Abstract
When oral tolerance was induced in either specific pathogen-free (SPF) or germ-free (GF) mice, ovalbumin (OVA) feeding before immunization induced oral tolerance successfully in SPF mice. On the other hand, OVA-specific immunoglobulin G1 (IgG1) and IgE titres in OVA-fed GF mice were comparable to those in phosphate-buffered saline-fed GF mice, thus demonstrating that oral tolerance could not be induced in GF mice. The frequencies of CD25+ CD4+/CD4+ cells in the mesenteric lymph node (MLN) and the absolute number of CD25+ CD4+ cells in the Peyer's patches and MLN of naive GF mice were significantly lower than those in naive SPF mice. In an in vitro assay, the CD25+ CD4+ cells from the naive SPF mice suppressed more effectively the proliferation of responder cells in a dose-dependent manner than those from the GF mice. In addition, the CD25+ CD4+ regulatory T (Treg) cells from the naive SPF mice produced higher amounts of interleukin (IL)-10 and transforming growth factor (TGF)-β than those from the GF mice. When anti-TGF-β neutralizing antibody, but not anti-IL-10 neutralizing antibody, was added to the in vitro proliferation assay, the suppressive effect of the CD25+ CD4+ Treg cells from the SPF mice was attenuated to the same level as that of the CD25+ CD4+ cells from the GF mice. In conclusion, the TGF-β-producing CD25+ CD4+ Treg cells from the MLN of SPF mice played a major role in oral tolerance induction. In addition, as the regulatory function of the CD25+ CD4+ cells from the naive GF mice was much lower than that of the CD25+ CD4+ Treg cells from the SPF mice, indigenous microbiota are thus considered to contribute to the induction and maintenance of CD25+ CD4+ Treg cells.
Keywords: germ-free mice, mesenteric lymph node, oral tolerance, regulatory T cell, TGF-β
Introduction
Oral tolerance is defined as an antigen-specific peripheral immune hyporesponsiveness following the mucosal, oral or nasal administration of an antigen [1–3]. A failure of oral tolerance induction leads to susceptibility to food and bacterial antigens in the gastrointestinal tract. The immune responses against food antigens and indigenous intestinal bacterial antigens lead to such diseases as food allergy and inflammatory bowel disease, and the number of patients suffering from these diseases is now increasing, especially in developed countries [4]. The purpose of this study was to investigate the pathogenesis of these diseases by examining the role of regulatory T (Treg) cells in oral tolerance induction.
Recently, the crucial role of antigen-specific CD4+ Treg cells in oral tolerance induction has been reported [2]. Treg cells are generated not only in the thymus, which may regulate the autoreactive T cells, but also in the peripheral immune system following systemic antigen administration. The induced CD25+ CD4+ Treg cells have been reported to contribute to the induction and maintenance of oral tolerance [5]. In fact, CD25+ CD4+ Treg cells have been reported to be generated in the mesenteric lymph nodes (MLN) in oral tolerance-induced ovalbumin–T cell receptor (OVA–TCR) transgenic mice [6], and antigen-specific CD25+ CD4+ Treg cell clones have been shown to be generated in oral tolerance-induced mice [7]. Recently, the oral tolerance-induced Treg cells were reported to include interleukin (IL)-10-secreting cells, transforming growth factor (TGF)-β-secreting cells and cells expressing a membrane-bound form of TGF-β with latency-associated peptide (LAP) [8–13]. In addition, it was revealed recently that regulatory CD25+ CD4+ T cells are unique in transcribing the forkhead box protein P3 (FoxP3) gene, thus indicating FoxP3 protein to be a reliable marker of Treg cells [14]. Therefore, recent studies have made it possible to recognize the Treg cells by their secretion of IL-10 and TGF-β and the expression levels of LAP and FoxP3 protein.
On the other hand, germ-free (GF) mice, which lack indigenous microbiota, have been reported to be resistant to oral tolerance induction in comparison with specific pathogen-free (SPF) mice [15]. It has been reported recently that regulatory function was impaired in the peripheral lymph nodes of untreated GF mice [16]. As mentioned above, Treg play a role in the induction and maintenance of oral tolerance [5–7], and it is plausible that the impaired function of Treg in GF mice is responsible for impaired oral tolerance induction in these mice. We have demonstrated herein that oral tolerance was induced only in SPF mice, but not GF mice. Therefore we evaluated the Treg function in oral tolerance induction in SPF mice and GF mice. Furthermore, we also evaluated the role of indigenous microbiota in oral tolerance induction using GF mice.
Materials and methods
Mice
Specific pathogen-free and GF Balb/c mice were purchased originally from Japan Clea Inc. (Tokyo, Japan), and were bred in our facility. The SPF mice were maintained under SPF conditions. The GF mice were housed in Trexler-Type flexible film plastic isolators with sterile food and water during the experiments. Surveillance for bacterial contamination was performed by a periodic bacteriological examination of faeces throughout the experiments. All experimental procedures were performed according to the guidelines of the Animal Care Committee of Tokai University.
Oral tolerance induction and immunization
To induce oral tolerance, 6–8-week-old SPF or GF Balb/c mice were given intragastrically either 5 mg/day OVA or phosphate-buffered saline (PBS) as control for 4 consecutive days from day −6 to day −3. To induce a systemic antibody production in response to OVA antigen, these mice were injected with 1 μg of OVA including 0·1 mg of aluminium hydroxide (alum) at day 0, 14, 28 and 42, and then blood samples to measure the OVA-specific antibody titre in the serum were collected on day 49 as described previously [17].
