 10.1161/01.cir.95.9.2303. [DOI] [PubMed] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY. Nuclear localization is required for Dishevelled function in Wnt/beta-catenin signaling. J Biol. 2005;4(1):3. doi: 10.1186/jbiol20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Takeishi Y, Nakada S, Miyamoto T, Tsunoda Y, Takahashi H, Kubota I, Tomoike H. Long-term treatment with angiotensin II type 1 receptor antagonist, CV-11974, restores beta-catenin mRNA expression in volume-overloaded rabbit hearts. Heart Vessels. 2002;17(1):36–41. doi: 10.1007/s003800200040. [DOI] [PubMed] [Google Scholar]
- Jackson GR, Wiedau-Pazos M, Sang TK, Wagle N, Brown CA, Massachi S, Geschwind DH. Human wild-type tau interacts with wingless pathway components and produces neurofibrillary pathology in Drosophila. Neuron. 2002;34(4):509–519. doi: 10.1016/s0896-6273(02)00706-7. [DOI] [PubMed] [Google Scholar]
- Jacobsen EA, Ananieva O, Brown ML, Chang Y. Growth, differentiation, and malignant transformation of pre-B cells mediated by inducible activation of v-Abl oncogene. J Immunol. 2006;176(11):6831–6838. doi: 10.4049/jimmunol.176.11.6831. [DOI] [PubMed] [Google Scholar]
- Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W, Harth J. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356(18):1842–1852. doi: 10.1056/NEJMoa066397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings CG, Dyer SM, Burden SJ. Muscle-specific trk-related receptor with a kringle domain defines a distinct class of receptor tyrosine kinases. Proc Natl Acad Sci U S A. 1993;90(7):2895–2899. doi: 10.1073/pnas.90.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Marsden I, Chen X, Liao X. Sequence specific collective motions in a winged helix DNA binding domain detected by 15N relaxation NMR. Biochemistry. 1998;37(17):6179–6187. doi: 10.1021/bi980031v. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Stewart HJ, Neal MJ. Modulated expression of secreted frizzled-related proteins in human retinal degeneration. Neuroreport. 2000;11(18):3963–3967. doi: 10.1097/00001756-200012180-00012. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68(5):1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak J, Wlodarski P. Hamartin and tuberin modulate gene transcription via beta-catenin. J Neurooncol. 2006;79(3):229–234. doi: 10.1007/s11060-006-9134-0. [DOI] [PubMed] [Google Scholar]
- Kameya S, Hawes NL, Chang B, Heckenlively JR, Naggert JK, Nishina PM. Mfrp, a gene encoding a frizzled related protein, is mutated in the mouse retinal degeneration 6. Hum Mol Genet. 2002;11(16):1879–1886. doi: 10.1093/hmg/11.16.1879. [DOI] [PubMed] [Google Scholar]
- Kanazawa A, Tsukada S, Sekine A, Tsunoda T, Takahashi A, Kashiwagi A, Tanaka Y, Babazono T, Matsuda M, Kaku K, Iwamoto Y, Kawamori R, Kikkawa R, Nakamura Y, Maeda S. Association of the gene encoding wingless-type mammary tumor virus integration-site family member 5B (WNT5B) with type 2 diabetes. Am J Hum Genet. 2004;75(5):832–843. doi: 10.1086/425340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Akt1 protects against inflammatory microglial activation through maintenance of membrane asymmetry and modulation of cysteine protease activity. J Neurosci Res. 2003a;74(1):37–51. doi: 10.1002/jnr.10740. [DOI] [PubMed] [Google Scholar]
- Kang JQ, Chong ZZ, Maiese K. Critical role for Akt1 in the modulation of apoptotic phosphatidylserine exposure and microglial activation. Mol Pharmacol. 2003b;64(3):557–569. doi: 10.1124/mol.64.3.557. [DOI] [PubMed] [Google Scholar]
- Karunakaran S, Diwakar L, Saeed U, Agarwal V, Ramakrishnan S, Iyengar S, Ravindranath V. Activation of apoptosis signal regulating kinase 1 (ASK1) and translocation of death-associated protein, Daxx, in substantia nigra pars compacta in a mouse model of Parkinson’s disease: protection by alpha-lipoic acid. Faseb J. 2007;21(9):2226–2236. doi: 10.1096/fj.06-7580com. [DOI] [PubMed] [Google Scholar]
- Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- Kengaku M, Twombly V, Tabin C. Expression of Wnt and Frizzled genes during chick limb bud development. Cold Spring Harb Symp Quant Biol. 1997;62:421–429. [PubMed] [Google Scholar]
- Kimura-Yoshida C, Tian E, Nakano H, Amazaki S, Shimokawa K, Rossant J, Aizawa S, Matsuo I. Crucial roles of Foxa2 in mouse anterior-posterior axis polarization via regulation of anterior visceral endoderm-specific genes. Proc Natl Acad Sci U S A. 2007;104(14):5919–5924. doi: 10.1073/pnas.0607779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida M, Hino S, Michiue T, Yamamoto H, Kishida S, Fukui A, Asashima M, Kikuchi A. Synergistic activation of the Wnt signaling pathway by Dvl and casein kinase Iepsilon. J Biol Chem. 2001;276(35):33147–33155. doi: 10.1074/jbc.M103555200. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Ray WJ, Glantschnig H, Nantermet PV, Yu Y, Leu CT, Harada S, Kato S, Freedman LP. A regulatory circuit mediating convergence between Nurr1 transcriptional regulation and Wnt signaling. Mol Cell Biol. 2007;27(21):7486–7496. doi: 10.1128/MCB.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130(4):704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104(47):18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Haendeler J, Badorff C, Brandes RP, Hoffmann J, Pandur P, Zeiher AM, Kuhl M, Dimmeler S. Non-canonical Wnt signaling enhances differentiation of human circulating progenitor cells to cardiomyogenic cells. J Biol Chem. 2005;280(17):16838–16842. doi: 10.1074/jbc.M500323200. [DOI] [PubMed] [Google Scholar]
