Abstract
Genetic changes in insects that lead to insecticide resistance include point mutations and up-regulation/amplification of detoxification genes. Here, we report a third mechanism, resistance caused by an absence of gene product. Mutations of the Methoprene-tolerant (Met) gene of Drosophila melanogaster result in resistance to both methoprene, a juvenile hormone (JH) agonist insecticide, and JH. Previous results have demonstrated a mechanism of resistance involving an intracellular JH binding protein that has reduced ligand affinity in Met flies. We show that a γ-ray induced allele, Met27, completely lacks Met transcript during the insecticide-sensitive period in development. Although Met27 homozygotes have reduced oogenesis, they are viable, demonstrating that Met is not a vital gene. Most target-site resistance genes encode vital proteins and thus have few mutational changes that permit both resistance and viability. In contrast, resistance genes such as Met that encode nonvital insecticide target proteins can have a variety of mutational changes that result in an absence of functional gene product and thus should show higher rates of resistance evolution.
Changes in insect genes that lead to insecticide resistance are beginning to be elucidated (1). The types of mutations seen can vary with the mechanism of resistance to a particular insecticide. Resistance caused by altered insecticide target molecules have been found to result from point mutations in their genes (2–4) whereas resistance associated with increased insecticide metabolism can result either from amplification of esterase genes responsible for insecticide sequestration/detoxification (5, 6) or from up-regulation of detoxification genes, especially cytochrome P450 (7). However, down-regulation of a insect gene, although a postulated mechanism (1, 8), has not been demonstrated.
We are interested in resistance to the juvenile hormone (JH) analog insecticides. These are agonist mimics of JH, an insect-specific hormone involved in a variety of critical functions in insects, including development, reproduction, caste determination, and behavior (9, 10). JH analogs (JHAs) kill dipteran insects during pupal development in a highly specific manner, probably by interfering with the ecdysone-mediated onset of metamorphosis (11). JHA insecticides have an advantage of low vertebrate toxicity (12), although a disturbing report recently showed an interaction of the JHA methoprene (isopropyl [2E, 4E]-11-methoxy-3, 7, 11-trimethyl-2, 4-dodecadienoate) with mammalian retinoid-X receptor molecules (13).
Insect resistance to JHA insecticides has been demonstrated after laboratory selection (14), as cross-resistance (15, 16), and, recently, in greenhouse populations of whiteflies (17) and salt-marsh mosquitoes (18), apparently in response to JHA applications. These results show that resistance to these compounds, once predicted difficult for insects to achieve (19), is possible. However, widespread control failure with JHAs has not been reported, possibly because of the limited use of these insecticides (20).
We identified the Methoprene-tolerant (Met) gene in Drosophila melanogaster and found that resistant mutants could be generated by chemical, x-ray, and transposable element mutagenesis (21–23). Met flies are resistant to the toxic and morphogenetic effects of JH and several JHAs (11, 21, 24) but not to other classes of insecticides (21), demonstrating that Met is not a general insecticide resistance gene. Biochemical studies showed a target-site insensitivity mechanism of resistance, that of reduced JH binding in cytosolic extracts from either of two JH target tissues in Met flies (25, 26). Molecular cloning of Met+ revealed homology with the basic helix-loop-helix (bHLH)-PER-AHR/ARNT-SIM (PAS) family of transcription factors (27). Because JH has been implicated in transcriptional regulation in a variety of insects (28, 29), this result and others (25) suggest that Met is involved in the transcriptional action of JH, perhaps encoding a JH receptor protein.
Previously, several alleles were shown to have reduced Met transcript levels, and one of these, Met27, appeared as a null allele (27). Here, we confirm that Met27 larvae and adults completely lack Met transcript, and we show that the absence results from the Met27 mutation. These results demonstrate that complete loss of a resistance gene product can result in resistance. Because Met27 homozygotes are viable, Met is not a vital gene, a conclusion that has implications for resistance to other insecticide targets that are nonvital to the insect.
EXPERIMENTAL PROCEDURES
Selection of Met27.
