Abstract
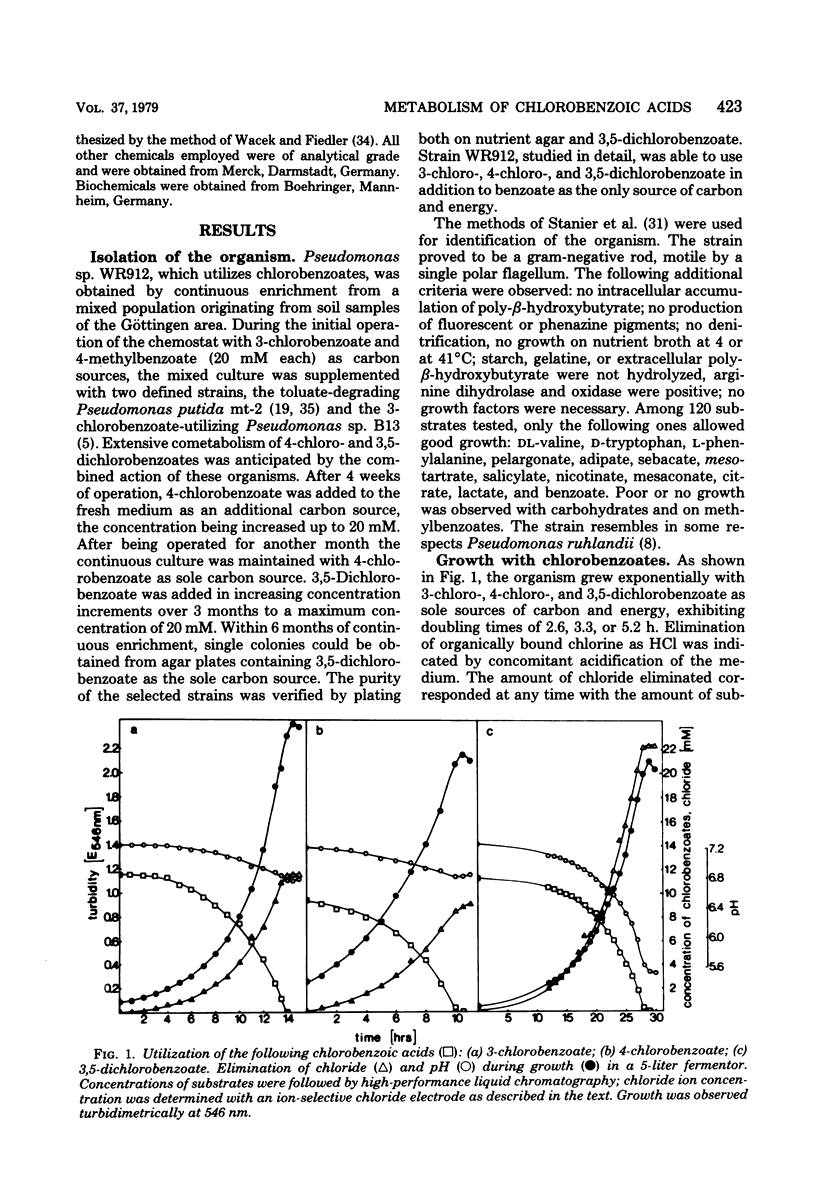
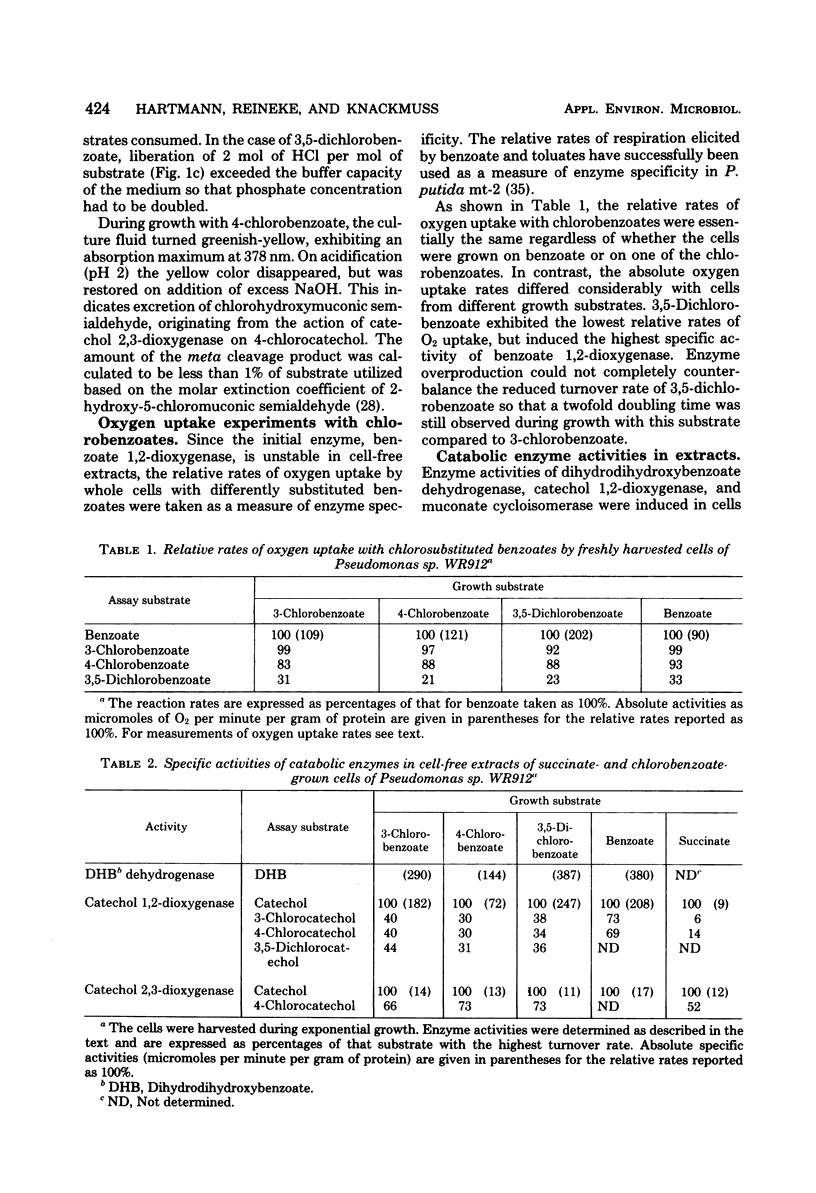
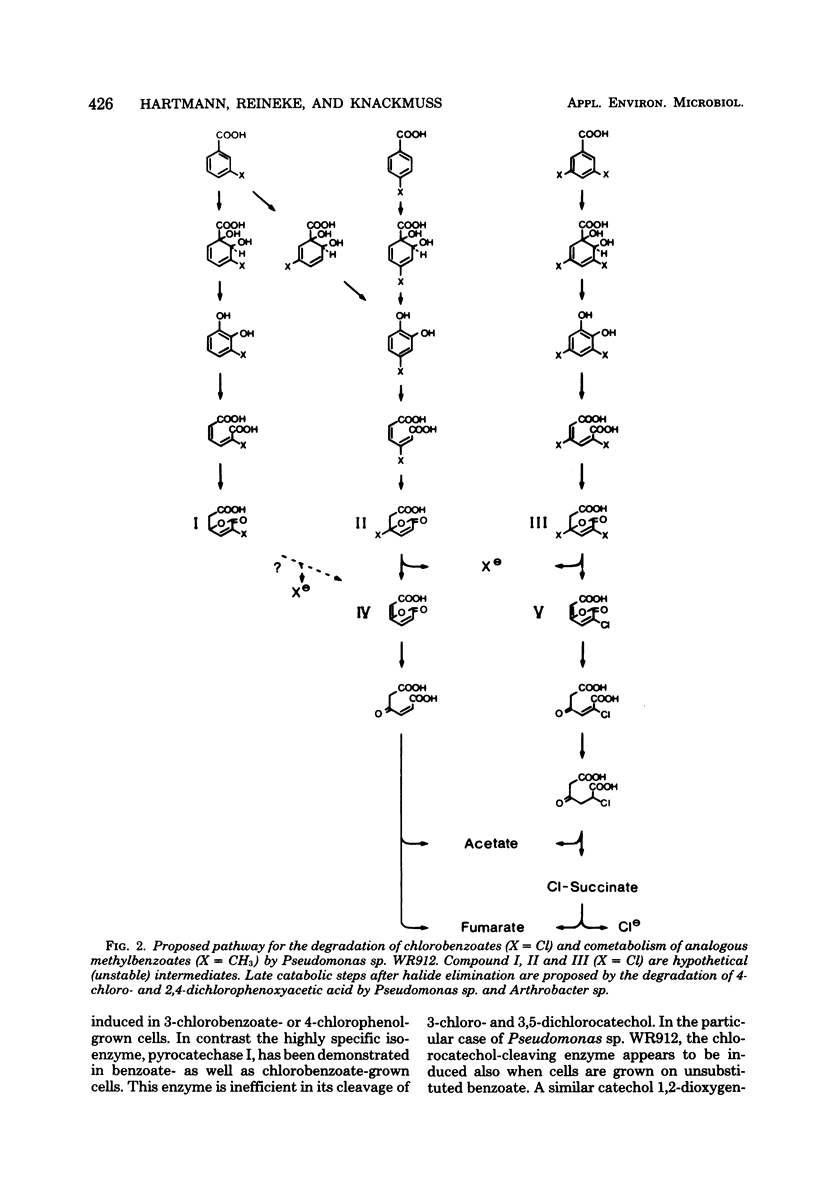
Pseudomonas sp. WR912 was isolated by continuous enrichment in three steps with 3-chloro-, 4-chloro-, and finally 3,5-dichlorobenzoate as sole source of carbon and energy. The doubling times of the pure culture with these growth substrates were 2.6, 3.3, and 5.2 h, respectively. Stoichiometric amounts of chloride were eliminated during growth. Oxygen uptake rates with chlorinated benzoates revealed low stereospecificity of the initial benzoate 1,2-dioxygenation. Dihydrodi-hydroxybenzoate dehydrogenase, catechol 1,2-dixoygenase, and muconate cycloisomerase activities were found in cell-free extracts. The ortho cleavage activity for catechols appeared to involve induction of isoenzymes with different stereospecificity towards chlorocatechols. A catabolic pathway for chlorocatechols was proposed on the basis of similarity to chlorophenoxyacetate catabolism, and cometabolism of 3,5-dimethylbenzoate by chlorobenzoate-induced cells yielded 2,5-dihydro-2,4-dimethyl-5-oxo-furan-2-acetic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZOUZ W. M., PARKE D. V., WILLIAMS R. T. Studies in detoxication. 62. The metabolism of halogenobenzenes; ortho- and para-dichlorobenzenes. Biochem J. 1955 Mar;59(3):410–415. doi: 10.1042/bj0590410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed M., Focht D. D. Degradation of polychlorinated biphenyls by two species of Achromobacter. Can J Microbiol. 