- Kratzsch J, Knerr I, Galler A, Kapellen T, Raile K, Korner A, Thiery J, Dotsch J, Kiess W. Metabolic decompensation in children with type 1 diabetes mellitus associated with increased serum levels of the soluble leptin receptor. Eur J Endocrinol. 2006;155(4):609–614. doi: 10.1530/eje.1.02261. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131(2):391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kunz M, Herrmann M, Wedlich D, Gradl D. Autoregulation of canonical Wnt signaling controls midbrain development. Dev Biol. 2004;273(2):390–401. doi: 10.1016/j.ydbio.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104(26):10894–10899. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med. 2001;249(3):225–235. doi: 10.1046/j.1365-2796.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: evidence for a two-signal model. Development. 1998;125(13):2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Larson ET, Eilers B, Menon S, Reiter D, Ortmann A, Young MJ, Lawrence CM. A winged-helix protein from sulfolobus turreted icosahedral virus points toward stabilizing disulfide bonds in the intracellular proteins of a hyperthermophilic virus. Virology. 2007 doi: 10.1016/j.virol.2007.06.040. [DOI] [PubMed] [Google Scholar]
- Lee DK, Nathan Grantham R, Trachte AL, Mannion JD, Wilson CL. Activation of the canonical Wnt/beta-catenin pathway enhances monocyte adhesion to endothelial cells. Biochem Biophys Res Commun. 2006a;347(1):109–116. doi: 10.1016/j.bbrc.2006.06.082. [DOI] [PubMed] [Google Scholar]
- Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303(5660):1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- Lee JL, Chang CJ, Chueh LL, Lin CT. Secreted frizzled related protein 2 (sFRP2) decreases susceptibility to UV-induced apoptosis in primary culture of canine mammary gland tumors by NF-kappaB activation or JNK suppression. Breast Cancer Res Treat. 2006b;100(1):49–58. doi: 10.1007/s10549-006-9233-9. [DOI] [PubMed] [Google Scholar]
- Lee JS, Ishimoto A, Yanagawa S. Characterization of mouse dishevelled (Dvl) proteins in Wnt/Wingless signaling pathway. J Biol Chem. 1999;274(30):21464–21470. doi: 10.1074/jbc.274.30.21464. [DOI] [PubMed] [Google Scholar]
- Lehman DM, Hunt KJ, Leach RJ, Hamlington J, Arya R, Abboud HE, Duggirala R, Blangero J, Goring HH, Stern MP. Haplotypes of Transcription Factor 7-Like 2 (TCF7L2) Gene and Its Upstream Region Are Associated With Type 2 Diabetes and Age of Onset in Mexican Americans. Diabetes. 2007;56(2):389–393. doi: 10.2337/db06-0860. [DOI] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125(5):987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Leuner K, Hauptmann S, Abdel-Kader R, Scherping I, Keil U, Strosznajder JB, Eckert A, Muller WE. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer’s disease? Antioxid Redox Signal. 2007;9(10):1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, Raible DW. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131(6):1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- Leytin V, Allen DJ, Mykhaylov S, Lyubimov E, Freedman J. Thrombin-triggered platelet apoptosis. J Thromb Haemost. 2006;4(12):2656–2663. doi: 10.1111/j.1538-7836.2006.02200.x. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Erythropoietin on a Tightrope: Balancing Neuronal and Vascular Protection between Intrinsic and Extrinsic Pathways. Neurosignals. 2004a;13(6):265–289. doi: 10.1159/000081963. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Navigating novel mechanisms of cellular plasticity with the NAD+ precursor and nutrient nicotinamide. Front Biosci. 2004b;9:2500–2520. doi: 10.2741/1412. [DOI] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Vital elements of the wnt-frizzled signaling pathway in the nervous system. Curr Neurovasc Res. 2005;2(4):331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Cell Life Versus Cell Longevity: The Mysteries Surrounding the NAD(+) Precursor Nicotinamide. Curr Med Chem. 2006a;13(8):883–895. doi: 10.2174/092986706776361058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Microglial integrity is maintained by erythropoietin through integration of Akt and its substrates of glycogen synthase kinase-3beta, beta-catenin, and nuclear factor-kappaB. Curr Neurovasc Res. 2006b;3(3):187–201. doi: 10.2174/156720206778018758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, Maiese K. Winding through the WNT pathway during cellular development and demise. Histol Histopathol. 2006c;21(1):103–124. doi: 10.14670/hh-21.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Yuan H, Xie W, Mao J, Caruso AM, McMahon A, Sussman DJ, Wu D. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J Biol Chem. 1999;274(1):129–134. doi: 10.1074/jbc.274.1.129. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Murthy S, Dou QP, Sheng S, Reddy GP, Sarkar FH. Regulation of FOXO3a/beta-catenin/GSK-3beta signaling by 3,3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in prostate cancer cells. J Biol Chem. 2007;282(29):21542–21550. doi: 10.1074/jbc.M701978200. [DOI] [PubMed] [Google Scholar]
- Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3(10):913–917. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- Lin CL, Wang JY, Huang YT, Kuo YH, Surendran K, Wang FS. Wnt/beta-catenin signaling modulates survival of high glucose-stressed mesangial cells. J Am Soc Nephrol. 2006;17(10):2812–2820. doi: 10.1681/ASN.2005121355. [DOI] [PubMed] [Google Scholar]
- Lin SH, Maiese K. The metabotropic glutamate receptor system protects against ischemic free radical programmed cell death in rat brain endothelial cells. J Cereb Blood Flow Metab. 2001;21(3):262–275. doi: 10.1097/00004647-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Lin SH, Vincent A, Shaw T, Maynard KI, Maiese K. Prevention of nitric oxide-induced neuronal injury through the modulation of independent pathways of programmed cell death. J Cereb Blood Flow Metab. 2000;20(9):1380–1391. doi: 10.1097/00004647-200009000-00013. [DOI] [PubMed] [Google Scholar]