Males from a stock of isogenic vermilion (v) flies were subjected to 3,000 R from a 60Co source and were mated with females homozygous for the Met2 allele. Met2 is an ethyl methanesulfonate-induced allele (21) that is useful for uncovering newly induced Met alleles by noncomplementation in F1 progeny. The v gene is a convenient closely linked marker for Met alleles. Progeny were raised on Drosophila Instant Food (Carolina Biological Supply) containing a dose of methoprene (1.6 μg/g food) that is toxic to susceptible larvae (22), and F1 resistant individuals were selected. Approximately 85,000 F1 were screened over a 3-month period. Lines were established from each survivor; each line resulted from a separate mutational event owing to the different parentage of each F1 survivor. From these lines, five new alleles of Met were recovered and confirmed as Met alleles having resistance to both the toxic and morphogenetic effects of methoprene in complementation tests with Met3. Before study, one of the alleles, Met27, was backcrossed to v males for five generations to exchange the majority of the background mutagenized genome with that of v. At each generation, v/v Met27 females were selected on a discriminating dose of methoprene, and the final cross was carried out with sibling matings to homozygose the v Met27 chromosome. The final genotype was confirmed by v eye-color and resistance to a higher dose of methoprene. This procedure ensured that the major portion of the background genome of v Met27 flies was the same as v, enabling a direct comparison between Met27 and Met+ in the two strains. Met27 heterozygotes were produced for resistance studies by crossing v/v females with v Met27 males and examining female progeny, all of which were v/v Met27 genotype.
Treatment of Larvae with Methoprene.
Methoprene (the biologically active ZR-2008 enantiomer) was obtained from Sandoz Pharmaceutical, was dissolved in ethanol, and was applied to the surface of the food contained in a 25- × 75-mm vial (Sarstedt) in a volume of 25 μl of solution as described (30) within one day of introduction of newly hatched larvae. The reproducibility of dose–response determinations made in this manner is similar to other methods of application (21) and is the preferred method. The lethal period is pharate adult to early adult (within 1 day of eclosion). Survival was expressed as adults eclosing and surviving >1 day.
Ovipositional and Oogenesis Analysis.
Females were isolated within 4 hours after eclosion at 25°C and were placed individually in a 28- × 95-mm vial (Capitol, Fonda, NY) containing a standard agar–molasses–cornmeal–yeast diet with baker’s yeast sprinkled on the surface. Two Oregon-RC wild-type males were added to each vial, and daily egg collections were counted and examined for hatching. Oogenesis was assessed by dissecting ovaries from females isolated at several times after eclosion and censusing vitellogenic oocytes as described (31).
Reverse Transcription–PCR.
RNA was isolated from late third-instar larvae by using TriReagent (Molecular Research Center, Cincinnati). One microgram of RNA was DNase-treated for 30 min and was heated to 65°C for 15 min. First strand synthesis was carried out in a Perkin–Elmer Thermocycler by using the Titan kit (Boehringer Mannheim) according to the manufacturer’s recommendations. Reverse transcription–PCR was carried out by using primers specific for Met gene ORF, 5′-ACAAGGAGGCAGTAACTC-3′ (forward) and 5′-GTCAAGCCCACTGTATCC-3′ (reverse); and for the 6K2A gene ORF, 5′-ACAACGAGGATCTGGAGAGCATAG-3′ (forward) and 5′-TGCGTGTGAGCGATTCCTTCTG-3′ (reverse). Samples then were denatured for 2 min at 94°C and were subjected to 10 cycles of initial amplification consisting of 94°C for 30 sec, 52°C for 30 sec, and extension 68°C for 1.5 min. A subsequent amplification of 25 cycles included a 10-sec additional extension at each cycle, ending with a 7-min extension at 68°C. Reaction products then were separated on an 0.8% agarose gel and were stained with ethidium bromide.
RESULTS AND DISCUSSION
Isolation of Met27.
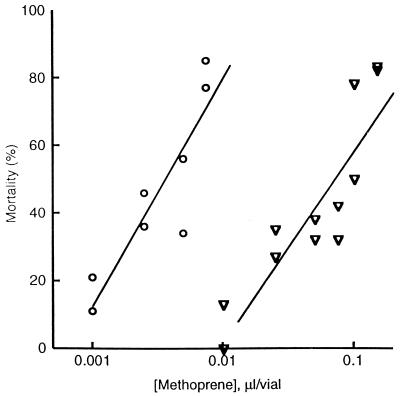
From a screen of progeny of irradiated methoprene-susceptible v males, five new alleles of Met were isolated. Each is resistant to both the toxic and morphogenetic effects (21, 32) of methoprene, as is characteristic of other Met alleles (21, 30). The dose–response relationship for methoprene toxicity is shown for one of the alleles, Met27, together with that of v flies (Fig. 1).