Measurement of OVA-specific antibody titre in serum
The OVA-specific immunoglobulin G1 (IgG1) titre in serum was measured by an enzyme-linked immunosorbent assay (ELISA). The OVA-specific IgE titre was determined using a fluorescence ELISA as described previously [15]. To make a pool of standard serum, 6–8-week-old SPF BALB/c mice were injected intraperitoneally (i.p.) three times with 10 μg of OVA plus 2 mg of alum at 0, 3 and 6 weeks; the mice were killed 7 days after the last injection to collect the serum and the pooled serum was used as the standard serum, which was assigned arbitrarily a value of 100 ELISA units/ml.
Cytokine production and proliferation in MLN and spleen cells
Single cell suspensions were prepared from the MLN and the spleens of the SPF and GF mice. These cells were plated at 2·5 × 105 cells/well in 96-well U-bottomed plates and were stimulated with 1 μg/ml anti-CD3 antibody (2C11) (BD Pharmingen, San Diego, CA, USA) in RPMI-1640 containing 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin and 10 mM Hepes (Sigma Aldrich Inc., St Louis, MO, USA). The supernatants, used to measure IL-2 production by these cells, were collected at 48 h after starting the culture, and the amount of IL-2 in the supernatants was measured using a mouse IL-2 ELISA development kit according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). To examine the proliferation of the cells at 48 h in culture, 1 μCi of [3H]-thymidine was added to each well and the cells were harvested 18 h later. The proliferation was detected using a β-counter.
Flow cytometry analysis
Flow cytometry analyses of cells from the Peyer's patches, MLN and spleen were performed using a fluorescence activated cell sorter (FACScan) with a Cellquest software program. The cells were stained in PBS with 2% bovine serum albumin (BSA), and then fixed with 1% paraformaldehyde. The antibodies used in this study were anti-CD16/CD32 antibody (2·4G2) as an Fc-blocker, fluorescein isothiocyanate-conjugated anti-CD4 antibody (L3T4), phycoerythrin (PE)-conjugated anti-CD25 antibody (PC61), PE-conjugated anti-CD152 antibody (UC10–4F10-11), allophycocianin (APC)-conjugated anti-CD25 antibody (PC61), APC-labelled streptavidin (BD Pharmingen), biotinylated anti-human LAP antibody (BAF246, lot no. XO02; R&D Systems) and a FoxP3 protein staining kit (eBioscience, San Diego, CA, USA). To stain the intracellular FoxP3 protein or CTLA-4 followed by surface molecule staining, the cell-fixation/cell-permeabilization kit included in the FoxP3 staining kit was used according to the manufacturer's protocol.
Cell purification and culture system
The CD25+ CD4+ or CD25- CD4+ cells were purified from the MLN using a CD25+ CD4+ Treg cell isolation kit (Miltenyi Biotec, Auburn, CA, USA), utilizing an immunomagnetic cell sorting (MACS) system in accordance with the manufacturer's instructions. To prepare the APC-depleted T cells, the spleen cells from naive SPF mice were stained with magnet-conjugated anti-CD90 antibody (Miltenyi Biotec), and the APCs were then isolated negatively using a MACS column. The preliminary experiments revealed that the magnetically retained cells were shown to be > 90% CD25+ CD4+ cells, and the flow-through cells were shown to be > 95% CD25- CD4+ cells and APCs. To examine the CD25+ CD4+ cells' suppressive function in co-cultures with responder cells (CD25- CD4+cells), titrated CD25+ CD4+ cells were plated with 2 × 105 responder cells and APCs and stimulated with 1 μg/ml anti-CD3 antibody in 96-well U-bottomed plates. The co-cultured cells were maintained for 48 h, and 1 μCi [3H]-thymidine was added to the culture at 18 h before harvesting. In some experiments, 100 μg/ml of the neutralizing antibodies anti-IL-10 antibody (JES052A5) or anti-TGF-β antibody (1D11), purchased from R&D Systems, was added to the culture system. For the cytokine assays, 5 × 105 CD25+ CD4+ cells were cultured with plate-bound 1 μg/ml anti-CD3 antibody in AIM-V medium (Invitrogen, Carlsbad, CA, USA), which is a serum-free medium, in 96-well U-bottomed plates. The culture supernatants were collected at 48 h for interferon (IFN)-γ and IL-10 and at 72 h for TGF-β and the amount of cytokine in the supernatants was determined using mouse IFN-γ and IL-10 ELISA development kits (R&D Systems) and a mouse/rat/porcine TGF-β immunoassay kit (R&D Systems), according to the manufacturer's instructions.
Statistical analysis
Statistical significance was calculated using the unpaired Student's t-test assuming unequal variance, and a P-value < 0·05 was considered to indicate significant difference.
Results
Failure of oral tolerance induction in GF mice
The SPF and GF mice were administered 5 mg/day OVA or PBS orally as a control for 4 consecutive days before i.p. challenge with 1 μg OVA plus 2 mg of alum on every other week for a total of four times. The serum was collected from the mice 1 week after the last challenge, and the OVA-specific Ig titres were measured. As shown in Fig. 1, the OVA-specific IgG1 and IgE titres in the serum in the SPF mice fed OVA before the immunization were significantly lower than those of the GF mice fed OVA. Moreover, in the SPF mice, both OVA-specific IgG1 and IgE in the OVA-fed mouse group were significantly reduced in comparison with those of the PBS-fed mouse group, thus suggesting that OVA feeding before immunization could induce oral tolerance in SPF mice. On the other hand, in the GF mice we could not find any significant difference in the production of OVA-specific IgG1 or IgE between the OVA-fed mouse group and the PBS-fed mouse group. The OVA-specific IgG2a titres in the serum of the tolerance-induced SPF mice were comparable to those of the GF mice (data not shown). These results all correlated closely with those of previous reports [15,17], and demonstrated that oral tolerance could be induced in SPF mice, but not in GF mice.
