Abstract
Scrub typhus is responsible for a large proportion of undifferentiated fevers in south-east Asia. The cellular tropism and pathophysiology of the causative agent, Orientia tsutsugamushi, remain poorly understood. We measured endothelial and leucocyte activation by soluble cell adhesion molecule enzyme-linked immunosorbent assays in 242 Lao and Thai patients with scrub or murine typhus, leptospirosis, dengue, typhoid and uncomplicated falciparum malaria on admission to hospital. Soluble E-selectin (sE-selectin) levels were lowest in dengue, sL-selectin highest in scrub typhus with a high sE-selectin to sL-selectin ratio in leptospirosis patients. In scrub typhus patients elevated sL-selectin levels correlated with the duration of skin rash (P = 0·03) and the presence of eschar (P = 0·03), elevated white blood cell (WBC) count (P = 0·007), elevated lymphocyte (P = 0·007) and neutrophil counts (P = 0·015) and elevated levels of sE-selectin correlated with the duration of illness before admission (P = 0·03), the presence of lymphadenopathy (P = 0·033) and eschar (P = 0·03), elevated WBC (P = 0·005) and neutrophil counts (P = 0·0003). In comparison, soluble selectin levels in murine typhus patients correlated only with elevated WBC counts (P = 0·03 for sE-selectin and sL-selectin). Soluble intercellular adhesion molecule-1 and soluble vascular adhesion molecule-1 levels were not associated significantly with any clinical parameters in scrub or murine typhus patients. The data presented suggest mononuclear cell activation in scrub typhus. As adhesion molecules direct leucocyte migration and induce inflammatory and immune responses, this may represent O. tsutsugamushi tropism during early dissemination, or local immune activation within the eschar.
Keywords: endothelial activation, Laos, pathophysiology, rickettsia, soluble cell adhesion molecule, typhus
Introduction
Typhus-like illnesses are significant diagnostic challenges; patients with rickettsiosis, leptospirosis, dengue fever (DF), dengue haemorrhagic fever (DHF), typhoid and malaria can present with similar symptoms and signs. Recent studies suggest that rickettsioses account for 20–35% of undifferentiated fevers [1,2] in south-east Asian adults, with scrub typhus being the most important. Rickettsial agents are divided into three antigenic groups: spotted fever (prototype Rickettsia rickettsii), typhus (prototype R. prowazekii) and scrub typhus groups (Orientia tsutsugamushi). There is strong evidence that endothelial cells (EC) are the principal target for R. rickettsii and R. conorii. However, the cellular tropism of O. tsutsugamushi remains controversial, and the pathophysiology of scrub typhus is poorly understood [3].
During the initial inflammatory response to infections, early response cytokines [tumour necrosis factor-α, interleukin (IL)-1α and IL-6] up-regulate cellular adhesion molecules (CAMs) on the surface of host leucocytes and EC, which co-ordinate leucocyte transmigration across the endothelium [4]. The selectins mediate initial leucocyte contact with EC, capturing cells from the bloodstream, followed by characteristic rolling and firm tethering to the endothelium. This requires higher-affinity leucocyte integrins (LFA-1 and Mac-1) binding to members of the immunoglobulin (Ig) superfamily, intercellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1), which are expressed on activated EC, enabling subsequent leucocyte diapedesis [5].
Soluble forms of these molecules (sCAMs) are released into the plasma either after differential splicing of their mRNA and/or proteolytic cleavage of their membrane-bound counterparts. Serum levels of sCAMs have been used as surrogate markers of systemic endothelial and leucocyte activation in a wide variety of neoplastic, inflammatory and infectious diseases [6,7].