Figure 1.
Dose-mortality relationship for methoprene-treated v (open circles) and v Met27 (open triangles) homozygotes. Each point represents the pupal and early adult mortality for 30 newly hatched larvae placed on medium containing methoprene at the indicated amounts. Because methoprene is a liquid, these amounts are reported in microliters. Surviving v adults, but not v Met27 adults, showed sternal bristle defects characteristic of methoprene treatment, as expected (21, 32, 49).
Transcriptional Analysis.
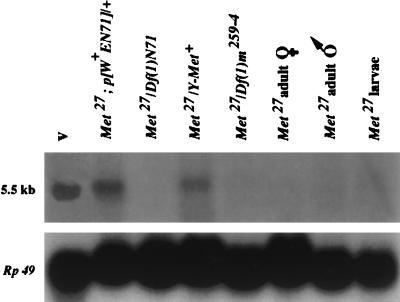
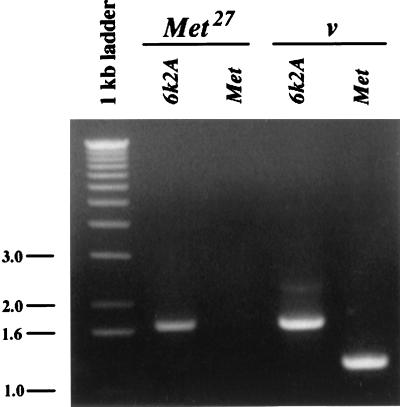
Previously, we identified a 5.5-kilobase (kb) Met transcript (27) expressed during the methoprene-sensitive period (32) during late larval–early pupal development. This transcript is not detected by Northern blot analysis of Met27 larvae and adults, suggesting that this allele is a null (Fig. 2). The sensitive method of reverse transcription–PCR (33) also failed to detect this transcript in late-stage third-instar larvae (Fig. 3). A smaller, 3.3-kb transcript present in adult ovaries and early embryos of Met+ flies (27) is also absent in Met27 females (Fig. 2). The function of this smaller transcript is unclear because flies are not sensitive to methoprene during either of these stages of development. However, a cDNA derived from this transcript is capable of restoring methoprene sensitivity to transgenic Met flies, showing that the cDNA encodes a functional Met+ protein (27).
Figure 2.
Northern blot of total RNA isolated from either v Met27 adults or wandering third-instar larvae of the indicated genotypes and probed with a [32P]UTP-labeled 331-bp riboprobe of the Met gene. Each lane was loaded with 40 μg of total RNA, was subjected to denaturing gel electrophoresis in a formaldehyde agarose gel, and was blotted onto Hybond-N membrane. The 3.3-kb Met transcript, which appears only in early embryos and adult females (27), is also absent in the Met27 adult female lane. Control loading (Lower) was evaluated by stripping the blot and reprobing with a [32P]dCTP random-primed cDNA of the Rp49 gene (50). p[w+EN71] designates an autosome carrying an ectopic copy of the Met+ gene in a w v Met27 genetic background. This transgene has been shown to rescue the Met phenotype (27) functionally. Df(1)N71 and Df(1)m259–4 are deficiency chromosomes lacking a Met+ gene (21); Y-Met+ is a (1; Y) translocation of the 9F to 10E Met+ region onto a Y chromosome (51).
Figure 3.
Reverse transcription–PCR products from primer pairs specific for either the Met or 6K2A ORF amplified from RNA of either v Met27 or v larvae. Each lane represents 1 μg of RNA isolated from late third-instar larvae homozygous for either v Met27 or v. The primer pairs were designed to amplify either a 1,215-bp fragment of the Met ORF sequence or a 1,718-bp fragment of the 6K2A ORF sequence. The 6K2A gene is located ≈2 kb distal to Met (T.G.W., unpublished data) and is used here as a positive control. After PCR, the reaction products were separated on an 0.8% agarose gel and were stained with ethidium bromide.