Fig. 1.
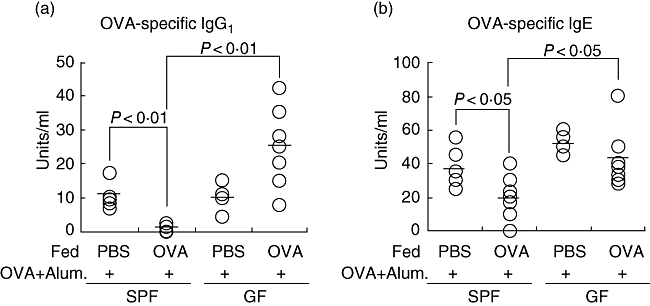
Failure of oral tolerance induction in germ-free mice. For oral tolerance induction, mice were fed 5 mg/day ovalbumin (OVA) (n = 7) or phosphate-buffered saline (n = 5) for consecutive days and were then immunized with OVA in alum at 3 days after the last feeding and every 2 weeks thereafter. Blood samples to measure antibodies in the serum were collected 1 week after the last immunization and were subjected to enzyme-linked immunosorbent assays. (a) OVA-specific immunoglobulin G1 (IgG1) in serum; (b) OVA-specific IgE in serum. Similar results were obtained in two independent experiments. SPF, specific pathogen-free.
The frequency of CD25+ CD4+ cells decreased in the MLN of GF mice
We next examined whether there were any differences in the cell populations of CD25+ CD4+ regulatory cells, which play important roles in the induction of oral tolerance, between the organs from naive SPF mice and naive GF mice. As shown in Table 1, the frequency of CD4+ cells in the GF mice decreased significantly in both the Peyer's patches and the MLN. Although the frequency of CD25+ CD4+/CD4+ cells in the Peyer's patches of the GF mice was comparable to that in the SPF mice (GF 7·1 ± 0·5 versus SPF 6·2 ± 0·9), that in the MLN of the GF mice was significantly lower than that in the SPF mice (GF 6·7 ± 0·1 versus SPF 8·6 ± 0·4, P < 0·05). We also calculated the absolute number of CD25+ CD4+ cells in each organ, because the organ size differed significantly between the SPF and GF mice. Interestingly, the absolute numbers of CD25+ CD4+ cells in the MLN, as well as in the Peyer's patches, decreased significantly in the GF mice in comparison with those in the SPF mice (Table 1). Thus, not only the frequency but also the total number of CD25+ CD4+ cells in the GF mice was significantly lower than those in the SPF mice. The frequencies and the absolute numbers of CD25+ CD4+ cells were not significantly different between the spleens of the SPF and GF mice.
Table 1.
Percentages and absolute cell number of the CD4+ cell population in Peyer's patches, mesenteric lymph nodes and spleen in naive mice.
| Peyer's patch | Mesenteric LN | Spleen | ||||
|---|---|---|---|---|---|---|
| SPF | GF | SPF | GF | SPF | GF | |
| CD4+/lymphocyte | 20·4 ± 1·2 | 13·1 ± 1·9** | 53·2 ± 2·0 | 49·2 ± 0·9** | 30·0 ± 3·6 | 27·5 ± 3·5 |
| CD4+ CD25+/CD4+ | 6·2 ± 0·9 | 7·1 ± 0·5 | 8·6 ± 0·4 | 6·7 ± 0·1** | 6·4 ± 0·5 | 6·1 ± 0·6 |
| CD4+ CD25+ cell number (×106) | 0·4 ± 0·04 | 0·2 ± 0·01** | 5·7 ± 1·0 | 3·0 ± 0·4** | 19 ± 2·2 | 17 ± 3·5 |
The mean and standard deviation from six mice/group is shown.
P < 0·01 in comparison with specific pathogen-free (SPF) mice by Student's t-test. GF, germ-free.
Next, the expression level of FoxP3 in the CD25+ CD4+ population was examined by FACS analysis. The results revealed that FoxP3 protein was observed in more than 95% of the CD25+ CD4+ cells in both the naive SPF and GF mice (Fig. 2).
Fig. 2.
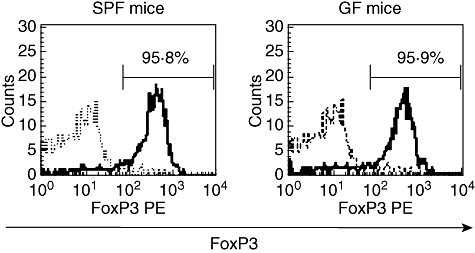
The expression of forkhead box P3 (FoxP3) in CD25+ CD4+ cells from specific pathogen-free (SPF) or germ-free (GF) mice. Single-cell suspensions were prepared from the mesenteric lymph node in naïve SPF and GF mice for fluorescence activated cell sorter analysis and were stained with fluorescein isothiocyanate–anti-CD4 antibody, phycoerythrin–anti-FoxP3 antibody and allophycocianin– anti-CD25 antibody. Intracellular FoxP3 expression in cells gated on the CD4+ CD25+ cells is presented. More than 95% of CD4+ CD25+ cells in both the SPF mice and GF mice groups expressed intracellular FoxP3. The results are from a representative experiment from three independent experiments.