Previous studies in adult Thai patients have suggested that O. tsutsugamushi induces a type 1 immune response, associated with elevation of interferon-γ, IL-18 and IL-15 levels [8]. Plasmodium falciparum malaria, DF and some spotted fever rickettsiosis are associated with cytokine-induced systemic endothelial activation [9–11]. However, comparative in vivo data on the spectrum of these markers in Asian ‘typhus-like’ illnesses are not available. The sCAM ‘fingerprint’ might allow differentiation between aetiologies of clinically similar patient groups and provide evidence of cellular tropism in the early stages of infection. Based on post-mortem findings and in vitro experiments, O. tsutsugamushi can target the endothelium but in vivo data are lacking [3]. However, O. tsutsugamushi has also been described within peripheral blood mononuclear cells of patients with scrub typhus [12]. We therefore hypothesized that markers of endothelial activation are raised in scrub typhus, and that measurement of both EC and leucocyte activation markers may allow us to distinguish between generalized systemic inflammatory responses or endothelial tropism of O. tsutsugamushi.
We measured levels of circulating soluble E-selectin (sE-selectin), soluble VCAM-1 (sVCAM-1) and soluble ICAM-1 (sICAM-1) and endoglin, a constitutive endothelial marker, in patients with typhus-like illnesses, and also measured sL-selectin to detect mononuclear cell activation. We aimed to evaluate their value in differentiating between typhus-like diseases, and determine whether systemic endothelial and leucocyte activation were present in south-east Asian adults with scrub typhus.
Materials and methods
Patients
A total of 242 admission serum samples were collected: scrub typhus (n = 66), murine typhus (n = 62), leptospirosis (n = 24), DF (n = 17), DHF (n = 16), typhoid (n = 22) and uncomplicated P. falciparum malaria (n = 25), as defined by World Health Organization (WHO) criteria 2000 and negative control sera (n = 10) from the healthy blood donors (Table 1). All patients provided informed written consent prior to sample collection. The admission samples of scrub and murine typhus, DF, typhoid, malaria and 13 leptospirosis patients were collected at Mahosot Hospital, Vientiane, Lao PDR. Leptospirosis samples were also collected from 11 Thai patients in Udon Thani, NE Thailand (90 km/56 miles from Vientiane), and 10 negative control samples from healthy Thai blood donors were provided generously by the blood bank of Mahidol University, Hospital for Tropical Diseases, Bangkok.
Table 1.
Soluble cell adhesion molecule serum levels in clinical groups.
| Patients | sE-selectin | sL-selectin | sICAM-1 | sVCAM-1 | Endoglin |
|---|---|---|---|---|---|
| Controls (n = 10) | 56·9 (30·9) | 328·3 (65·8) | 246·9 (140·8) | 806·6 (477·7) | 4·4 (2·4) |
| Murine typhus (n = 62) | 60·8 (37·7) | 517·9 (104·5) | 473·8 (265·8) | 1347·9 (636·2) | 5·8 (1·3) |
| Scrub typhus (n = 66) | 79 (46·3) | 586·1 (120·6) | 458·1 (244·8) | 1326·4 (658) | 6·6 (1·6) |
| Leptospirosis (n = 24) | 92·9 (50·9) | 428·6 (126·6) | 500·9 (270·4) | 1340·2 (522·7) | 6·9 (2·8) |
| Dengue fever (n = 17) | 36·9 (8·5) | 514·4 (73·6) | 309·1 (181) | 1312 (575·5) | 6·3 (3·1) |
| Dengue haemorrhagic fever (n = 16) | 37·2 (15·9) | 472·3 (59·3) | 389·8 (224·7) | 1423·9 (605·2) | 7·2 (3·2) |
| Typhoid (n = 22) | 61·2 (36·3) | 572·8 (99·5) | 504·5 (291·5) | 1384 (748·3) | 7·1 (3·4) |
| Uncomplicated malaria (n = 25) | 68·2 (35·6) | 447·6 (123·3) | 500·9 (242·3) | 1252 (547·0) | 6·3 (2·9) |
Results are shown as the mean level (standard deviation) of sCAM level in serum in ng/ml. sICAM-1, soluble intercellular adhesion molecule; sVCAM, soluble vascular adhesion molecule.
The study was approved by the National Ethics Committee For Health Research, Ministry of Public Health, Lao PDR, the Thai Ministry of Public Health and the Oxford Tropical Research Ethics Committee.
