Abstract
AIMS
The primary aim of this study was to describe the use of prescribed drugs in both mothers and fathers before and during pregnancy in Norway.
METHODS
This population-based cohort study was based on data retrieved from the Medical Birth Registry of Norway and the Norwegian Prescription Database. These registries cover the entire population of Norway. Information on >100 000 births during 2004–2006 in the birth registry was linked to prescription data. Prescriptions issued to mothers just prior to, during and after the pregnancies as well as prescriptions to fathers just prior to conception were identified.
RESULTS
Among mothers, 83% were prescribed drugs during the period 3 months prior to estimated conception until 3 months after giving birth. The mothers who received drugs were prescribed on average 3.3 different Anatomical Therapeutic Chemical (ATC) codes (range 1–38). During pregnancy, 57% were prescribed drugs. In the first trimester, 33% of mothers were dispensed drugs, while the figure was 29% for mothers in the last trimester. Among fathers, 25% used prescribed drugs during the 3 months prior to conception, with on average 1.9 different ATC codes (range 1–22).
CONCLUSION
Large proportions of both fathers and mothers were dispensed drugs prior to conception or during pregnancy. While there is a high awareness of the issues involved in maternal drug use in pregnancy, possible teratogenic effects of drug use in fathers shortly before conception should be further explored.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Mothers are using medicines during pregnancies; the extent varies across the world and is generally difficult to compare.
In this registry-based study, we examined more than 100 000 Norwegian pregnancies and described the drug prescription pattern of both fathers and mothers around conception and during pregnancy (mothers).
WHAT THIS STUDY ADDS
In every trimester of pregnancy, about 30% of the mothers was dispensed a drug.
The total drug exposure did not seem to diminish throughout pregnancy.
One-quarter of the fathers was dispensed drugs during the last 3 months prior to conception.
Keywords: drug use, medical birth registry, pharmacoepidemiology, pharmacy data, pregnancy, prescription database
Introduction
During the last 40 years, several studies have been published on possible associations between maternal drug use and birth defects [1]. Physicians and pregnant women are, in many cases, aware of possible teratogenic effects connected to the use of specific drugs. However, a number of pregnancies are not planned. This may lead to exposure to drugs which are not recommended for pregnant women before the women are aware of their pregnancies and typically during organogenesis in the first trimester. Some women, however, need drugs during pregnancy to treat chronic conditions, e.g. asthma, epilepsy, diabetes mellitus or psychiatric disorders. In addition to possible birth defects, drug use later in pregnancy may also influence the course of the pregnancy and the health of both mother and child before and during birth and in the neonatal period.
Because pregnant women or women at risk of becoming pregnant are normally excluded from clinical trials, and ethical reasons almost always prevent testing possible negative effects of drug use during pregnancy in pregnant women [2], there is still very limited information about the risk and safety of prescription drugs during pregnancy. Nevertheless, for reasons of safety, it is often recommended that certain drugs are not used by pregnant women, even though no harmful effects have been observed [3]. As noted by Koren [2], the best way to achieve better knowledge of possible teratogenic effect of drugs during pregnancy is collection and follow-up of observational data.
Epidemiological studies may be important for identifying possible negative effects on mother and child of drug use during and after pregnancy, i.e. short-term as well as long-term effects of drug use during pregnancy. Many women will take prescription drugs during pregnancy. Different studies are difficult to compare because of differences in study design [4]. The use of drugs among fathers prior to conception has received less attention [5, 6]. Fewer restrictions have been put on the use of drugs in fertile males than in females.
Large prospective studies may be particularly useful in exploring the use of medication in relation to pregnancies [1]. In Norway, we are able to perform large prospective studies using population-based registries of births and prescriptions, which cover the entire population.
It is important to know which drugs are most commonly used during or around pregnancy, because the potential risks of these drugs may have major clinical and public health impact.