- Ling PR, Mueller C, Smith RJ, Bistrian BR. Hyperglycemia induced by glucose infusion causes hepatic oxidative stress and systemic inflammation, but not STAT3 or MAP kinase activation in liver in rats. Metabolism. 2003;52(7):868–874. doi: 10.1016/s0026-0495(03)00057-x. [DOI] [PubMed] [Google Scholar]
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira II, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317(5839):803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22(4):361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Rao S, Carroll TJ, Vallance JE, Ito M, Ondr JK, Kurup S, Glass DA, Patel MS, Shu W, Morrisey EE, McMahon AP, Karsenty G, Lang RA. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437(7057):417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J Biol Chem. 2002;277(41):38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119(1):97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Luchsinger JA, Tang MX, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154(7):635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22(4):1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Wang HY. Suppression of cyclic GMP-dependent protein kinase is essential to the Wnt/cGMP/Ca2+ pathway. J Biol Chem. 2006;281(41):30990–31001. doi: 10.1074/jbc.M603603200. [DOI] [PubMed] [Google Scholar]
- Maiese K. The dynamics of cellular injury: transformation into neuronal and vascular protection. Histol Histopathol. 2001;16(2):633–644. doi: 10.14670/HH-16.633. [DOI] [PubMed] [Google Scholar]
- Maiese K, Ahmad I, TenBroeke M, Gallant J. Metabotropic glutamate receptor subtypes independently modulate neuronal intracellular calcium. J Neurosci Res. 1999;55:472–485. doi: 10.1002/(SICI)1097-4547(19990215)55:4<472::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Maiese K, Boccone L. Neuroprotection by peptide growth factors against anoxia and nitric oxide toxicity requires modulation of protein kinase C. J Cereb Blood Flow Metab. 1995;15(3):440–449. doi: 10.1038/jcbfm.1995.55. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Nicotinamide: necessary nutrient emerges as a novel cytoprotectant for the brain. Trends Pharmacol Sci. 2003;24(5):228–232. doi: 10.1016/S0165-6147(03)00078-6. [DOI] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ. Insights into oxidative stress and potential novel therapeutic targets for Alzheimer disease. Restor Neurol Neurosci. 2004;22(2):87–104. [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Li F. Driving cellular plasticity and survival through the signal transduction pathways of metabotropic glutamate receptors. Curr Neurovasc Res. 2005a;2(5):425–446. doi: 10.2174/156720205774962692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Mechanisitic insights into diabetes mellitus and oxidative stress. Curr Med Chem. 2007a;14:1689–1699. doi: 10.2174/092986707781058968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC. Sly as a FOXO”: New paths with Forkhead signaling in the brain. Curr Neurovasc Res. 2007b;4(4):295–302. doi: 10.2174/156720207782446306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. Erythropoietin in the brain: can the promise to protect be fulfilled? Trends Pharmacol Sci. 2004;25(11):577–583. doi: 10.1016/j.tips.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005b;293(1):90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Morhan SD, Chong ZZ. Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res. 2007c;4(1):63–71. doi: 10.2174/156720207779940653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, TenBroeke M, Kue I. Neuroprotection of lubeluzole is mediated through the signal transduction pathways of nitric oxide. J Neurochem. 1997;68(2):710–714. doi: 10.1046/j.1471-4159.1997.68020710.x. [DOI] [PubMed] [Google Scholar]
- Maiese K, Vincent A, Lin SH, Shaw T. Group I and Group III metabotropic glutamate receptor subtypes provide enhanced neuroprotection. J Neurosci Res. 2000;62(2):257–272. doi: 10.1002/1097-4547(20001015)62:2<257::AID-JNR10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Critical temporal modulation of neuronal programmed cell injury. Cell Mol Neurobiol. 2000a;20(3):383–400. doi: 10.1023/A:1007070311203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Vincent AM. Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res. 2000b;59(4):568–580. doi: 10.1002/(SICI)1097-4547(20000215)59:4<568::AID-JNR13>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Maiorana A, O’Driscoll G, Goodman C, Taylor R, Green D. Combined aerobic and resistance exercise improves glycemic control and fitness in type 2 diabetes. Diabetes Res Clin Pract. 2002;56(2):115–123. doi: 10.1016/s0168-8227(01)00368-0. [DOI] [PubMed] [Google Scholar]
- Mallat M, Marin-Teva JL, Cheret C. Phagocytosis in the developing CNS: more than clearing the corpses. Curr Opin Neurobiol. 2005;15(1):101–107. doi: 10.1016/j.conb.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315(5816):1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao C, Malek OT, Pueyo ME, Steg PG, Soubrier F. Differential expression of rat frizzled-related frzb-1 and frizzled receptor fz1 and fz2 genes in the rat aorta after balloon injury. Arterioscler Thromb Vasc Biol. 2000;20(1):43–51. doi: 10.1161/01.atv.20.1.43. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell. 2001;7(4):801–809. doi: 10.1016/s1097-2765(01)00224-6. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21(8):1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mari C, Karabiyikoglu M, Goris ML, Tait JF, Yenari MA, Blankenberg FG. Detection of focal hypoxic-ischemic injury and neuronal stress in a rodent model of unilateral MCA occlusion/reperfusion using radiolabeled annexin V. Eur J Nucl Med Mol Imaging. 2004;31(5):733–739. doi: 10.1007/s00259-004-1473-5. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15(3):316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki H, Tamatani M, Mitsuda N, Namikawa K, Kiyama H, Miyake S, Tohyama M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J Neurochem. 1999;73(5):2037–2046. [PubMed] [Google Scholar]