Interleukin-2 production and proliferation were impaired in the whole MLN cells from GF mice
Fluorescence activated cell sorter analysis demonstrated the frequency and absolute number of CD25+ CD4+/CD4+ cells to differ significantly between the naive SPF and GF mice in the MLN, but not in the spleen. To determine the function of this population, the IL-2 production and proliferation of whole MLN cells were examined. When these cells were stimulated in vitro with anti-CD3 antibody, the IL-2 production by the MLN cells in the GF mice was significantly higher than that in the SPF mice (Fig. 3a, left panel). However, no such difference was observed in the spleen cells (Fig. 3a, right panel). Correspondingly, as shown in Fig. 3b, the proliferation of MLN cells in the GF mice also increased significantly compared with that of the SPF mice. However, such a difference could not be observed between the spleen cells from the SPF and GF mice (Fig. 3a and b, right panels). These results suggest that CD25+ CD4+ cells were functionally active in the MLN from the naive SPF mice, and that the population was functionally impaired in the naive GF mice.
Fig. 3.
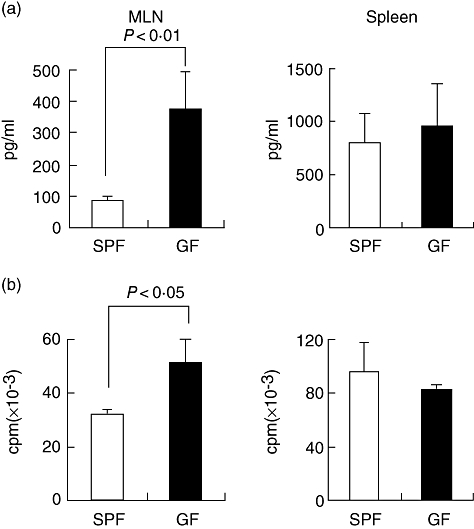
Interleukin (IL)-2 production and proliferation of mesenteric lymph node (MLN) and spleen cells in specific pathogen-free (SPF) and germ-free (GF) mice. The MLN or spleen was removed from naive SPF and GF mice and single-cell suspensions were prepared. A total of 250 000 cells were cultured with 1 μg/ml anti-CD3 antibody for 48 h and the supernatants were then collected to measure the IL-2 production. For the proliferation assays, 1 μCi [3H]-thymidine was added to the culture during the last 18 h of culture. (a) IL-2 production; (b) proliferation assay. Five mice were used in each group. The mean and standard deviation are shown in the figures. These results are representative of two independent experiments.
CD25+ CD4+ cells in GF mice had impaired suppressive functions compared with those in SPF mice
To confirm that the CD25+ CD4+ population was responsible for the difference in the in vitro suppressive function noted in whole MLN cells from the naive SPF and GF mice, purified CD25+ CD4+ cells (regulator cells) from the MLN were co-cultured with CD25- CD4+ cells (responder cells) and APCs and treated with soluble anti-CD3 antibody (1 μg/ml). As shown in Fig. 4, the CD25+ CD4+ cells from both the SPF mice and GF mice were anergic to stimulation by the anti-CD3 antibody (ratio of 0:1 in Fig. 4a and b). When these CD25+ CD4+ cells were cultured with responder cells from naive SPF mice, the SPF CD25+ CD4+ cells suppressed the responder cell proliferation in a dose-dependent fashion (Fig. 4a, open columns). On the other hand, the suppressive function of the GF CD25+ CD4+ cells was significantly weaker in comparison with that of the SPF CD25+ CD4+ cells, and the GF CD25+ CD4+ cells at ratios of 1 : 0·25 and 1 : 0·125 (CD25- CD4+ : CD25+ CD4+) were no longer able to suppress the responder cell proliferation (Fig. 4a, closed columns). Similar results were obtained in the experiments using GF CD25- CD4+ cells as responder cells (Fig. 4b). The SPF CD25+ CD4+ cells suppressed the proliferation of the GF responder cells more effectively (Fig. 4b, open columns) than the GF CD25+ CD4+ cells (Fig. 4b, closed columns). Interestingly, the GF responder cells were suppressed more easily by the SPF CD25+ CD4+ cells than the SPF responder cells (Fig. 4b, open columns versusFig. 4a, open columns, P < 0·05 for 1 : 0·125–1 : 1). These data suggest that the CD25+ CD4+ cells from naive GF mice demonstrate an impaired suppressive function in comparison with those from SPF mice.
Fig. 4.
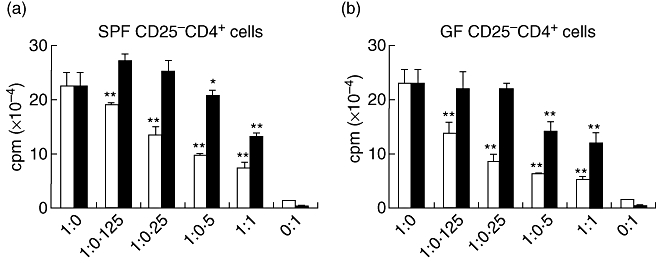
Suppressive function of CD25+ CD4+ cells obtained from germ-free (GF) mice is impaired compared with that of CD25+ CD4+ cells obtained from specific pathogen-free (SPF) mice. Either CD25+ CD4+ or CD25– CD4+ cells were purified from the mesenteric lymph node in naive SPF or GF mice and antigen presenting cell (APC)-depleted T cells were isolated from the spleens of SPF mice, as described in the Materials and methods. The co-cultured cells were maintained for 48 h and 1 μCi [3H]-thymidine was added to the culture 18 h before harvesting. (a) The titrated CD25+ CD4+ cells were co-cultured with 2·5 × 105 CD25– CD4+ cells as responder cells, which were obtained from SPF mice in the presence of APC and anti-CD3 antibody. (b) The titrated CD25+ CD4+ cells were co-cultured with 2·5 × 105 CD25– CD4+ cells derived from GF mice in the presence of APC and anti-CD3 antibody. □: CD25+CD4+ derived from SPF mice; ▪: CD25+ CD4+ derived from GF mice. The ratio of CD25– CD4+ : CD25+ CD4+ is indicated. The mean and standard deviation are shown in the figures and are representative of three experiments. *P < 0·05, **P < 0·01 compared with responder cell proliferation.