The aim of this study was to describe drug use among mothers in the 3 months prior to conception, during pregnancy and during the 3 months after the end of pregnancy. We looked at pregnancies in 2004–2006 using population-based registries of births and prescriptions. We also wanted to describe drug use among fathers just prior to conception.
Materials and methods
Data sources
The Norwegian Prescription Database
The Norwegian Prescription Database (NorPD) [7] is a research database which captures all dispensed prescriptions in Norway from 1 January 2004 and covers the entire population of Norway (4.7 million). NorPD contains information on all prescribed drugs, reimbursed or not, dispensed at pharmacies to individual patients treated in ambulatory care. Data on use in institutionalized patients in nursing homes and hospitals are also collected, but these figures are registered only at an institutional level and not at the patient level. Therefore, drugs dispensed to institutions are not included in our study. For each prescription, sex, age (patient and prescriber), demographic information, prescriber speciality, pharmacy identifier, dispensing date and detailed drug information are registered. The indication for prescribing is not recorded. However, the code of reimbursement is recorded and this may in some cases function as a proxy of diagnosis. Classification of drugs was based on the Anatomical Therapeutic Chemical (ATC) classification system as of 2007 [8].
The Medical Birth Registry of Norway
The Medical Birth Registry of Norway (MBRN) is a population-based registry containing information on all births in Norway since 1967 (more than 2.3 million births) [9]. MBRN is based on compulsory notification of every birth or late abortion from 12 weeks of gestation onwards. MBRN includes identification of the mother and the father in terms of their national identification numbers, demographic information on the mother and father, the mother's health before and during pregnancy, complications during pregnancy and delivery, length of pregnancy as well as information on the infant, including birth defects and other perinatal problems [9]. A standard antenatal form is completed at visits to a general practitioner or a midwife during pregnancy and is brought by the mother to the place of birth. The midwife enters additional data recorded at the time of birth. Follow-up data are added to the form until discharge of hospital births. Since 1998, data obtained in neonatal wards on congenital conditions for infants transferred to such units after birth have also been included.
Study subjects
Data in MBRN were linked to NorPD using the unique 11-digit identification number assigned to all individuals living in Norway after 1960. In this study, all singleton pregnancies registered in MBRN were included, beginning 30 March 2004 or later and ending before 1 January 2007. Only the first pregnancy during the study period was included for each parent. Prescription data from NorPD from January 2004 until and including March 2007 were used. Thirtieth March 2004 was defined as the starting date of pregnancies to ensure that information on all prescriptions dispensed from 3 months before conception until the end of pregnancy was available.
Methods
Length of pregnancy was mostly determined by ultrasound (97%), and a date for start of pregnancy was estimated. Each pregnancy was divided into trimesters (weeks 0–12, 13–26, 27 onwards). In addition, prescriptions during the last 3 months before conception and, when the child was live born, 3 months after delivery were studied.
We calculated the proportion of prescriptions by ATC codes and within the categories suggested by Bakker et al.[4]: drugs for chronic conditions, drugs for occasional and short-time use and pregnancy-related drugs.
Ethics
Linkage between the two registries generated anonymous files for research purposes, as regulated by Norwegian law for health registers.
Results
Prescription drug use in mothers
The total number of pregnancies included was 106 329, with known identity of the mother, and with mother still living in Norway, at childbirth. These pregnancies resulted in 105 282 live-births, 256 induced abortions and 791 stillbirths. The mean age of the mothers was 30 (range 13–52), 33 (19–45) and 31 years (15–46), respectively.