1973 Jan;19(1):47–52. doi: 10.1139/m73-007. [DOI] [PubMed] [Google Scholar]
- Dorn E., Hellwig M., Reineke W., Knackmuss H. J. Isolation and characterization of a 3-chlorobenzoate degrading pseudomonad. Arch Microbiol. 1974;99(1):61–70. doi: 10.1007/BF00696222. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1,2-dioxygenation of catechol. Biochem J. 1978 Jul 15;174(1):85–94. doi: 10.1042/bj1740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Fernley H. N., Davies J. I. Bacterial metabolism of 2,4-dichlorophenoxyacetate. Biochem J. 1971 May;122(4):543–551. doi: 10.1042/bj1220543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Smith B. S., Moss P., Fernley H. N. Bacterial metabolism of 4-chlorophenoxyacetate. Biochem J. 1971 May;122(4):509–517. doi: 10.1042/bj1220509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. D. Metabolism of the herbicide pronamide in soil. J Agric Food Chem. 1974 Jul-Aug;22(4):606–608. doi: 10.1021/jf60194a023. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Tonomura K., Kamibayashi A. Effect of chlorine substitution on the biodegradability of polychlorinated biphenyls. Appl Environ Microbiol. 1978 Feb;35(2):223–227. doi: 10.1128/aem.35.2.223-227.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt J. K., Evans W. C. Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Preliminary evidence for the metabolic pathway. Biochem J. 1971 May;122(4):519–526. doi: 10.1042/bj1220519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt J. K., Evans W. C. Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Ring-fission, lactonizing and delactonizing enzymes. Biochem J. 1971 May;122(4):533–542. doi: 10.1042/bj1220533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackmuss H. J., Hellwig M. Utilization and cooxidation of chlorinated phenols by Pseudomonas sp. B 13. Arch Microbiol. 1978 Apr 27;117(1):1–7. doi: 10.1007/BF00689343. [DOI] [PubMed] [Google Scholar]
- Murray K., Duggleby C. J., Sala-Trepat J. M., Williams P. A. The metabolism of benzoate and methylbenzoates via the meta-cleavage pathway by Pseudomonas arvilla mt-2. Eur J Biochem. 1972 Jul 24;28(3):301–310. doi: 10.1111/j.1432-1033.1972.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Pemberton J. M., Fisher P. R. 2,4-D plasmids and persistence. Nature. 1977 Aug 25;268(5622):732–733. doi: 10.1038/268732a0. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Chemical structure and biodegradability of halogenate aromatic compounds. Substituent effects on 1,2-dioxygenation of benzoic acid. Biochim Biophys Acta. 1978 Sep 6;542(3):412–423. doi: 10.1016/0304-4165(78)90372-0. [DOI] [PubMed] [Google Scholar]