The lack of transcript in Met27 could result either from a lesion in the Met gene or from another gene that blocks Met transcript expression or stabilization. To determine whether the absence maps to the Met locus, we used two deficiency chromosomes that lack the 10C cytogenetic region, where Met was localized by recombinational mapping (21) and in situ hybridization (34). These deficiencies were found previously to uncover the methoprene-resistance phenotype in Met/Df flies (21). Both Met27/Df(1) m259–4 and Met27/Df(1)N71 larvae lack Met transcript (Fig. 2). Met males carrying a Y chromosome with a translocated portion of an X chromosome that includes Met+ lose resistance to methoprene (21); likewise, Met27 males carrying this Y translocation produce transcript (Fig. 2). Finally, transgenic Met27 males carrying an autosomal ectopic copy of a 7.6-kb genomic fragment that includes a functional copy of Met+ (27) produce transcript (Fig. 2). Therefore, it is clear that the failure of Met27 flies to produce transcript results from the lesioned Met gene instead of from a secondary effect at another locus.
Because Met27 was recovered from a γ-irradiated parent, we suspected a chromosomal break in or near the Met27 allele. To better understand the lesion, we subjected genomic DNA from these flies to PCR amplification by using forward primers located either 42 or 128 bp 5′-upstream of the ATG start site of the Met ORF and a reverse primer located 1,162 bp into the ORF but found no size change in the amplified fragments relative to those from v flies, ruling out a large deletion. However, when the forward primer was located 380 bp 5′-upstream of the ATG start site, no amplification product was obtained, suggesting that Met27 has a chromosomal break in the transcriptional regulatory region of the gene, accounting for the lack of transcript. We note that a transposable P-element insertion located 424 bp 5′-upstream of the ATG start site interferes with Met transcription in a P-element allele of Met (27).
Met27 Phenotype.
The certainty of Met27 as a null allele now allows an evaluation of the phenotype for Met deficiency. First, it is clear that Met27 homozygotes are resistant to both the toxic and morphogenetic effects of methoprene (Fig. 1 and unpublished data). Although the level of resistance is not as high as other target-site resistance genes in other insects (35, 36), not all resistance genes show such high (>1,000-fold) resistance ratios. For example, Drosophila resistant to the insecticide cyromazine were found to have <5-fold resistance over susceptible strains (37). Another possibility to explain the low resistance is the presence of multiple targets for methoprene in Drosophila, with only one of them altered in Met27 flies.
Met27 heterozygotes show partial resistance to the morphogenetic effects of methoprene, which is a characteristic of other alleles (21, 30), but otherwise appear wild-type. Homozygotes easily survive and, although the cultures are not as vigorous as those of v, can be maintained readily. Therefore, Met is not a vital gene.
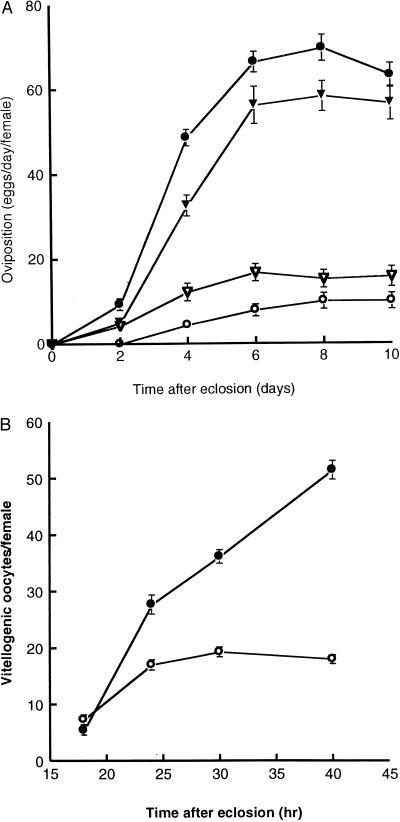
The most obvious defect is reduced oviposition compared with that of females homozygous for v (Fig. 4). Homozygous Met27 females lag behind v females both for the onset of oviposition and for daily oviposition during a 10-day period after eclosion. That this phenotype maps to the Met locus is evident from reduced oviposition in Met27/Df(1)N71 females as well as from the ability of an ectopic copy of Met+ to rescue the ovipositional defect in transgenic flies (Fig. 4A). Therefore, the rather mild reduction in oogenesis previously seen in Met1 (38) becomes exaggerated in the null allele flies.
Figure 4.