- Mayr T, Deutsch U, Kuhl M, Drexler HC, Lottspeich F, Deutzmann R, Wedlich D, Risau W. Fritz: a secreted frizzled-related protein that inhibits Wnt activity. Mech Dev. 1997;63(1):109–125. doi: 10.1016/s0925-4773(97)00035-x. [DOI] [PubMed] [Google Scholar]
- McCormick WC, Hardy J, Kukull WA, Bowen JD, Teri L, Zitzer S, Larson EB. Healthcare utilization and costs in managed care patients with Alzheimer’s disease during the last few years of life. J Am Geriatr Soc. 2001;49(9):1156–1160. doi: 10.1046/j.1532-5415.2001.49231.x. [DOI] [PubMed] [Google Scholar]
- Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. 2000;18(4–5):423–431. doi: 10.1016/s0736-5748(00)00012-5. [DOI] [PubMed] [Google Scholar]
- Melkonyan HS, Chang WC, Shapiro JP, Mahadevappa M, Fitzpatrick PA, Kiefer MC, Tomei LD, Umansky SR. SARPs: a family of secreted apoptosis-related proteins. Proc Natl Acad Sci U S A. 1997;94(25):13636–13641. doi: 10.1073/pnas.94.25.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memisogullari R, Bakan E. Levels of ceruloplasmin, transferrin, and lipid peroxidation in the serum of patients with Type 2 diabetes mellitus. J Diabetes Complications. 2004;18(4):193–197. doi: 10.1016/S1056-8727(03)00032-1. [DOI] [PubMed] [Google Scholar]
- Mendiondo MS, Kryscio RJ, Schmitt FA. Models of progression in AD: predicting disability and costs. Neurology. 2001;57(6):943–944. doi: 10.1212/wnl.57.6.943. [DOI] [PubMed] [Google Scholar]
- Miki T, Miura T, Yano T, Takahashi A, Sakamoto J, Tanno M, Kobayashi H, Ikeda Y, Nishihara M, Naitoh K, Ohori K, Shimamoto K. Alteration in erythropoietin-induced cardioprotective signaling by postinfarct ventricular remodeling. J Pharmacol Exp Ther. 2006;317(1):68–75. doi: 10.1124/jpet.105.095745. [DOI] [PubMed] [Google Scholar]
- Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaoka T, Seno H, Ishino H. Increased expression of Wnt-1 in schizophrenic brains. Schizophr Res. 1999;38(1):1–6. doi: 10.1016/s0920-9964(98)00179-0. [DOI] [PubMed] [Google Scholar]
- Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295(14):1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316(3):999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Kawachi K, Kamakura S, Masuyama N, Yamanaka H, Matsumoto K, Kikuchi A, Nishida E. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase/stress-activated protein kinase activation and the axis formation in vertebrates. J Biol Chem. 1999;274(43):30957–30962. doi: 10.1074/jbc.274.43.30957. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Medina M, Semenov M, Brown AM, Kosik KS. Wnt-1 expression in PC12 cells induces exon 15 deletion and expression of L-APP. Neurobiol Dis. 2004;16(1):59–67. doi: 10.1016/j.nbd.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Mudher A, Chapman S, Richardson J, Asuni A, Gibb G, Pollard C, Killick R, Iqbal T, Raymond L, Varndell I, Sheppard P, Makoff A, Gower E, Soden PE, Lewis P, Murphy M, Golde TE, Rupniak HT, Anderton BH, Lovestone S. Dishevelled regulates the metabolism of amyloid precursor protein via protein kinase C/mitogen-activated protein kinase and c-Jun terminal kinase. J Neurosci. 2001;21(14):4987–4995. doi: 10.1523/JNEUROSCI.21-14-04987.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A. 1995;92(7):3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroyama Y, Kondoh H, Takada S. Wnt proteins promote neuronal differentiation in neural stem cell culture. Biochem Biophys Res Commun. 2004;313(4):915–921. doi: 10.1016/j.bbrc.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Mussmann R, Geese M, Harder F, Kegel S, Andag U, Lomow A, Burk U, Onichtchouk D, Dohrmann C, Austen M. Inhibition of glycogen synthase kinase (GSK) 3 promotes replication and survival of pancreatic beta cells. J Biol Chem. 2007 doi: 10.1074/jbc.M609637200. [DOI] [PubMed] [Google Scholar]
- Nacher V, Carretero A, Navarro M, Armengol C, Llombart C, Blasi J, Ruberte J. beta-Catenin expression during vascular development and degeneration of avian mesonephros. J Anat. 2005;206(2):165–174. doi: 10.1111/j.1469-7580.2005.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito AT, Akazawa H, Takano H, Minamino T, Nagai T, Aburatani H, Komuro I. Phosphatidylinositol 3-kinase-Akt pathway plays a critical role in early cardiomyogenesis by regulating canonical Wnt signaling. Circ Res. 2005;97(2):144–151. doi: 10.1161/01.RES.0000175241.92285.f8. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A. 2006;103(52):19812–19817. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagami Y, Nishimura S, Murasugi T, Kubo T, Kaneko I, Meguro M, Marumoto S, Kogen H, Koyama K, Oda T. A novel compound RS-0466 reverses beta-amyloid-induced cytotoxicity through the Akt signaling pathway in vitro. Eur J Pharmacol. 2002;457(1):11–17. doi: 10.1016/s0014-2999(02)02657-2. [DOI] [PubMed] [Google Scholar]
- Nangaku M, Fliser D. Erythropoiesis-stimulating agents: past and future. Kidney Int. 2007;(Suppl 107):S1–3. doi: 10.1038/sj.ki.5002480. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Haber EP, Hirabara SM, Rebelato EL, Procopio J, Morgan D, Oliveira-Emilio HC, Carpinelli AR, Curi R. Diabetes associated cell stress and dysfunction: role of mitochondrial and non-mitochondrial ROS production and activity. J Physiol. 2007;583(Pt 1):9–24. doi: 10.1113/jphysiol.2007.135871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann S, Zhao C, Pascu F, Stahl U, Aulepp U, Niswander L, Weber JL, Muller U. Homozygous WNT3 mutation causes tetra-amelia in a large consanguineous family. Am J Hum Genet. 2004;74(3):558–563. doi: 10.1086/382196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noria S, Cowan DB, Gotlieb AI, Langille BL. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85(6):504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- Nurmi A, Goldsteins G, Narvainen J, Pihlaja R, Ahtoniemi T, Grohn O, Koistinaho J. Antioxidant pyrrolidine dithiocarbamate activates Akt-GSK signaling and is neuroprotective in neonatal hypoxia-ischemia. Free Radic Biol Med. 2006;40(10):1776–1784. doi: 10.1016/j.freeradbiomed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Wnt genes. Cell. 1992;69(7):1073–1087. doi: 10.1016/0092-8674(92)90630-u. [DOI] [PubMed] [Google Scholar]