- Reineke W., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on dehydrogenation of 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid. Biochim Biophys Acta. 1978 Sep 6;542(3):424–429. doi: 10.1016/0304-4165(78)90373-2. [DOI] [PubMed] [Google Scholar]
- Reiner A. M. Metabolism of aromatic compounds in bacteria. Purification and properties of the catechol-forming enzyme, 3,5-cyclohexadiene-1,2-diol-1-carboxylic acid (NAD + ) oxidoreductase (decarboxylating). J Biol Chem. 1972 Aug 25;247(16):4960–4965. [PubMed] [Google Scholar]
- SCHMIDT K., LIAAENJENSEN S., SCHLEGEL H. G. DIE CAROTINOIDE DER THIORHODACEAE. I. OKENON ALS HAUPTEAROTINOID VON CHROMATIUM OKENII PERTY. Arch Mikrobiol. 1963 Aug 1;46:117–126. [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Tyler J. E., Finn R. K. Growth rates of a pseudomonad on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol. Appl Microbiol. 1974 Aug;28(2):181–184. doi: 10.1128/am.28.2.181-184.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. A., Worsey M. J. Plasmids and catabolism. Biochem Soc Trans. 1976;4(3):466–468. doi: 10.1042/bst0040466. [DOI] [PubMed] [Google Scholar]
- Worsey M. J., Williams P. A. Metabolism of toluene and xylenes by Pseudomonas (putida (arvilla) mt-2: evidence for a new function of the TOL plasmid. J Bacteriol. 1975 Oct;124(1):7–13. doi: 10.1128/jb.124.1.7-13.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