Altogether, 83% of the mothers were dispensed drugs during the 3 months prior to conception, during pregnancy or during the 3 months after birth. There was no age difference between those who had dispensed drugs and those who did not. The mothers who received drugs received on average 3.3 different ATC codes (range 1–38) and 4.6 prescriptions (1.6% of these received ≥20 prescriptions). Twenty-two percent of the women received only one prescription. The prescribed drugs are tabulated by ATC groups and by the dispensing period of prescriptions (Table 1). During the last 3 months prior to conception, 39% of the mothers were dispensed drugs. During the first trimester, 33% received drugs, and during both the second and the third trimester 29% were dispensed drugs. During pregnancy, 61 019 (57% of the women) received at least one prescription drug. During the first 3 months after birth, 57% of mothers were dispensed prescriptions. The percentage of pregnant women using prescribed drugs in different periods is illustrated in Figure 1.
Table 1.
Prescriptions dispensed to mothers 3 months prior to pregnancy to 3 months after pregnancy (n = 106 329 pregnancies)
| Women with ≥1 prescription | ||||||||
|---|---|---|---|---|---|---|---|---|
| Whole period | Percentage of pregnant women | |||||||
| Drug (ATC group) | Number of prescriptions | Number | Percentage | 3 months before conception | 1. trimester | 2. trimester | 3. trimester* | 3 months after pregnancy† |
| Alimentary tract and metabolism (A) | 24 631 | 12 098 | 11.4 | 2.9 | 4.4 | 2.7 | 2.6 | 3.6 |
| Antacids and drugs for peptic ulcer (A02) | 3 329 | 2 058 | 1.9 | 0.7 | 0.6 | 0.4 | 0.5 | 0.3 |
| Drugs for functional gastrointestinal disorders (A03) | 5 659 | 3 944 | 3.7 | 0.2 | 2.3 | 0.8 | 0.2 | 0.5 |
| Antidiarrhoeals, intest. anti-inflam./anti-infect. agents (A07) | 4 012 | 2 662 | 2.5 | 0.3 | 0.3 | 0.3 | 0.3 | 1.9 |
| Antiobesity preparations, excl. diet products (A08) | 1 442 | 729 | 0.7 | 0.5 | 0.2 | 0.0 | 0.0 | 0.2 |
| Drugs for diabetes (A10) | 6 081 | 1 254 | 1.2 | 0.7 | 0.6 | 0.6 | 0.7 | 0.3 |
| Blood and blood forming organs (B) | 15 852 | 6 733 | 6.3 | 0.8 | 2.3 | 2.2 | 2.5 | 1.7 |
| Antithrombotic agents (B01) | 9 466 | 2 295 | 2.2 | 0.2 | 0.9 | 1.1 | 1.3 | 0.9 |
| Vitamin B12 and folic acid (B03B) | 3 816 | 2 762 | 2.6 | 0.4 | 1.2 | 0.6 | 0.6 | 0.4 |
| Cardiovascular system (C) | 12 156 | 7 675 | 7.2 | 0.9 | 0.7 | 0.7 | 1.8 | 4.9 |
| Vasoprotectives (C05) | 7 808 | 5 731 | 5.4 | 0.3 | 0.2 | 0.4 | 1.2 | 3.8 |
| β-Blockers (C07) | 1 982 | 1 196 | 1.1 | 0.2 | 0.2 | 0.1 | 0.3 | 0.7 |
| Agents acting on the renin–angiotensin system (C09) | 404 | 263 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.1 |