- Okouchi M, Ekshyyan O, Maracine M, Aw TY. Neuronal apoptosis in neurodegeneration. Antioxid Redox Signal. 2007;9(8):1059–1096. doi: 10.1089/ars.2007.1511. [DOI] [PubMed] [Google Scholar]
- Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133(5):833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- Palpant NJ, Yasuda S, MacDougald O, Metzger JM. Non-canonical Wnt signaling enhances differentiation of Sca1+/c-kit+ adipose-derived murine stromal vascular cells into spontaneously beating cardiac myocytes. J Mol Cell Cardiol. 2007;43(3):362–370. doi: 10.1016/j.yjmcc.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panhuysen M, Vogt Weisenhorn DM, Blanquet V, Brodski C, Heinzmann U, Beisker W, Wurst W. Effects of Wnt1 signaling on proliferation in the developing mid-/hindbrain region. Mol Cell Neurosci. 2004;26(1):101–111. doi: 10.1016/j.mcn.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Papkoff J, Aikawa M. WNT-1 and HGF regulate GSK3 beta activity and beta-catenin signaling in mammary epithelial cells. Biochem Biophys Res Commun. 1998;247(3):851–858. doi: 10.1006/bbrc.1998.8888. [DOI] [PubMed] [Google Scholar]
- Parsa CJ, Matsumoto A, Kim J, Riel RU, Pascal LS, Walton GB, Thompson RB, Petrofski JA, Annex BH, Stamler JS, Koch WJ. A novel protective effect of erythropoietin in the infarcted heart. J Clin Invest. 2003;112(7):999–1007. doi: 10.1172/JCI18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 2000;10(3):392–399. doi: 10.1016/s0959-4388(00)00100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl R. The rate of living. University of London Press; London: 1928. [Google Scholar]
- Perreault N, Katz JP, Sackett SD, Kaestner KH. Foxl1 controls the Wnt/beta-catenin pathway by modulating the expression of proteoglycans in the gut. J Biol Chem. 2001;276(46):43328–43333. doi: 10.1074/jbc.M104366200. [DOI] [PubMed] [Google Scholar]
- Perreault N, Sackett SD, Katz JP, Furth EE, Kaestner KH. Foxl1 is a mesenchymal Modifier of Min in carcinogenesis of stomach and colon. Genes Dev. 2005;19(3):311–315. doi: 10.1101/gad.1260605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. Frzb modulates Wnt-9a-mediated beta-catenin signaling during avian atrioventricular cardiac cushion development. Dev Biol. 2005;278(1):35–48. doi: 10.1016/j.ydbio.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407(6803):535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Playford MP, Bicknell D, Bodmer WF, Macaulay VM. Insulin-like growth factor 1 regulates the location, stability, and transcriptional activity of beta-catenin. Proc Natl Acad Sci U S A. 2000;97(22):12103–12108. doi: 10.1073/pnas.210394297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl BS, Knochel W. Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mech Dev. 2001;103(1–2):93–106. doi: 10.1016/s0925-4773(01)00334-3. [DOI] [PubMed] [Google Scholar]
- Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131(3):476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- Price RM, Tulsyan N, Dermody JJ, Schwalb M, Soteropoulos P, Castronuovo JJ., Jr Gene expression after crush injury of human saphenous vein: using microarrays to define the transcriptional profile. J Am Coll Surg. 2004;199(3):411–418. doi: 10.1016/j.jamcollsurg.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Qu J, Zhou J, Yi XP, Dong B, Zheng H, Miller LM, Wang X, Schneider MD, Li F. Cardiac-specific haploinsufficiency of beta-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. J Mol Cell Cardiol. 2007;43(3):319–326. doi: 10.1016/j.yjmcc.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachek LI, Thornley NP, Grishko VI, LeDoux SP, Wilson GL. Protection of INS-1 cells from free fatty acid-induced apoptosis by targeting hOGG1 to mitochondria. Diabetes. 2006;55(4):1022–1028. doi: 10.2337/diabetes.55.04.06.db05-0865. [DOI] [PubMed] [Google Scholar]
- Regulska M, Leskiewicz M, Budziszewska B, Kutner A, Jantas D, Basta-Kaim A, Kubera M, Jaworska-Feil L, Lason W. Inhibitory effects of 1,25-dihydroxyvitamin D(3) and its low-calcemic analogues on staurosporine-induced apoptosis. Pharmacol Rep. 2007;59(4):393–401. [PubMed] [Google Scholar]
- Rehn M, Pihlajaniemi T. Identification of three N-terminal ends of type XVIII collagen chains and tissue-specific differences in the expression of the corresponding transcripts. The longest form contains a novel motif homologous to rat and Drosophila frizzled proteins. J Biol Chem. 1995;270(9):4705–4711. doi: 10.1074/jbc.270.9.4705. [DOI] [PubMed] [Google Scholar]
- Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, Hintz L, Nusse R, Weissman IL. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423(6938):409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386(6626):671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- Roberts E, Jr, Chih CP. The influence of age of pH regulation in hippocampal slices before, during, and after anoxia. J Cereb Blood Flow Metab. 1997;17(5):560–566. doi: 10.1097/00004647-199705000-00010. [DOI] [PubMed] [Google Scholar]