Diagnosis and origin of specimens
Scrub typhus (pooled Karp, Kato, Gilliam antigens) and murine typhus (R. typhi Wilmington strain antigens) IgM and IgG antibodies were detected using the indirect micro immunofluorescence assay on Australian Rickettsial Reference Laboratory slides. A regionally determined positivity criterium of ≥ 1 : 400 IgM or a fourfold (or greater) rising titre was indicative of an active infection [13]. Leptospirosis was diagnosed by either microscopic agglutination test (of ≥ 1 : 400 or more, or a fourfold rise in titre between acute and convalescence samples) or/and culture-proven (WHO/FAO/OIE Collaborating Centre for Reference and Research on Leptospirosis, Brisbane, Australia). Dengue was diagnosed by IgM antibody capture enzyme-linked immunosorbent assay (ELISA) (cat. no. E-DEN01M; Panbio Pty Ltd, Brisbane, Australia), performed according to the manufacturer's instructions. Results were calculated as ‘Panbio units’ with results < 9·0, 9·0–11·0 and > 11·0 defined as negative, equivocal and positive respectively. Samples that initially returned an equivocal result were retested to confirm the result. DHF was diagnosed clinically according to the WHO criteria, 1997 [14]. Falciparum malaria was diagnosed by a minimum of two positive blood films and a rapid diagnostic test (Paracheck; Orchid Biosystems, Goa, India) and Salmonella enterica serovar Typhi infection was diagnosed by blood culture [15].
Assays for quantification of circulating sCAMs in serum
Peripheral venous blood was processed immediately and serum aliquots stored at −80°C until use. ELISA kits used in this study [human sL-selectin (cat. no. BBE 4B), sE-selectin (BBE 2B), sICAM-1 (BBE 1B), sVCAM-1 (DVC00) and sEndoglin/CD105 (DNDG00)] were commercial sandwich enzyme immunoassays (R&D Systems, Inc., Minneapolis, MN, USA) performed in accordance with the manufacturer's instructions. Optical density (OD) results were measured at absorbance 465 nm and converted to serum concentrations by interpolation of sample ODs with standard curves constructed from supplied quantitative standards. Patient samples were run in duplicate and selected samples assessed at five- or 10-fold dilutions to control for intra- and interplate variability.
Statistical analysis
Statistical calculations were performed with Stata/SE version 9 (College Station, TX, USA). Significance was calculated using analysis of variance or non-parametric tests (Kruskal–Wallis) where appropriate. Soluble CAMs were correlated to clinical parameters using Spearman's rho or Kruskal–Wallis tests. For the clinical subgroup analysis of scrub and murine typhus the following parameters were included: (a) severity [fever clearance time, fever days and total fever time expressed as area under the curve (minimum 37·5°C)]; (b) history (days of illness, fever and skin rash); (c) clinical examination (tympanic temperature, blood pressure, jaundice, lymphadenopathy, anaemia, pulse rate, hepato-splenomegaly, skin rash and the presence of an eschar); and (d) peripheral blood cell count [white blood cell (WBC) count, haematocrit, neutrophil, lymphocyte and eosinophil counts]. A P < 0·05 was used to designate associations as significant. Although this cut-off does not take into account the multiple comparisons made, we opted for this less conservative approach, as most of the associations examined in this study are regarded as hypothesis-generating rather than definitive.
Results
Comparison of sCAM levels between disease groups
Mean values for sCAM levels in all clinical groups are shown in Table 1. Levels of the inducible sCAMs and the constitutive endothelial marker sEndoglin showed no significant differences between clinical groups or controls.
As expected, sICAM-1 was increased in uncomplicated malaria, but levels of EC-specific sCAMs were not significantly different to controls [10]. DF and DHF patients showed moderate, but non-significant, increases of sICAM-1 and sVCAM-1, while sE-selectin levels were similar to controls [11]. Leptospirosis demonstrated the highest sICAM-1 and sE-selectin levels, with a significantly raised ratio of sE-selectin to sEndoglin when compared with the other disease groups (P < 0·017) except malaria, and the lowest sL-selectin levels.