| Dermatologicals (D) | 2 7447 | 17 297 | 16.3 | 4.2 | 3.2 | 3.4 | 2.9 | 6.9 |
| Antifungals for dermatological use (D01) | 6 049 | 5 107 | 4.8 | 0.6 | 0.4 | 0.5 | 0.5 | 3.1 |
| Antibiotics and chemother. for dermatological use (D06) | 4 898 | 4 292 | 4.0 | 1.0 | 0.7 | 0.7 | 0.6 | 1.4 |
| Dermal corticosteroids (D07) | 12 305 | 7 622 | 7.2 | 2.0 | 1.7 | 1.9 | 1.5 | 2.2 |
| Anti-acne preparations (D10) | 2 748 | 1 725 | 1.6 | 0.6 | 0.4 | 0.4 | 0.3 | 0.4 |
| Genitourinary system and sex hormones (G) | 71 253 | 46 848 | 44.1 | 12.7 | 6.1 | 1.4 | 1.0 | 33.7 |
| Gynaecological anti-infectives (G01) | 5 105 | 4 393 | 4.1 | 0.8 | 0.8 | 1.1 | 0.9 | 0.9 |
| Other gynaecologicals (G02) | 8 493 | 8 150 | 7.7 | 0.6 | 0.2 | 0.0 | 0.0 | 7.1 |
| Sex hormones (G03) | 57 557 | 38 148 | 35.9 | 11.5 | 5.2 | 0.2 | 0.1 | 26.7 |
| Systemic hormonal preparations, excl. sex hormones and insulins (H) | 26 336 | 13 008 | 12.2 | 3.2 | 2.0 | 1.8 | 1.9 | 9.6 |
| Posterior pituitary lobe hormones (H01B) | 12 689 | 8 546 | 8.0 | 0.1 | 0.0 | 0.0 | 0.1 | 8.0 |
| Thyroid therapy (H03) | 9 383 | 2 312 | 2.2 | 1.4 | 1.4 | 1.6 | 1.6 | 1.4 |
| Anti-infectives for systemic use (J) | 84 545 | 46 558 | 43.8 | 11.1 | 10.0 | 12.2 | 13.0 | 16.3 |
| Antibacterials for systemic use (J01) | 80 146 | 45 240 | 42.5 | 10.1 | 9.5 | 12.0 | 12.6 | 16.0 |
| Tetracyclines (J01A) | 1 972 | 1 769 | 1.7 | 1.1 | 0.3 | 0.0 | 0.0 | 0.2 |
| β-Lactam antibacterials, penicillins (J01C) | 54 107 | 35 781 | 33.7 | 5.9 | 6.8 | 9.3 | 9.8 | 12.2 |
| Sulphonamides and trimethoprim (J01E) | 4 972 | 4 278 | 4.0 | 1.3 | 0.5 | 0.6 | 0.9 | 1.0 |
| Macrolides, lincosamides and streptosamins (J01F) | 11 568 | 9 319 | 8.8 | 2.3 | 1.6 | 1.7 | 1.3 | 2.9 |
| Other antibacterials (J01X) | 5 185 | 4 056 | 3.8 | 0.3 | 0.8 | 1.4 | 1.5 | 0.3 |
| Antineoplastic and immunomodulating agents (L) | 1 264 | 852 | 0.8 | 0.7 | 0.1 | 0.0 | 0.0 | 0.1 |
| Musculoskeletal system (M) | 17 574 | 11 331 | 10.7 | 6.0 | 2.3 | 0.4 | 0.2 | 3.4 |
| Anti-inflammatory and antirheumatic products, nonsteroids (M01A) | 13 875 | 10 497 | 9.9 | 5.5 | 2.0 | 0.3 | 0.1 | 3.2 |
| Muscle relaxants (M03) | 2 921 | 891 | 0.8 | 0.6 | 0.3 | 0.0 | 0.0 | 0.2 |
| Nervous system (N) | 43 888 | 15 197 | 14.3 | 7.3 | 5.0 | 2.9 | 2.8 | 4.3 |
| Opioids (N02A) | 12 785 | 6 235 | 5.9 | 2.5 | 1.3 | 0.9 | 1.0 | 1.8 |
| Anti-epileptics (N03) | 2 826 | 615 | 0.6 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 |
| Antipsychotics (N05A) | 3 322 | 1 736 | 1.6 | 0.3 | 0.9 | 0.5 | 0.2 | 0.2 |
| Anxiolytics, hypnotics and sedatives (N05B eller N05C) | 9 690 | 3 520 | 3.3 | 1.8 | 1.0 | 0.5 | 0.6 | 0.8 |
| Antidepressants (N06A) | 6 516 | 2 792 | 2.6 | 1.8 | 1.1 | 0.5 | 0.4 | 0.7 |