(A) Ovipositional rates of homozygous v and Met27 females. Each point represents the mean (± SEM bars) daily oviposition of single females (n = 25) of various genotypes at the indicated times after eclosion. Open circles, v Met27; open triangles, v Met27/Df(1)N71; solid triangles, w v Met27; p, [w+EN71]/+; solid circles, v. (B) Total vitellogenic oocytes per female as a function of time after eclosion. Each point represents the mean number of total stage 8–14 oocytes per female contained in staged females (n = 40) at the indicated times. Solid circles, v; open circles, v Met27.
The ovipositional defect could be caused by a defect in either oogenesis or oviposition. We examined the status of oogenesis by dissecting ovaries from v and Met27 females at several times after eclosion and censusing the vitellogenic oocytes in each female. Met27 females clearly had fewer vitellogenic (stages 8–14) oocytes than those of v females (Fig. 4B), suggesting a defect in vitellogenic oocyte development rather than in oviposition. A role for JH in vitellogenic oocyte development has been demonstrated clearly (31, 39, 40).
Although one might expect a gene involved in JH reception, as we believe Met is, to be a vital gene, clearly the phenotype of Met27 shows it to be nonvital. Two observations are helpful in reconciling the phenotype with the expectation: (i) No role for JH in preadult development in Drosophila has been found. The lethality caused by methoprene application results from the action of this JH agonist at an inappropriate time in development (early pupariation), apparently interfering with expression of the early ecdysone-regulated genes (11). (ii) Perhaps, a partner gene is providing “functional redundancy” for the needed critical function during development. A similar result recently was described in mice carrying an inactivated steroid receptor coactivator-1 (SRC-1) gene. This basic helix-loop-helix (bHLH)-PER-AHR/ARNT-SIM (PAS) gene is a coactivator for members of the steroid receptor superfamily. When SRC-1 was silenced by gene targeting, the mice became partially resistant to steroid hormone but otherwise were fertile and viable (41). In these mice, another SRC-1 family member, TIF-2, showed elevated expression, which the authors postulate to partially compensate for the SRC-1 deficiency (41). When the presumed partner of Met is identified, this possibility of functional redundancy can be tested directly.
What can Met27 tell us about insecticide resistance? Most insecticides target a critical protein, typically one involved in neurophysiology, in insects. For example, organophosphate and carbamate insecticides target acetylcholinesterase (42), and cyclodienes target a γ-aminobutyric acid channel protein (43); neither are dispensable proteins. To achieve resistance by a target-site mechanism, insects must modify the target protein slightly so that endogenous function is not impaired severely, yet binding of insecticide is poorer. Point mutations have been shown to be responsible for this modification in two instances of insecticide resistance (3, 4). Met resistance is target-site resistance (26), yet the present study clearly shows that the Met gene product is dispensable for viability. Therefore, null mutants are permissible, although, in the absence of insecticide, the null allele will be favored less in a population. This conclusion is based on results after mixing wild-type and Met1 in a population cage; Met1 was displaced in the population in the absence of methoprene in only a few generations (38).
Another insecticide target protein that may be dispensable is the midgut epithelial receptor protein(s) of insect species that are susceptible to the Cry insecticidal crystal proteins from the soil bacterium Bacillus thuringiensis. Resistance of several lepidopteran insects has been shown to be correlated with reduced Cry protein binding to midgut brush border membrane preparations (35, 44, 45), although the identity of the receptor protein is unclear (46). Some of these B. thuringiensis resistant insects have a fitness cost associated with the resistance (35, 47), but at least one apparently has little or no associated fitness cost (48). These results suggest that loss of midgut receptor protein or protein function may result in resistance but not entail a severe loss in fitness. Therefore, we may expect instances of resistance to B. thuringiensis insecticides, and perhaps newer insecticides with unknown target molecules, to include genetic nulls, greatly widening the spectrum of permissible mutations leading to resistance. Such latitude might result in rapid evolution of resistance to insecticides that target nonvital gene products.
Acknowledgments
We thank J. Herbers, A. Reddy, and two anonymous reviewers for comments on the manuscript. Bingyan Meng assisted with the Northern blot. This work was supported by grants from the National Science Foundation and the U.S. Department of Agriculture Competitive Grants Program to T.G.W.