Specific pathogen-free CD25+ CD4+ cells produce higher levels of IL-10 and TGF-β than GF CD25+ CD4+ cells
To characterize further the CD25+ CD4+ cells in naive SPF or GF mice, purified CD25+ CD4+ cells from the MLN were stimulated with plate-bound 1 μg/ml anti-CD3 antibody and the amounts of the cytokines in the serum-free AIM-V medium were measured by ELISA, as described in Materials and methods. As shown in Fig. 5, the SPF CD25+ CD4+ cells produced larger amounts of the regulatory cytokines IL-10 and TGF-β than the GF CD25+ CD4+ cells (Fig. 5, left and middle panels). Interestingly, the level of TGF-β produced by the GF CD25+ CD4+ cells was under the limit of detection (15 pg/ml). On the other hand, no difference was noted in IFN-γ production between the SPF and GF CD25+ CD4+ cells. In addition to TGF-β secretion, we also analysed a membrane-bound form of TGF-β with LAP by FACS. The expression level of membrane-bound surface TGF-β on the SPF CD25+ CD4+ cells in the MLN was significantly higher than that in the GF CD25+ CD4+ cells (SPF 6·8 ± 1·7 versus GF 4·5 ± 0·6, P < 0·05), whereas the expression level of intracellular CTLA-4 in the SPF CD25+ CD4+ cells was comparable to that in the GF CD25+ CD4+ cells (data not shown).
Fig. 5.
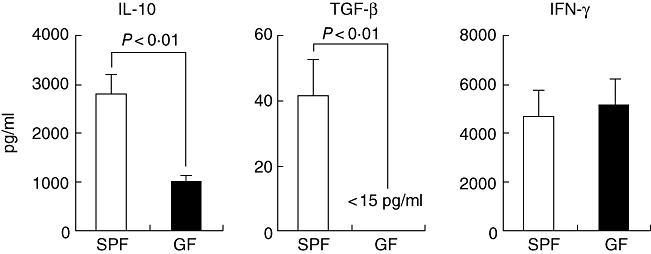
Regulatory cytokine production by CD25+ CD4+ cells derived from specific pathogen-free (SPF) or germ-free (GF) mice. CD25+ CD4+ cells were purified from the mesenteric lymph node in naive SPF and GF mice and 5 × 105 CD4+ CD25+ cells were cultured with plate-bound 1 μg/ml anti-CD3 antibody in 96-well U-bottomed plates. The culture supernatants were collected at 48 h for interleukin-10 and interferon-γ, at 72 h for transforming growth factor-β. The amounts of cytokines in the cultures were measured by enzyme-linked immunosorbent assay. The mean and standard deviation are shown. Similar results were obtained in three independent studies. IL, interleukin; IFN, interferon; TGF, transforming growth factor.
Suppressive function level of SPF CD25+ CD4+ cells was reduced to the same level of that of GF CD25+ CD4+ cells by TGF-β neutralization in vitro
As the SPF CD25+ CD4+ cells produced higher amounts of such regulatory cytokines as IL-10 and TGF-β than did the GF CD25+ CD4+ cells (Fig. 5), we next examined which cytokine mediated the suppressive function of the SPF CD25+ CD4+ cells. The CD25+ CD4+ cells were co-cultured with responder cells and APCs and stimulated with anti-CD3 antibody in the presence of neutralizing anti-IL-10 antibody and/or anti-TGF-β antibody. As shown in Fig. 6, the anti-IL-10 antibody and anti-TGF-β antibody had no effect on the responder cell proliferation. In the co-culture systems, the SPF CD25+ CD4+ cells had more suppressive function than the GF CD25+ CD4+ cells, as shown above. When anti-IL-10 antibody was added to this co-culture system, it had no effect on the proliferation of responder cells. In contrast, neutralizing anti-TGF-β antibody reduced the suppressive function of the SPF CD25+ CD4+ cells to the same level as that of the GF CD25+ CD4+ cells. No synergistic or additive effects were noted for the combination of anti-IL-10 antibody and anti-TGF-β antibody. These results suggest that TGF-β plays an important role in the acquisition of the full suppressive function by CD25+ CD4+ cells.
Fig. 6.
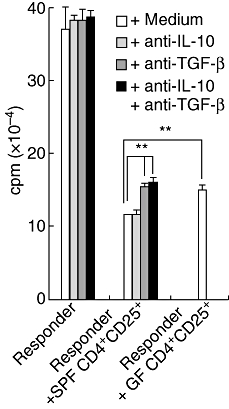
Neutralization of transforming growth factor (TGF)-β reversed suppressive function of specific pathogen-free (SPF) CD25+ CD4+ cells. A total of 250 000 CD25+ CD4+ cells obtained from the mesenteric lymph node (MLN) of naive SPF and germ-free mice were co-cultured with 2 × 105 responder cells purified from the MLN of naive SPF mice and antigen presenting cells and then stimulated with 1 μg/ml anti-CD3 antibody. To neutralize interleukin (IL)-10 and/or TGF-β, 100 μg/ml anti-IL-10 antibody and/or anti-TGF-β antibody was added to the cultures. The co-cultured cells were incubated for 48 h and 1 μCi [3H]-thymidine was added to the culture 18 h before harvest. **P < 0·01.