| Antiparasitic products, insecticides and repellents (P) | 3 022 | 2 696 | 2.5 | 1.0 | 0.4 | 0.2 | 0.1 | 1.0 |
| Antiprotozoals (P01) | 2 907 | 2 597 | 2.4 | 1.0 | 0.4 | 0.2 | 0.1 | 0.9 |
| Respiratory system (R) | 57 423 | 25 071 | 23.6 | 8.4 | 7.9 | 7.4 | 7.1 | 5.0 |
| Nasal preparations (R01) | 12 983 | 8 773 | 8.3 | 2.9 | 2.0 | 2.1 | 1.9 | 1.8 |
| Anti-asthmatics (R03) | 15 330 | 5 489 | 5.2 | 2.0 | 1.7 | 1.9 | 2.0 | 1.3 |
| Cough and cold preparations (R05) | 8 085 | 6 514 | 6.1 | 2.0 | 1.2 | 1.3 | 1.5 | 0.7 |
| Antihistamines (R06) | 21 025 | 13 319 | 12.5 | 3.8 | 4.6 | 3.4 | 3.0 | 2.2 |
| Sensory organs and various (S + V) | 19 340 | 13 083 | 12.3 | 3.4 | 2.6 | 2.9 | 2.8 | 3.6 |
| Total | 404 731 | 88 173 | 82.9 | 39.3 | 32.8 | 28.6 | 29.0 | 57.2 |
Eight hundred and eighty-six pregnancies did not last until the third trimester and are therefore not included here.
One thousand and forty-seven pregnancies resulted in induced abortions or stilldeaths and are therefore not included here.
Figure 1.
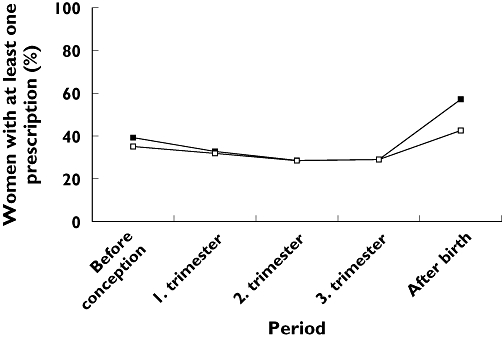
Percentage of mothers who had dispensed prescriptions 3 months prior to pregnancy to 3 months after pregnancy (based on n = 106 329 pregnancies). (All drugs, (▪); Excl. Contraceptives, (□))
The number of women with prescriptions in ATC group A (alimentary tract and metabolism) increased >50% from the period prior to conception to first trimester. The increase was due to the prescription of drugs for functional gastrointestinal disorders (ATC group A03), and the main drug prescribed was metoclopramide (ATC code A03FA01). In the 3 months prior to conception, 235 women had such prescriptions, whereas 2464 women received metoclopramide in the first trimester. Compared with the period prior to conception, the number of women with prescriptions in ATC group B (blood and blood forming organs) was higher during pregnancy. The increase was in prescription of antithrombotic agents, vitamin B12 and folic acid. The proportion of women using folic acid (ATC code B03BB01) was more than four times higher in the first trimester than in the 3 months prior to conception (0.2% and 1.0%, respectively). In ATC group C (cardiovascular system), a large increase in the number of women with prescriptions was seen from second to third trimester. Most of the increase was observed in vasoprotectives (ATC group C05). The prescription of dermatologicals (ATC group D) was reduced during pregnancy. Anti-acne preparations were dispensed to women throughout the pregnancies, but decreased during pregnancy. There were six prescriptions of isotretinoin in six women (ATC code D10BA01) during the study period, of which four were >2 months prior to conception and two were after birth.