In contrast, when compared with controls sL-selectin levels were raised significantly in scrub typhus, murine typhus and typhoid (P < 0·0001), DF (P < 0·001) and DHF (P < 0·03) (Fig. 1), whereas in leptospirosis and malaria they were not. Scrub typhus patients had the highest median sL-selectin values and were significantly higher than patients with murine typhus (P = 0·012), leptospirosis (P < 0·0001), DHF (P < 0·006), malaria (P < 0·0001) and controls (P < 0·0001). Levels of sE-selectins were significantly lower in DF and DHF patients than in scrub typhus (P < 0·004) and leptospirosis (P < 0·0005) patients (data not shown).
Fig. 1.
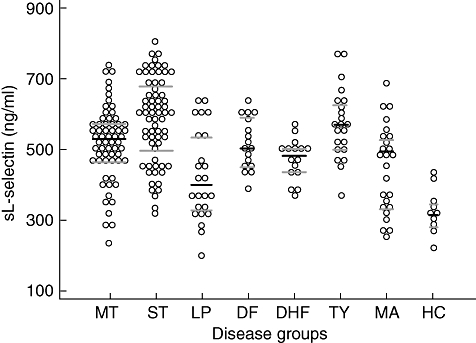
Dot plot diagram demonstrating 242 admission serum levels of circulating soluble L-selectin (sL-selectin) in the eight patient groups studied. Abbreviations used: murine typhus (MT), scrub typhus (ST), leptospirosis (LP), dengue fever (DF), dengue haemorrhagic fever (DHF), typhoid (TY), uncomplicated malaria (MA) and healthy controls (HC). Bars indicate median and inter-quartile range.
Correlation of sCAM levels with clinical findings in scrub and murine typhus
Elevated levels of sE-selectin in scrub typhus patients correlated with duration of illness, lymphadenopathy, escharformation, elevated WBC count and neutrophil counts (Table 2), whereas elevated sL-selectin levels were associated with skin rash, eschar and elevated WBC count. In comparison, levels of soluble selectins in murine typhus patients were associated only with elevated WBC counts. sICAM-1 and sVCAM-1 levels were not associated significantly with any clinical parameters in scrub or murine typhus patients.
Table 2.
Overview of clinical parameters with a significant association with either sE- or sL-selectins in patients with scrub or murine typhus on admission.
| Cellular adhesion molecule | Disease group | Clinical parameters | Probability of association |
|---|---|---|---|
| sE-selectin | ST | Neutrophil count | P = 0·0003 |
| ST | WBC count | P = 0·005 | |
| ST | Duration of illness | P = 0·03 | |
| ST | Presence of eschar | P = 0·03 | |
| ST | Lymphadenopathy | P = 0·033 | |
| MT | WBC count | P = 0·03 | |
| sL-selectin | ST | Duration of skin rash | P = 0·03 |
| ST | Presence of eschar | P = 0·03 | |
| ST | WBC count | P = 0·007 | |
| ST | Lymphocyte counts | P = 0·007 | |
| ST | Neutrophil counts | P = 0·015 | |
| MT | WBC count | P = 0·03 |
Soluble intercellular adhesion molecule-1 (sICAM) and soluble vascular adhesion molecule-1 (sVCAM-1) levels were not associated significantly with any clinical parameters in scrub typhus (ST) or murine typhus (MT) patients at the P < 0·05 level. WBC, white blood cell.
Discussion
This study investigated the patterns of sCAM release in Asian adults on admission to hospital, with emphasis on tropical rickettsial diseases. Different patterns of markers for endothelial and mononuclear cell activation were distinguished in infections with typhus-like illnesses, i.e. in sera from patients with uncomplicated malaria had a (non-significant) rise in all sCAMs, whereas sera from patients with leptospirosis demonstrated a pattern of high sE-selectin and low sL-selectin concentrations.
In scrub typhus elevation of sE-selectin with modest sICAM-1 and sVCAM-1 levels were accompanied by marked elevation of sL-selectin levels in admission samples. A similar but less pronounced pattern was seen in patients with murine typhus and typhoid infection, which are also intracellular infections. The finding of significantly raised sL-selectin levels in scrub typhus implies mononuclear cell activation, as sL-selectin release from peripheral blood leucocytes accompanies cellular activation and transmigration [5]. The high sL-selectin levels in typhoid patients probably reflects the well-established mononuclear cellular tropism of this agent as well as lymphocyte trafficking in the gastrointestinal tract [16].