ABBREVIATIONS
- JH
juvenile hormone
- JHA
JH analog
- kb
kilobase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Feyereisen R. Toxicol Lett. 1995;82:83–90. doi: 10.1016/0378-4274(95)03470-6. [DOI] [PubMed] [Google Scholar]
- 2.Newcomb R D, Campbell P M, Ollis D L, Cheah E, Russell R J, Oakeshott J G. Proc Natl Acad Sci USA. 1997;94:7464–7468. doi: 10.1073/pnas.94.14.7464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ffrench-Constant R E, Steichen J C, Aronstein K, Roush R T. Proc Natl Acad Sci USA. 1993;90:1957–1961. doi: 10.1073/pnas.90.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mutero A, Pralavorio M, Bride J-M, Fournier D. Proc Natl Acad Sci USA. 1994;91:5922–5926. doi: 10.1073/pnas.91.13.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mouches C, Pasteur N, Berge J B, Hyrien O, Raymond M, De Saint Vincent B R, De Silvestri M, Georghiou G P. Science. 1986;233:778–780. doi: 10.1126/science.3755546. [DOI] [PubMed] [Google Scholar]
- 6.Field L M, Devonshire A L, Forde B G. Biochem J. 1988;251:309–312. doi: 10.1042/bj2510309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carino F A, Koener J F, Plapp F W J, Feyereisen R. Insect Biochem Mol Biol. 1994;24:411–418. doi: 10.1016/0965-1748(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 8.Feyereisen R. Proc Natl Acad Sci USA. 1998;95:2725–2726. doi: 10.1073/pnas.95.6.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nijhout H F, Wheeler D E. Q Rev Biol. 1982;57:109–133. [Google Scholar]
- 10.Riddiford L M. In: Hormone Action at the Cellular Level. Kerkut G, Gilbert L I, editors. Vol. 8. Oxford: Pergamon; 1985. pp. 37–84. [Google Scholar]
- 11.Restifo L L, Wilson T G. Dev Genet (Amsterdam) 1998;22:141–159. doi: 10.1002/(SICI)1520-6408(1998)22:2<141::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Wright J E. Environ Health Perspect. 1976;14:127–132. doi: 10.1289/ehp.7614127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harmon M A, Boehm M F, Heyman R A, Mangelsdorf D J. Proc Natl Acad Sci USA. 1995;92:6157–6160. doi: 10.1073/pnas.92.13.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown T M, Brown A W A. J Econ Entomol. 1974;67:799–801. doi: 10.1093/jee/67.6.799a. [DOI] [PubMed] [Google Scholar]
- 15.Cerf D C, Georghiou G P. Pestic Sci. 1974;5:759–767. [Google Scholar]
- 16.Zhang L, Harada K, Shono T. Appl Entomol Zool. 1998;33:195–197. [Google Scholar]
- 17.Ishaaya I, Horowitz R. Pestic Sci. 1995;43:227–232. [Google Scholar]
- 18.Dame D A, Wichterman G J, Hornby J A. J Am Mosquito Control Assoc. 1998;14:200–203. [PubMed] [Google Scholar]
- 19.Williams C M. Sci Am. 1967;217:13–17. doi: 10.1038/scientificamerican0767-13. [DOI] [PubMed] [Google Scholar]
- 20.Dhadialla T S, Carlson G R, Le D P. Annu Rev Entomol. 1998;43:545–569. doi: 10.1146/annurev.ento.43.1.545. [DOI] [PubMed] [Google Scholar]
- 21.Wilson T G, Fabian J. Dev Biol. 1986;118:190–201. doi: 10.1016/0012-1606(86)90087-4. [DOI] [PubMed] [Google Scholar]
- 22.Wilson T G, Fabian J. In: Molecular Entomology. Law J, editor. Vol. 49. Los Angeles: UCLA Symposium on Molecular and Cellular Biology; 1987. pp. 179–188. [Google Scholar]
- 23.Wilson T G, Turner C. In: Molecular Mechanisms of Insecticide Resistance. Mullin C A, Scott J A, editors. Vol. 505. Washington, DC: Am. Chem. Soc.; 1992. pp. 99–112. [Google Scholar]
- 24.Riddiford L M, Ashburner M. Gen Comp Endocrinol. 1991;82:172–183. doi: 10.1016/0016-6480(91)90181-5. [DOI] [PubMed] [Google Scholar]