Discussion
It has been reported previously that oral tolerance could not be induced in GF mice [15]. We have investigated herein the reason why oral tolerance cannot be induced in GF mice, which provided an ideal system for determining the organ(s) and cells responsible for oral tolerance induction. In this study, we demonstrated that TGF-β-producing CD25+ CD4+ Treg cells in the MLN played a crucial role in oral tolerance induction, and that the indigenous microbiota augmented the number and suppressive function of the cells in the MLN.
It has been demonstrated unequivocally that several organs, including Peyer's patches, MLN and the spleen, are the inductive sites of oral tolerance. Among them, the Peyer's patches and the MLN have been reported to be the main organs for oral tolerance induction. Antigen-specific suppressive cells have been observed previously in the Peyer's patches in both low- and high-dose tolerance [7,18]. In addition, the Peyer's patches have been identified as a major site for the differentiation of IL-10-producing Treg cells [19]. However, other reports have demonstrated that oral tolerance is fully inducible even after selective elimination of Peyer's patches [20–22]. Worbs et al. reported recently that oral tolerance was impeded in mesenteric lymphadenectomized mice and thus concluded that the MLN were essential for oral tolerance induction [23]. Our results in this study also support their conclusion. The discrepancy among these studies may have been caused by the different experimental conditions used, because oral tolerance involves redundant mechanisms, including clonal anergy, clonal deletion and/or suppression by Treg. In addition, the localization of the Treg cells seemed to be different even in the same protocol. In our study Treg was noted in the MLN, but not in other such lymphoid organs as the spleen. It has recently been reported that the absence of gut flora did not alter the generation, proliferation or maintenance of Treg cells [24]. In their proliferation assay the pooled cells from various lymphoid organs were used as Treg cells, whereas Treg from the MLN were used in our study. The discrepancy in the organ of the Treg source may explain the adverse conclusion.
Although it has not been defined clearly [25–27] and it remains controversial whether cell–cell contact is required to demonstrate the suppressive function [28–31], the main suppressive mechanism may be mediated by IL-10 and/or TGF-β secretion. In the present study, the CD25+ CD4+ Treg cells from the SPF mice produced both IL-10 and TGF-β upon in vitro stimulation with anti-CD3 antibody. The neutralization of TGF-β produced by the SPF CD25+ CD4+ cells reduced their suppressive activity to the same level as that of the GF CD25+ CD4+ cells, suggesting that TGF-β plays a key role as a suppressive cytokine for the CD25+ CD4+ cells Treg functions. The TGF-β-producing Treg cells [T helper 3 (Th3) cells] play a major role both in inducing mucosal tolerance and preventing autoimmune diseases [1,3,9,32,33]. TGF-β also participates in immune homeostasis by inhibiting the development of both Th1 and Th2 immunity, and it has been reported that normal CD25+ CD4+ Treg cells could suppress the polyclonal in vitro activation of T cells by inhibiting IL-2 production [34,35]. Indeed, we observed that the MLN cells from the SPF mice suppressed the production of IL-2 and T cell proliferation in comparison with those from GF mice. These results also suggest that the MLN cells in the GF mice were incomplete for maturation, including the recruitment or expansion of functional regulatory CD25+ CD4+ cells. Therefore, the indigenous microbiota may be required for the maturation and recruitment of functional CD25+ CD4+ Treg cells.
It has been shown that the FoxP3 expression in mouse CD4 cells is sufficient to mark these cells as Treg cells, and that the FoxP3-expresssing CD4 T cells play a role in peripheral tolerance [14]. In the present study, to our surprise, almost all CD25+ CD4+ MLN cells expressed FoxP3, even in the CD25+ CD4+ MLN cells from the GF mice with impaired suppressive function. It has also been reported recently that membrane-bound TGF-β with LAP on CD4+ T cells is important in mucosal tolerance induction [10–13,36], and that the CD4 T cells expressing TGF-β with LAP on their surface can contribute to the development of tolerance by cell–cell contact and/or by secreting soluble TGF-β. In the present study, the expression level of membrane-bound TGF-β with LAP on GF CD25+ CD4+ cells correlated with the production of TGF-β and the suppressive function. Although the TGF-β produced by CD25+ CD4+ Treg cells possesses the suppressor activity, particularly the ability to suppress inflammation [37], the most controversial aspect of FoxP3 gene regulation is the role of TGF-β[14], On the other hand, the induction of regulatory activity has been reported to correlate with an increased expression of FoxP3 [38], and that only FoxP3-expressing T cells possess a suppressive activity [39]. Based on the findings of both these reports and the results of our study, although FoxP3 might be a marker of Treg its suppressive activity is considered to correlate with the amount of production of such suppressive cytokines as TGF-β.
As for cytokine production, a manuscript contrary to our results and conclusions has been published recently [40]. In that study, they examined T cell oral tolerance using the transfer of T cells having an OVA-specific TCR (OVA–TCR Tg), and then concluded that T cell oral tolerance was intact in GF mice. In our study, although the IL-10 production was suppressed in the control GF mice in comparison with that in the SPF mice, IFN-γ production in the GF mice was comparable to that in the SPF mice (Fig. 5). In contrast, Walton et al. observed that not only IL-10 but also IFN-γ production in the OVA–TCR Tg T cells were suppressed in the GF recipient mice in comparison with those in the SPF recipient mice, while also describing that the T cell recall responses could be measured by IFN-γ secretion. It is therefore plausible that not only the Treg function but also the T cell recall responses were suppressed in their protocol to induce T cell oral tolerance. The counterbalance of these two effects might result in the intact oral tolerance in the GF mice in their protocol.