Prescriptions in ATC group G (genitourinary system and sex hormones) were mainly sex hormones (G03). The number of women using these drugs decreased from 12 222 (11.5%) in the 3 months prior to conception to 5503 in the first, 229 in the second and 121 in the third trimester. Of all the pregnant women, 1.5% received contraceptives (G03A) during the first trimester. About 2.6% received hormones for in vitro fertilization (G03G) during the first trimester. The increase in prescription of ATC group G to 34% after delivery was due mainly to the use of oral contraceptives (ATC group G03A).
The decrease in use of systemic hormonal preparations during pregnancy, except for sex hormones and insulins (ATC group H), was caused mainly by a decrease in the use of hypothalamic hormones (ATC group H01C). No prescriptions of these drugs were dispensed in the last two trimesters of pregnancy.
The increase in use of systemic anti-infectives (ATC group J) during pregnancy was due mainly to an increase in the use of antibacterials for systemic use (J01). Of the women using systemic anti-infectives, 80% used penicillins (ATC group J01C). Tetracyclines (ATC group J01A) were dispensed to 364 (0.3%) of all pregnant women in the first trimester, and 29 received it in the second and 22 in the third trimesters. Between 1.0% and 1.5% of the pregnant women used the macrolide antibiotic erythromycin (ATC code J01FA01) in each trimester. Altogether, 383 women (0.4%) were dispensed fluoroquinolones (ATC code J01MA) in connection with pregnancy. The number of women receiving fluoroquinolones decreased from 202 in the 3 months prior to conception to 69, 14 and 1 in the trimesters of pregnancy and increased again to 104 in the 3 months after birth. A total of 920 women (0.9%) used antivirals for systemic use (ATC code J05) in connection with pregnancy.
Few women were dispensed drugs in ATC group L (antineoplastic and immunomodulating agents) during the last two trimesters. Also, the number of women using drugs in group M (musculoskeletal system), consisting mostly of nonsteroidal anti-inflammatory and antirheumatic products (ATC group M01A), decreased during pregnancy. Anti-inflammatory and antirheumatic products were used by 5.5% of the women in the 3 months prior to pregnancy, but the proportion decreased to 0.1% in the last trimester.
The number of women using nervous system drugs (ATC group N) decreased with 7.3% of women in the period prior to conception to 2.8% of women during the third trimester. This decrease was due to a reduction in opioids (ATC group N02A) and antidepressants (ATC group N06A). Of the 615 women using antiepileptics (ATC group N03) in connection with pregnancy, 141 used carbamazepine (ATC code N03AF01), 107 used valproic acid (ATC code N03AG01) and 251 used lamotrigine (ATC code N03AX09). Whereas 36, 33 and 39 women used valproic acid in the first, second and third trimester, respectively, the numbers for carbamazepine were 75, 66 and 67, and for lamotrigine 161, 141 and 137. The proportion of women using anxiolytics and hypnotics (ATC groups N05B and N05C) decreased from 1.8% before conception to 1% in the first trimester and further to 0.5% in the second and 0.6% in the third trimester. Also, the use of antidepressants (ATC group N06A) dropped from 1.8% before conception to about 1% in the first trimester. Overall, about 71% of the antidepressant prescriptions were selective serotonin reuptake inhibitors (SSRIs) (ATC group N06AB). Prescriptions for SSRIs were dispensed to 1.3% of mothers during the last 3 months prior to conception and 0.8% of mothers during the first trimester. In the last two trimesters of pregnancy 0.4% and 0.3% of pregnant women were prescribed SSRIs.
A modest decrease in the number of women being dispensed drugs for diseases of the respiratory system (ATC group R) during pregnancy was observed. The proportion of women using antiasthmatics (ATC group R03) was about 2% during the pregnancy period and decreased to 1.3% after delivery. Antihistamine (ATC group R06) use increased from 3.8% before conception to 4.6% in the first trimester, but decreased again in the following trimesters.