- Robitaille J, MacDonald ML, Kaykas A, Sheldahl LC, Zeisler J, Dube MP, Zhang LH, Singaraja RR, Guernsey DL, Zheng B, Siebert LF, Hoskin-Mott A, Trese MT, Pimstone SN, Shastry BS, Moon RT, Hayden MR, Goldberg YP, Samuels ME. Mutant frizzled-4 disrupts retinal angiogenesis in familial exudative vitreoretinopathy. Nat Genet. 2002;32(2):326–330. doi: 10.1038/ng957. [DOI] [PubMed] [Google Scholar]
- Rodova M, Islam MR, Maser RL, Calvet JP. The polycystic kidney disease-1 promoter is a target of the beta-catenin/T-cell factor pathway. J Biol Chem. 2002;277(33):29577–29583. doi: 10.1074/jbc.M203570200. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289(5481):950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Rosso SB, Sussman D, Wynshaw-Boris A, Salinas PC. Wnt signaling through Dishevelled, Rac and JNK regulates dendritic development. Nat Neurosci. 2005;8(1):34–42. doi: 10.1038/nn1374. [DOI] [PubMed] [Google Scholar]
- Rowe MK, Wiest C, Chuang DM. GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder. Neurosci Biobehav Rev. 2007;31(6):920–931. doi: 10.1016/j.neubiorev.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadot E, Simcha I, Shtutman M, Ben-Ze’ev A, Geiger B. Inhibition of beta-catenin-mediated transactivation by cadherin derivatives. Proc Natl Acad Sci U S A. 1998;95(26):15339–15344. doi: 10.1073/pnas.95.26.15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas M, Diaz R, Abraham NG, Ruiz de Galarreta CM, Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J Biol Chem. 2003;278(16):13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- Salins P, Shawesh S, He Y, Dibrov A, Kashour T, Arthur G, Amara F. Lovastatin protects human neurons against Abeta-induced toxicity and causes activation of beta-catenin-TCF/LEF signaling. Neurosci Lett. 2007;412(3):211–216. doi: 10.1016/j.neulet.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353(2):312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- Sanz O, Acarin L, Gonzalez B, Castellano B. NF-kappaB and IkappaBalpha expression following traumatic brain injury to the immature rat brain. J Neurosci Res. 2002;67(6):772–780. doi: 10.1002/jnr.10140. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Kesari S, Stiles CD. Molecular pathogenesis of adult brain tumors and the role of stem cells. Neurol Clin. 2007;25(4):891–924. vii. doi: 10.1016/j.ncl.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Schnaider Beeri M, Goldbourt U, Silverman JM, Noy S, Schmeidler J, Ravona-Springer R, Sverdlick A, Davidson M. Diabetes mellitus in midlife and the risk of dementia three decades later. Neurology. 2004;63(10):1902–1907. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- Schneider S, Buchert M, Georgiev O, Catimel B, Halford M, Stacker SA, Baechi T, Moelling K, Hovens CM. Mutagenesis and selection of PDZ domains that bind new protein targets. Nat Biotechnol. 1999;17(2):170–175. doi: 10.1038/6172. [DOI] [PubMed] [Google Scholar]
- Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28(10):518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Schumann H, Holtz J, Zerkowski HR, Hatzfeld M. Expression of secreted frizzled related proteins 3 and 4 in human ventricular myocardium correlates with apoptosis related gene expression. Cardiovasc Res. 2000;45(3):720–728. doi: 10.1016/s0008-6363(99)00376-4. [DOI] [PubMed] [Google Scholar]
- Scott LJ, Bonnycastle LL, Willer CJ, Sprau AG, Jackson AU, Narisu N, Duren WL, Chines PS, Stringham HM, Erdos MR, Valle TT, Tuomilehto J, Bergman RN, Mohlke KL, Collins FS, Boehnke M. Association of transcription factor 7-like 2 (TCF7L2) variants with type 2 diabetes in a Finnish sample. Diabetes. 2006;55(9):2649–2653. doi: 10.2337/db06-0341. [DOI] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8(2):126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shein NA, Tsenter J, Alexandrovich AG, Horowitz M, Shohami E. Akt phosphorylation is required for heat acclimation-induced neuroprotection. J Neurochem. 2007;103(4):1523–1529. doi: 10.1111/j.1471-4159.2007.04862.x. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Neuritic plaque evolution in Alzheimer’s disease is accompanied by transition of activated microglia from primed to enlarged to phagocytic forms. Acta Neuropathol (Berl) 1997;94(1):1–5. doi: 10.1007/s004010050664. [DOI] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473(4):496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Iaina A, Schwartz D. The interaction between heart failure and other heart diseases, renal failure, and anemia. Semin Nephrol. 2006;26(4):296–306. doi: 10.1016/j.semnephrol.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Silverberg DS, Wexler D, Sheps D, Blum M, Keren G, Baruch R, Schwartz D, Yachnin T, Steinbruch S, Shapira I, Laniado S, Iaina A. The effect of correction of mild anemia in severe, resistant congestive heart failure using subcutaneous erythropoietin and intravenous iron: a randomized controlled study. J Am Coll Cardiol. 2001;37(7):1775–1780. doi: 10.1016/s0735-1097(01)01248-7. [DOI] [PubMed] [Google Scholar]
- Singh AM, Li FQ, Hamazaki T, Kasahara H, Takemaru K, Terada N. Chibby, an antagonist of the Wnt/beta-catenin pathway, facilitates cardiomyocyte differentiation of murine embryonic stem cells. Circulation. 2007;115(5):617–626. doi: 10.1161/CIRCULATIONAHA.106.642298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390(6658):410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- Smith K, Bui TD, Poulsom R, Kaklamanis L, Williams G, Harris AL. Up-regulation of macrophage wnt gene expression in adenoma-carcinoma progression of human colorectal cancer. Br J Cancer. 1999;81(3):496–502. doi: 10.1038/sj.bjc.6690721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Fricker LD. Cloning and expression of human carboxypeptidase Z, a novel metallocarboxypeptidase. J Biol Chem. 1997;272(16):10543–10550. doi: 10.1074/jbc.272.16.10543. [DOI] [PubMed] [Google Scholar]