However, leptospirosis showed an opposite pattern, with a high sE-selectin : sL-selectin ratio, implying significant endothelial, but not leucocyte, activation. This finding is in accordance with a recent report of a leptospiral protein capable of up-regulating the expression of EC adhesion molecules in vitro[17].
The pattern of sCAM release in O. tsutsugamushi-infected patients is consistent with intracellular tropism to mononuclear cells during the early dissemination phase, presumably because of invasion and consequent activation of these cells. Alternatively, it may reflect immune activation and localization in response to initial rickettsial replication within the eschar. Supporting this second explanation, comparison in scrub typhus of sCAMs with clinical and laboratory findings showed that sE-selectin values correlate with elevated neutrophil counts and sL-selectin with elevated lymphocyte counts, and both are associated weakly with the presence of an eschar. The mean concentration of serum endothelial-specific sCAMs, such as sE-selectin, in patients with murine typhus were similar to controls, but was significantly higher in scrub typhus than in both DF and DHF groups. This supports the importance of endothelial involvement in the early dissemination of O. tsutsugamushi. Soluble CAM patterns have been described previously in patients with Mediterranean spotted fever (R. conorii), an endothelium-tropic spotted fever group rickettsiosis of Europe, where elevated sE- and sL-selectins were observed early on admission, combined with a rise of sICAM and sVCAM levels later in the disease process [11]. We found little evidence that sICAM-1 and sVCAM-1 levels differ between the disease groups or with patients' clinical features, although the highest values were recorded in DHF.
The pathogenesis of the initial local host immune response at the inoculation site and the mode of early dissemination of the obligate intracellular O. tsutsugamushi is still poorly understood. These results imply that both EC and leucocyte activation occur early in the course of scrub typhus, releasing sE-selectin and L-selectin into the systemic circulation. This may reflect direct infection of both cell types by O. tsutsugamushi. Endothelial recruitment of neutrophils and lymphocytes from the eschar would encourage localized inflammation and contribute potentially to subsequent dissemination, by localized increased vascular permeability and direct haematogenous spread, by circulation of pre-apoptotic infected EC, or by infection of lymphocytes, which could recirculate into the lymphatic system. In addition, high levels of sL-selectin could also reflect the homing potential of lymphocytes to peripheral lymph nodes and/or immune modulation through inhibition of endothelial-leucocyte attachment [18].
Identifying the cellular sites of replication in the eschar, such as endothelium, leucocytes or intrinsic fibroblasts/dendritic cells, will be important in determining which of these potential routes the organism takes to disseminate from the initial inoculation site.
The comparison of sCAM levels in this large cohort of Lao/Thai patients with typhus-like illnesses showed significantly higher levels of sL-selectin in both scrub and murine typhus patients than in leptospirosis, DHF and malaria, suggesting early lymphocyte activation and recruitment. In addition, the raised sE-selectin is indicative of background endothelial activation. The pattern and levels of sCAM release differed between disease groups but are not specific enough to be of use in the acute differential diagnosis of ‘typhus-like’ illnesses. The information they give on cellular activation and tropism of rickettsial agents suggests a role for early leucocyte and endothelial activation in the immune response and subsequent dissemination of the bacteria.
Acknowledgments
Financial Support for this study came from the Swiss National Science Foundation (PBZHB-106270) and the Wellcome Trust of Great Britain, as part of the Wellcome Trust–Mahidol University–Oxford Tropical Medicine Research Programme and the Wellcome Trust–Mahosot Hospital–Oxford Tropical Medicine Research Collaboration. D. H. P. is a Wellcome Trust Clinical Training Fellow (grant no. 078990/Z/06/Z). We are very grateful to the staff of the Microbiology Laboratory and all the ward staff of Mahosot Hospital for their assistance. This work was presented in part at the 5th European Congress on Tropical Medicine and International Health, 24–28 May 2007, Amsterdam, the Netherlands.