Figure 2 shows the percentage of women who were dispensed three categories of drugs during the study period. These categories constituted 69% of all prescriptions given to women in the study period. Between 30 and 40% used pregnancy-related drugs in all the periods studied here. The use of pregnancy-related drugs was stable, but most frequent during the 3 months prior to conception and the 3 months after giving birth. The use of drugs for chronic conditions was also stable, whereas a decrease in the use of drugs for occasional and short-time use was observed after the first trimester.
Figure 2.
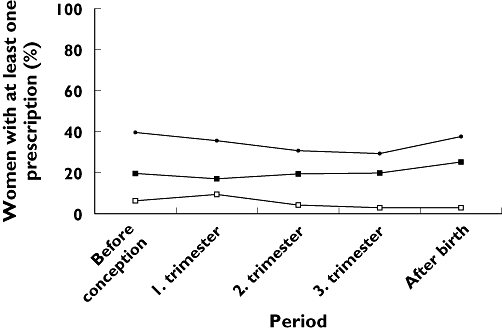
Percentage of women who had dispensed prescriptions for three categories of drugs, defined by Bakker et al.[4], from 3 months prior to pregnancy to 3 months after pregnancy (based on n = 106 329 pregnancies). (Drugs for chronic conditions, (▪); Drugs for occasional and short-time use, (□); Pregnancy-related drugs, (•))
Prescription drug use in fathers
A total of 109 725 pregnancies were initially included in this study. Information about the fathers was available in 96% of pregnancies. After including only the first pregnancy for each father, the total number of pregnancies included in the analyses was 103 302. These pregnancies resulted in 102 698 live-births, 31 abortions and 573 stillbirths. The mean age of the fathers was 33 (range 16–72), 33 (24–49) and 35 years (17–87), respectively.
During the last 3 months prior to conception, 75% of fathers were not dispensed any prescribed drug, while 12% received only one drug. The prescribed drugs are tabulated by ATC groups (Table 2). Fathers receiving drugs were prescribed on average 1.9 different ATC codes (range 1–22).
Table 2.
Prescriptions dispensed to fathers in the last 3 months prior to pregnancy (n = 103 302 pregnancies)
| Drug (ATC group) | Number of prescriptions | Number of fathers with ≥1 prescription | Percentage of fathers with ≥1 prescription |
|---|---|---|---|
| Alimentary tract and metabolism (A) | 4 349 | 2 838 | 2.7 |
| Antacids and drugs for peptic ulcer (A02) | 1 808 | 1 438 | 1.4 |
| Antidiarrhoeals, intest. anti-inflam./anti-infect. agents (A07) | 530 | 383 | 0.4 |
| Drugs for diabetes (A10) | 1 387 | 656 | 0.6 |
| Blood and blood forming organs (B) | 657 | 515 | 0.5 |
| Antithrombotic agents (B01) | 498 | 394 | 0.4 |
| Cardiovascular system (C) | 3 012 | 1 934 | 1.9 |
| β-Blockers (C07) | 502 | 424 | 0.4 |
| Lipid-modifying agents (C10) | 744 | 644 | 0.6 |
| Dermatologicals (D) | 4 685 | 3 396 | 3.3 |
| Antifungals for dermatological use (D01) | 932 | 776 | 0.8 |
| Antibiotics and chemother. for dermatological use (D06) | 667 | 628 | 0.6 |
| Dermal corticosteroids (D07) | 2 300 | 1 787 | 1.7 |
| Anti-acne preparations (D10) | 213 | 151 | 0.1 |
| Genitourinary system and sex hormones (G) | 1 166 | 681 | 0.7 |
| Urologicals (G04) | 1 009 | 587 | 0.6 |
| Systemic hormonal preparations, excl. sex hormones and insulins (H) | 1 305 | 1 089 | 1.1 |
| Corticosteroids for systemic use (H02) | 922 | 780 | 0.8 |
| Anti-infectives for systemic use (J) | 7 627 | 6 365 | 6.2 |
| Antibacterials for systemic use (J01) | 7 025 | 5 965 | 5.8 |
| Tetracyclines (J01A) | 1 022 | 934 | 0.9 |
| β-Lactam antibacterials, penicillins (J01C) | 3 537 | 3 283 | 3.2 |
| Macrolides, lincosamides and streptosamins (J01F) | 1 857 | 1 697 | 1.6 |
| Antineoplastic and immunomodulating agents (L) | 311 | 198 | 0.2 |