- Soriano S, Kang DE, Fu M, Pestell R, Chevallier N, Zheng H, Koo EH. Presenilin 1 negatively regulates beta-catenin/T cell factor/lymphoid enhancer factor-1 signaling independently of beta-amyloid precursor protein and notch processing. J Cell Biol. 2001;152(4):785–794. doi: 10.1083/jcb.152.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandou E, Tsouchnikas I, Karkavelas G, Dounousi E, Simeonidou C, Guiba-Tziampiri O, Tsakiris D. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant. 2006;21(2):330–336. doi: 10.1093/ndt/gfi177. [DOI] [PubMed] [Google Scholar]
- Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30(6):268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372(6507):679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Stegh AH, Barnhart BC, Volkland J, Algeciras-Schimnich A, Ke N, Reed JC, Peter ME. Inactivation of caspase-8 on mitochondria of Bcl-xL-expressing MCF7-Fas cells: role for the bifunctional apoptosis regulator protein. J Biol Chem. 2002;277(6):4351–4360. doi: 10.1074/jbc.M108947200. [DOI] [PubMed] [Google Scholar]
- Su F, Overholtzer M, Besser D, Levine AJ. WISP-1 attenuates p53-mediated apoptosis in response to DNA damage through activation of the Akt kinase. Genes Dev. 2002;16(1):46–57. doi: 10.1101/gad.942902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Nakamura S, Asano K, Kinouchi M, Ishida-Yamamoto A, Iizuka H. Fas antigen modulates ultraviolet B-induced apoptosis of SVHK cells: sequential activation of caspases 8, 3, and 1 in the apoptotic process. Exp Cell Res. 1999;249(2):291–298. doi: 10.1006/excr.1999.4476. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407(6803):530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Shan W, Phillips GR, Arndt K, Bozdagi O, Shapiro L, Huntley GW, Benson DL, Colman DR. Molecular modification of N-cadherin in response to synaptic activity. Neuron. 2000;25(1):93–107. doi: 10.1016/s0896-6273(00)80874-0. [DOI] [PubMed] [Google Scholar]
- Teo JL, Ma H, Nguyen C, Lam C, Kahn M. Specific inhibition of CBP/beta-catenin interaction rescues defects in neuronal differentiation caused by a presenilin-1 mutation. Proc Natl Acad Sci U S A. 2005;102(34):12171–12176. doi: 10.1073/pnas.0504600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry S, Yang X, Chen MW, Vacherot F, Buttyan R. Multifaceted interaction between the androgen and Wnt signaling pathways and the implication for prostate cancer. J Cell Biochem. 2006;99(2):402–410. doi: 10.1002/jcb.20983. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398(6726):422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tickenbrock L, Schwable J, Strey A, Sargin B, Hehn S, Baas M, Choudhary C, Gerke V, Berdel WE, Muller-Tidow C, Serve H. Wnt signaling regulates transendothelial migration of monocytes. J Leukoc Biol. 2006;79(6):1306–1313. doi: 10.1189/jlb.0905539. [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3beta activity. Dev Cell. 2003;4(3):407–418. doi: 10.1016/s1534-5807(03)00063-7. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Hong Z, Kuzuya T, Tada M, Hori M. Wnt/frizzled-2 signaling induces aggregation and adhesion among cardiac myocytes by increased cadherin-beta-catenin complex. J Cell Biol. 2000;150(1):225–241. doi: 10.1083/jcb.150.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Schans VA, van den Borne SW, Strzelecka AE, Janssen BJ, van der Velden JL, Langen RC, Wynshaw-Boris A, Smits JF, Blankesteijn WM. Interruption of Wnt signaling attenuates the onset of pressure overload-induced cardiac hypertrophy. Hypertension. 2007;49(3):473–480. doi: 10.1161/01.HYP.0000255946.55091.24. [DOI] [PubMed] [Google Scholar]
- van Gijn ME, Blankesteijn WM, Smits JF, Hierck B, Gittenberger-de Groot AC. Frizzled 2 is transiently expressed in neural crest-containing areas during development of the heart and great arteries in the mouse. Anat Embryol (Berl) 2001;203(3):185–192. doi: 10.1007/s004290000152. [DOI] [PubMed] [Google Scholar]
- Verdaguer E, Susana Gde A, Clemens A, Pallas M, Camins A. Implication of the transcription factor E2F-1 in the modulation of neuronal apoptosis. Biomed Pharmacother. 2007;61(7):390–399. doi: 10.1016/j.biopha.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Direct temporal analysis of apoptosis induction in living adherent neurons. J Histochem Cytochem. 1999a;47(5):661–672. doi: 10.1177/002215549904700508. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Maiese K. Nitric oxide induction of neuronal endonuclease activity in programmed cell death. Exp Cell Res. 1999b;246(2):290–300. doi: 10.1006/excr.1998.4282. [DOI] [PubMed] [Google Scholar]
- Vincent AM, TenBroeke M, Maiese K. Metabotropic glutamate receptors prevent programmed cell death through the modulation of neuronal endonuclease activity and intracellular pH. Exp Neurol. 1999a;155(1):79–94. doi: 10.1006/exnr.1998.6966. [DOI] [PubMed] [Google Scholar]
- Vincent AM, TenBroeke M, Maiese K. Neuronal intracellular pH directly mediates nitric oxide-induced programmed cell death. J Neurobiol. 1999b;40(2):171–184. doi: 10.1002/(sici)1097-4695(199908)40:2<171::aid-neu4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338(6212):263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- Wagner U, Brownlees J, Irving NG, Lucas FR, Salinas PC, Miller CC. Overexpression of the mouse dishevelled-1 protein inhibits GSK-3beta-mediated phosphorylation of tau in transfected mammalian cells. FEBS Lett. 1997;411(2–3):369–372. doi: 10.1016/s0014-5793(97)00733-3. [DOI] [PubMed] [Google Scholar]
- Wang H, Gilner JB, Bautch VL, Wang DZ, Wainwright BJ, Kirby SL, Patterson C. Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. J Biol Chem. 2007a;282(1):782–791. doi: 10.1074/jbc.M606610200. [DOI] [PubMed] [Google Scholar]