Östman et al. have demonstrated recently that the CD25+ CD4+ cells from the lymph nodes of GF mice were significantly less effective than those from conventional mice in suppressing the proliferation of CD25- CD4+ effector cells in vitro[16]. When they used lymph node cells from untreated GF and conventional NMRI mice (H-2q), similar results were obtained. It was also observed that the GF responder cells were suppressed more easily by the SPF CD25+ CD4+ cells than the SPF responder cells, as shown in Fig. 4 of this study. As mentioned in their report, GF mice may have a higher ratio of naive T cells in comparison with either SPF or conventional mice, which have a higher proportion of memory-type T cells. As it is possible that naive cells are suppressed more easily than memory cells, the GF responder cells may therefore be suppressed more easily than the SPF responder cells. Although the strains of mice and experimental protocols were different, this may also have been true in our study.
The last question regards how the microbiota controls the induction and maintenance of Treg. It has been reported that bacterial antigens can directly modify the host immune system through Toll-like receptors (TLRs) and other microbial pattern recognition molecules [41]. IL-2 has been reported to play a crucial role in the expansion and survival of regulatory CD25+ CD4+ cells [42]. In addition, IL-2-deficient and IL-2 receptor-deficient mice suffer lethal autoimmune diseases [43,44]. It is plausible that constant IL-2 production by T cells activated by bacterial antigens in mucosal sites is required to generate and to maintain Treg cells peripherally. Wannemuhler et al. have reported that the failure of oral tolerance induction in Balb/c GF mice could be restored by oral administration of lipopolysaccharide (LPS), even when GF mice are given LPS orally at adult ages [45].
Dendritic cells (DC) play an important role in controlling oral tolerance, tolerance towards commensal microbiota and immunity against pathogens. DC reportedly express tight junction protein and penetrate gut epithelial monolayers to recognize bacterial product through TLR [46,47], and DC may then induce T cell unresponsiveness by stimulating naive T cell differentiation into Treg[48]. In GF mice, the lack of the signals through TLR on the DC may result in disturbance of induction and maintenance of Treg. In addition, it has been reported recently that TLRs expressed not only on DC but also on Treg themselves. Regulatory CD25+ CD4+ cells have also been reported to express TLR-4, which recognizes LPS, heat shock protein 60 and other factors derived from bacteria and that this signalling can activate CD25+ CD4+ cells [49,50]. Furthermore, TLR-4 mutant/knock-out mice are highly susceptible to the development of food allergies, which is correlated with high levels of Th2 cytokines such as IL-4 and IL-13 [51]. The regulatory CD25+ CD4+ cells also express TLR-2, which recognizes lipoteichoic acid, peptidoglycan and other bacterial components. In addition, in TLR-2-deficient mice, the number of CD25+ CD4+ cells was decreased significantly in comparison with that in wild-type mice, and the exogenous administration of TLR-2 ligands increased the CD25+ CD4+ cells [52]. The bacterial stimulation via TLR, not only on the DC but also on Treg, may therefore also be involved in the generation and expansion of CD25+ CD4+ cells.
In conclusion, this work provides evidence that the microbiota play an important role in generating fully functional CD25+ CD4+ Treg cells. In this study, oral tolerance depended on TGF-β-producing Treg in the MLN. In developed countries, the number of patients with allergies, autoimmune diseases and inflammatory bowel diseases is increasing. Approaches using GF mice may be a useful tool for studies trying to elucidate how to prevent these diseases through the induction of oral tolerance. An interesting subject for future research would be the generation and expansion of Treg using intestinal microbiota.
Acknowledgments
We thank Brian Quinn for his careful proofreading. This work was supported by a Grant-in-Aid for Young Scientists (B) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (to H. I., 60433918).