References
- 1.Phongmany S, Rolain JM, Phetsouvanh R, et al. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–62. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suttinont C, Losuwanaluk K, Niwatayakul K, et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100:363–70. doi: 10.1179/136485906X112158. [DOI] [PubMed] [Google Scholar]
- 3.Moron CG, Popov VL, Feng HM, Wear D, Walker DH. Identification of the target cells of Orientia tsutsugamushi in human cases of scrub typhus. Mod Pathol. 2001;14:752–9. doi: 10.1038/modpathol.3880385. [DOI] [PubMed] [Google Scholar]
- 4.Lasky LA. Selectin–carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–39. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 5.Alon R, Chen S, Puri KD, Finger EB, Springer TA. The kinetics of 1-selectin tethers and the mechanics of selectin-mediated rolling. J Cell Biol. 1997;138:1169–80. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-Amaro R, Díaz-González F, Sánchez-Madrid F. Adhesion molecules in inflammatory diseases. Drugs. 1998;56:977–88. doi: 10.2165/00003495-199856060-00003. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Boelte KC, Lin PC. Endothelial cell adhesion molecules and cancer progression. Curr Med Chem. 2007;14:377–86. doi: 10.2174/092986707779941032. [DOI] [PubMed] [Google Scholar]
- 8.Chierakul W, de Fost M, Suputtamongkol Y, et al. Differential expression of interferon-gamma and interferon-gamma-inducing cytokines in Thai patients with scrub typhus or leptospirosis. Clin Immunol. 2004;113:140–4. doi: 10.1016/j.clim.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Khongphatthanayothin A, Phumaphuti P, Thongchaiprasit K, Poovorawan Y. Serum levels of sICAM-1 and sE-selectin in patients with dengue virus infection. Jpn J Infect Dis. 2006;59:186–8. [PubMed] [Google Scholar]
- 10.Turner GD, Ly VC, Nguyen TH, et al. Systemic endothelial activation occurs in both mild and severe malaria. Correlating dermal microvascular endothelial cell phenotype and soluble cell adhesion molecules with disease severity. Am J Pathol. 1998;152:1477–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Vitale G, Mansueto S, Gambino G, et al. Differential up-regulation of circulating soluble selectins and endothelial adhesion molecules in Sicilian patients with Boutonneuse fever. Clin Exp Immunol. 1999;117:304–8. doi: 10.1046/j.1365-2249.1999.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh DS, Myint KS, Kantipong P, Jongsakul K, Watt G. Orientia tsutsugamushi in peripheral white blood cells of patients with acute scrub typhus. Am J Trop Med Hyg. 2001;65:899–901. doi: 10.4269/ajtmh.2001.65.899. [DOI] [PubMed] [Google Scholar]
- 13.Coleman RE, Sangkasuwan V, Suwanabun N, et al. Comparative evaluation of selected diagnostic assays for the detection of IgG and IgM antibody to Orientia tsutsugamushi in Thailand. Am J Trop Med Hyg. 2002;67:497–503. doi: 10.4269/ajtmh.2002.67.497. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO) Dengue hemorrhagic fever: diagnosis, treatment, prevention and control. 2. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 15.Phetsouvanh R, Phongmany S, Soukaloun D, et al. Causes of community-acquired bacteremia and patterns of antimicrobial resistance in Vientiane, Laos. Am J Trop Med Hyg. 2006;75:978–85. [PMC free article] [PubMed] [Google Scholar]
- 16.Kantele A, Arvilommi H, Iikkanen K, et al. Unique characteristics of the intestinal immune system as an inductive site after antigen reencounter. J Infect Dis. 2005;191:312–17. doi: 10.1086/426943. [DOI] [PubMed] [Google Scholar]
- 17.Vieira ML, D'Atri LP, Schattner M, et al. A novel leptospiral protein increases ICAM-1 and E-selectin expression in human umbilical vein endothelial cells. FEMS Microbiol Lett. 2007;276:172–80. doi: 10.1111/j.1574-6968.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 18.Kishimoto TK, Jutila MA, Butcher EC. Identification of a human peripheral lymph node homing receptor: a rapidly down-regulated adhesion molecule. Proc Natl Acad Sci USA. 1990;87:2244–8. doi: 10.1073/pnas.87.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]