- Wang HY, Malbon CC. Wnt signaling, Ca2+, and cyclic GMP: visualizing Frizzled functions. Science. 2003;300(5625):1529–1530. doi: 10.1126/science.1085259. [DOI] [PubMed] [Google Scholar]
- Wang HY, Malbon CC. Wnt-frizzled signaling to G-protein-coupled effectors. Cell Mol Life Sci. 2004;61(1):69–75. doi: 10.1007/s00018-003-3165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Xiao Y, Mou Y, Zhao Y, Blankesteijn WM, Hall JL. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ Res. 2002;90(3):340–347. doi: 10.1161/hh0302.104466. [DOI] [PubMed] [Google Scholar]
- Wang XL, Yang YJ, Xie M, Yu XH, Liu CT, Wang X. Proliferation of neural stem cells correlates with Wnt-3 protein in hypoxic-ischemic neonate rats after hyperbaric oxygen therapy. Neuroreport. 2007b;18(16):1753–1756. doi: 10.1097/WNR.0b013e3282f0ec09. [DOI] [PubMed] [Google Scholar]
- Wehrli M, Dougan ST, Caldwell K, O’Keefe L, Schwartz S, Vaizel-Ohayon D, Schejter E, Tomlinson A, DiNardo S. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407(6803):527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- Wexler EM, Geschwind DH, Palmer TD. Lithium regulates adult hippocampal progenitor development through canonical Wnt pathway activation. Mol Psychiatry. 2007 doi: 10.1038/sj.mp.4002093. [DOI] [PubMed] [Google Scholar]
- Wiedau-Pazos M, Wong E, Solomon E, Alarcon M, Geschwind DH. Wnt-pathway activation during the early stage of neurodegeneration in FTDP-17 mice. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijchers PJ, Burbach JP, Smidt MP. In control of biology: of mice, men and Foxes. Biochem J. 2006;397(2):233–246. doi: 10.1042/BJ20060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Wilson C, Goberdhan DC, Steller H. Dror, a potential neurotrophic receptor gene, encodes a Drosophila homolog of the vertebrate Ror family of Trk-related receptor tyrosine kinases. Proc Natl Acad Sci U S A. 1993;90(15):7109–7113. doi: 10.1073/pnas.90.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson FH, Hariri A, Farhi A, Zhao H, Petersen KF, Toka HR, Nelson-Williams C, Raja KM, Kashgarian M, Shulman GI, Scheinman SJ, Lifton RP. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306(5699):1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- Woll PS, Morris JK, Painschab MS, Marcus RK, Kohn AD, Biechele TL, Moon RT, Kaufman DS. Wnt signaling promotes hemato-endothelial cell development from human embryonic stem cells. Blood. 2007 doi: 10.1182/blood-2007-04-084186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem Biophys Res Commun. 1999;263(2):384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- Wright WS, Longo KA, Dolinsky VW, Gerin I, Kang S, Bennett CN, Chiang SH, Prestwich TC, Gress C, Burant CF, Susulic VS, Macdougald OA. Wnt10b Inhibits Obesity in ob/ob and Agouti Mice. Diabetes. 2007;56(2):295–303. doi: 10.2337/db06-1339. [DOI] [PubMed] [Google Scholar]
- Wu B, Crampton SP, Hughes CC. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity. 2007a;26(2):227–239. doi: 10.1016/j.immuni.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Shang Y, Sun S, Liang H, Liu R. Erythropoietin prevents PC12 cells from 1-methyl-4-phenylpyridinium ion-induced apoptosis via the Akt/GSK-3beta/caspase-3 mediated signaling pathway. Apoptosis. 2007b;12(8):1365–1375. doi: 10.1007/s10495-007-0065-9. [DOI] [PubMed] [Google Scholar]
- Xu B, Dong GH, Liu H, Wang YQ, Wu HW, Jing H. Recombinant human erythropoietin pretreatment attenuates myocardial infarct size: a possible mechanism involves heat shock Protein 70 and attenuation of nuclear factor-kappaB. Ann Clin Lab Sci. 2005;35(2):161–168. [PubMed] [Google Scholar]
- Xu HT, Wei Q, Liu Y, Yang LH, Dai SD, Han Y, Yu JH, Liu N, Wang EH. Overexpression of Axin Downregulates TCF-4 and Inhibits the Development of Lung Cancer. Ann Surg Oncol. 2007;14(11):3251–3259. doi: 10.1245/s10434-007-9555-9. [DOI] [PubMed] [Google Scholar]
- Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungsholmen project: a 6-year follow-up study. Neurology. 2004;63(7):1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274(21):14926–14935. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- Yano M, Hasegawa G, Ishii M, Yamasaki M, Fukui M, Nakamura N, Yoshikawa T. Short-term exposure of high glucose concentration induces generation of reactive oxygen species in endothelial cells: implication for the oxidative stress associated with postprandial hyperglycemia. Redox Rep. 2004;9(2):111–116. doi: 10.1179/135100004225004779. [DOI] [PubMed] [Google Scholar]
- Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol. 2004;274(2):233–244. doi: 10.1016/j.ydbio.2004.07.026. [DOI] [PubMed] [Google Scholar]
- You L, He B, Uematsu K, Xu Z, Mazieres J, Lee A, McCormick F, Jablons DM. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res. 2004;64(10):3474–3478. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- You Z, Saims D, Chen S, Zhang Z, Guttridge DC, Guan KL, MacDougald OA, Brown AM, Evan G, Kitajewski J, Wang CY. Wnt signaling promotes oncogenic transformation by inhibiting c-Myc-induced apoptosis. J Cell Biol. 2002;157(3):429–440. doi: 10.1083/jcb.200201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui R, Matsuura ET. Detection of deletions flanked by short direct repeats in mitochondrial DNA of aging Drosophila. Mutat Res. 2006;594(1–2):155–161. doi: 10.1016/j.mrfmmm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Zamora M, Manner J, Ruiz-Lozano P. Epicardium-derived progenitor cells require beta-catenin for coronary artery formation. Proc Natl Acad Sci U S A. 2007;104(46):18109–18114. doi: 10.1073/pnas.0702415104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Park TS, Gidday JM. Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol. 2007;292(6):H2573–2581. doi: 10.1152/ajpheart.01098.2006. [DOI] [PubMed] [Google Scholar]