References
- 1.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol Rev. 2001;182:207–14. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsuji NM. Antigen-specific CD4(+) regulatory T cells in the intestine. Inflamm Allergy Drug Targets. 2006;5:191–201. doi: 10.2174/187152806778256043. [DOI] [PubMed] [Google Scholar]
- 3.Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5:179–90. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 4.Strachan DP. Family size, infection and atopy: the first decade of the ‘hygiene hypothesis’. Thorax. 2000;55(Suppl. 1):S2–10. doi: 10.1136/thorax.55.suppl_1.s2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung Y, Lee SH, Kim DH, Kang CY. Complementary role of CD4+CD25+ regulatory T cells and TGF-beta in oral tolerance. J Leukoc Biol. 2005;77:906–13. doi: 10.1189/jlb.1004599. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+) CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–53. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 7.Tsuji NM, Mizumachi K, Kurisaki J. Antigen-specific, CD4+CD25+ regulatory T cell clones induced in Peyer's patches. Int Immunol. 2003;15:525–34. doi: 10.1093/intimm/dxg051. [DOI] [PubMed] [Google Scholar]
- 8.Groux H, O'Garra A, Bigler M, et al. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–42. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–40. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+) CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629–44. doi: 10.1084/jem.194.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oida T, Zhang X, Goto M, et al. CD4+CD25– T cells that express latency-associated peptide on the surface suppress CD4+CD45RBhigh-induced colitis by a TGF-beta-dependent mechanism. J Immunol. 2003;170:2516–22. doi: 10.4049/jimmunol.170.5.2516. [DOI] [PubMed] [Google Scholar]
- 12.Ochi H, Abraham M, Ishikawa H, et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+ CD25– LAP+ T cells. Nat Med. 2006;12:627–35. doi: 10.1038/nm1408. [DOI] [PubMed] [Google Scholar]
- 13.Ishikawa H, Ochi H, Chen ML, Frenkel D, Maron R, Weiner HL. Inhibition of autoimmune diabetes by oral administration of anti-CD3 monoclonal antibody. Diabetes. 2007;56:2103–9. doi: 10.2337/db06-1632. [DOI] [PubMed] [Google Scholar]
- 14.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 15.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45. [PubMed] [Google Scholar]
- 16.Ostman S, Rask C, Wold AE, Hultkrantz S, Telemo E. Impaired regulatory T cell function in germ-free mice. Eur J Immunol. 2006;36:2336–46. doi: 10.1002/eji.200535244. [DOI] [PubMed] [Google Scholar]
- 17.Maeda Y, Noda S, Tanaka K, et al. The failure of oral tolerance induction is functionally coupled to the absence of T cells in Peyer's patches under germfree condition. Immunobiol. 2001;204:442–57. doi: 10.1078/0171-2985-00054. [DOI] [PubMed] [Google Scholar]
- 18.Tsuji NM, Mizumachi K, Kurisaki J. Interleukin-10-secreting Peyer's patch cells are responsible for active suppression in low-dose oral tolerance. Immunology. 2001;103:458–64. doi: 10.1046/j.1365-2567.2001.01265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jump RL, Levine AD. Murine Peyer's patches favor development of an IL-10-secreting, regulatory T cell population. J Immunol. 2002;168:6113–19. doi: 10.4049/jimmunol.168.12.6113. [DOI] [PubMed] [Google Scholar]
- 20.Spahn TW, Fontana A, Faria AM, et al. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur. J Immunol. 2001;31:1278–87. doi: 10.1002/1521-4141(200104)31:4<1278::aid-immu1278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 21.Spahn TW, Weiner HL, Rennert PD, et al. Mesenteric lymph nodes are critical for the induction of high-dose oral tolerance in the absence of Peyer's patches. Eur J Immunol. 2002. pp. 1109–13. [DOI] [PubMed]
- 22.Kraus TA, Brimnes J, Muong C, et al. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–43. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worbs T, Bode U, Yan S, et al. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–27. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Min B, Thornton A, Caucheteux SM, et al. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–23. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 25.Piccirillo CA, Letterio JJ, Thornton AM, et al. CD4(+) CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–46. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan TJ, Letterio JJ, van Elsas A, et al. Lack of a role for transforming growth factor-beta in cytotoxic T lymphocyte antigen-4-mediated inhibition of T cell activation. Proc Natl Acad Sci USA. 2001;98:2587–92. doi: 10.1073/pnas.051632398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Q, Boden EK, Henriksen KJ, Bour-Jordan H, Bi M, Bluestone JA. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996–3005. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 28.La Cava A, Van Kaer L, Fu Dong S. CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–7. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Read S, Greenwald R, Izcue A, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–83. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD. Horwitz DA. TGF-beta requires CTLA-4 early after T cell activation to induce FoxP3 and generate adaptive CD4+CD25+ regulatory cells. J Immunol. 2006;176:3321–9. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 31.Fahlen L, Read S, Gorelik L, et al. T cells that cannot respond to TGF-beta escape control by CD4(+) CD25(+) regulatory T cells. J Exp Med. 2005;201:737–46. doi: 10.1084/jem.20040685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohtsuka Y, Sanderson IR. Transforming growth factor-beta: an important cytokine in the mucosal immune response. Curr Opin Gastroenterol. 2000;16:541–5. doi: 10.1097/00001574-200011000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. II. TGF-beta-transgenic Th3 cells rescue IL-2-deficient mice from autoimmunity. J Immunol. 2007;178:172–8. doi: 10.4049/jimmunol.178.1.172. [DOI] [PubMed] [Google Scholar]
- 34.Suri-Payer E, Amar AZ, Thornton AM, Shevach EM. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–18. [PubMed] [Google Scholar]
- 35.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ostroukhova M, Seguin-Devaux C, Oriss TB, et al. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura K, Kitani A, Fuss I, et al. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–42. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 38.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 39.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci USA. 2005;102:5126–31. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walton KL, Galanko JA, Balfour Sartor R, Fisher NC. T cell-mediated oral tolerance is intact in germ-free mice. Clin Exp Immunol. 2006;143:503–12. doi: 10.1111/j.1365-2249.2006.03019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cario E. Bacterial interactions with cells of the intestinal mucosa: Toll-like receptors and NOD2. Gut. 2005;54:1182–93. doi: 10.1136/gut.2004.062794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–4. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki H, Kundig TM, Furlonger C, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–6. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 45.Wannemuehler MJ, Kiyono H, Babb JL, Michalek SM, McGhee JR. Lipopolysaccharide (LPS) regulation of the immune response: LPS converts germfree mice to sensitivity to oral tolerance induction. J Immunol. 1982;129:959–65. [PubMed] [Google Scholar]
- 46.Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40. doi: 10.1016/S0140-6736(07)60750-8. [DOI] [PubMed] [Google Scholar]
- 47.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–7. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 48.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 49.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–73. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express Toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–11. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bashir ME, Louie S, Shi HN, Nagler-Anderson C. Toll-like receptor 4 signaling by intestinal microbes influences susceptibility to food allergy. J Immunol. 2004;172:6978–87. doi: 10.4049/jimmunol.172.11.6978. [DOI] [PubMed] [Google Scholar]
- 52.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–94. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]