- 25.Shemshedini L, Lanoue M, Wilson T G. J Biol Chem. 1990;265:1913–1918. [PubMed] [Google Scholar]
- 26.Shemshedini L, Wilson T G. Proc Natl Acad Sci USA. 1990;87:2072–2076. doi: 10.1073/pnas.87.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashok M, Turner C, Wilson T G. Proc Natl Acad Sci USA. 1998;95:2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riddiford L M. Adv Insect Physiol. 1994;24:213–274. [Google Scholar]
- 29.Jones G. Annu Rev Entomol. 1995;40:147–169. doi: 10.1146/annurev.en.40.010195.001051. [DOI] [PubMed] [Google Scholar]
- 30.Wilson T G. Arch Insect Biochem Physiol. 1996;32:641–649. doi: 10.1002/(SICI)1520-6327(1996)32:3/4<641::AID-ARCH35>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Wilson T G. Wilhelm Roux Arch Entwicklungsmech Org. 1982;191:257–263. doi: 10.1007/BF00848413. [DOI] [PubMed] [Google Scholar]
- 32.Postlethwait J H. Drosophila Inform Serv. 1973;50:135–138. [Google Scholar]
- 33.Zhang L, Medina D. Mol Carcinogen. 1993;8:123–126. doi: 10.1002/mc.2940080209. [DOI] [PubMed] [Google Scholar]
- 34.Turner C, Wilson T G. Arch Insect Biochem Physiol. 1995;30:133–147. doi: 10.1002/arch.940300205. [DOI] [PubMed] [Google Scholar]
- 35.Tabashnik B E, Finson N, Groeters F R, Moar W J, Johnson M W, Luo K, Adang M J. Proc Natl Acad Sci USA. 1994;91:4120–4124. doi: 10.1073/pnas.91.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.ffrench-Constant R H. Insect Biochem Mol Biol. 1994;24:335–345. doi: 10.1016/0965-1748(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 37.Adcock G J, Batterham P, Kelly L E, McKenzie J A. J Econ Entomol. 1993;86:1001–1008. doi: 10.1093/jee/86.4.1001. [DOI] [PubMed] [Google Scholar]
- 38.Minkoff C, III, Wilson T G. Genetics. 1992;131:91–97. doi: 10.1093/genetics/131.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jowett T, Postlethwait J H. Dev Biol. 1980;80:225–234. doi: 10.1016/0012-1606(80)90510-2. [DOI] [PubMed] [Google Scholar]
- 40.Bownes M, Ronaldson E, Mauchline D. Dev Biol. 1996;173:475–489. doi: 10.1006/dbio.1996.0041. [DOI] [PubMed] [Google Scholar]
- 41.Xu J, Qiu Y, DeMayo F J, Tsai S Y, Tsai M-J, O’Malley B W. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 42.Pralavorio M, Fournier D. Biochem Genet. 1992;30:77–83. doi: 10.1007/BF00554429. [DOI] [PubMed] [Google Scholar]
- 43.ffrench-Constant R H, Mortlock D P, Shaffer C D, MacIntyre R J, Roush R T. Proc Natl Acad Sci USA. 1991;88:7209–7213. doi: 10.1073/pnas.88.16.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferre J, Real M D, Van Rie J, Jansens S, Peferoen M. Proc Natl Acad Sci USA. 1991;88:5119–5123. doi: 10.1073/pnas.88.12.5119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferre J, Escriche B, Bel Y, Van Rie J. FEMS Microbiol Lett. 1995;132:1–7. [Google Scholar]
- 46.Luo K, Tabashnik B E, Adang M J. Appl Environ Microbiol. 1997;63:1024–1027. doi: 10.1128/aem.63.3.1024-1027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groeters F R, Tabashnik B E, Finson N, Johnson M W. Evolution. 1994;48:197–201. doi: 10.1111/j.1558-5646.1994.tb01306.x. [DOI] [PubMed] [Google Scholar]
- 48.Tang J D, Gilboa S, Roush R T, Shelton A M. J Econ Entomol. 1997;90:732–741. [Google Scholar]
- 49.Ashburner M. Nature (London) 1970;227:187–189. doi: 10.1038/227187a0. [DOI] [PubMed] [Google Scholar]
- 50.O’Connell P, Rosbash M. Nucleic Acids Res. 1984;12:5495–5512. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schalet A. Drosophila Inform Serv. 1969;44:123. [Google Scholar]