| Musculoskeletal system (M) | 8 674 | 6 244 | 6.0 |
| Anti-inflammatory drugs (M01) | 7 237 | 5 797 | 5.6 |
| Nervous system (N) | 13 046 | 6 164 | 6.0 |
| Opioids (N02A) | 4 616 | 2 725 | 2.6 |
| Anti-epileptics (N03) | 680 | 408 | 0.4 |
| Antipsychotics, anxiolytics, hypnotics and sedatives (N05) | 3 853 | 1 814 | 1.8 |
| Antidepressants (N06A) | 2 035 | 1 414 | 1.4 |
| Antiparasitic products, insecticides and repellents (P) | 415 | 386 | 0.4 |
| Respiratory system (R) | 11 648 | 7 168 | 6.9 |
| Nasal preparations (R01) | 3 077 | 2 628 | 2.5 |
| Anti-asthmatics (R03) | 3 265 | 1 934 | 1.9 |
| Cough and cold preparations (R05) | 1 570 | 1 370 | 1.3 |
| Antihistamines (R06) | 3 736 | 3 335 | 3.2 |
| Sensory organs and various (S + V) | 3 230 | 2 599 | 2.5 |
| Total | 60 125 | 26 182 | 25.3 |
Almost 2% of fathers used drugs in ATC group C (cardiovascular system). Anti-acne preparations were dispensed to 151 men. Of these, 13 were prescribed isotretinoin (ATC code D10BA01). The 13 men received isotretinoin between 5 and 84 days prior to conception. Of the 408 men using antiepileptics (ATC group N03) prior to conception, 116 used carbamazepine (ATC code N03AF01), 84 used valproic acid (ATC code N03AG01) and 89 used lamotrigine (ATC code N03AX09). Antidepressants (ATC group N06A) were dispensed to 1.4% of fathers. More than 60% of these were prescribed SSRIs (ATC group N06AB).
Discussion
In this study, which included 106 000 Norwegian pregnancies during 2004–2006, we observed that most mothers (83%) were prescribed medicines in the period from 3 months prior to conception until 3 months after giving birth. Antibacterial drugs for systemic use and drugs for the respiratory system constituted the major part of these drugs. During pregnancy, 57% of mothers used prescribed drugs, whereas 33% and 29% of mothers used drugs during the first and last trimester, respectively. Every fourth father used prescribed medicines during the last 3 months prior to conception. Use of anti-inflammatory drugs and drugs for the nervous system were frequent in both mothers and fathers in the 3 months prior to conception.
This study is based on data from population-based registries covering the entire population of Norway. The unique identification number and population-based registries on births, deaths, emigrations and prescriptions give a high quality of linkage between the registries. Recall bias was avoided by using prospectively collected data, not collected by interviews but by central registries. Since the data were extracted from the general population of Norway, not a selected population, our study may be used to evaluate the public impact of drug use in relation to pregnancies. However, registry-based studies also have shortcomings. Although the drugs, the date of dispensing and the dosage of drugs dispensed to each person were known, we did not know whether the individuals used the drugs, or, if they did, when they used them. For this reason, the drug use may have been overestimated. The NorPD does not contain individual information on prescriptions made in institutions, so these prescriptions were not included in the study. Also, drugs sold over the counter were not included. This may lead to an underestimation of the use of certain drugs. The time of conception was calculated on the basis of birth date and information on the length of pregnancy. The length of pregnancy was mostly determined on the basis of ultrasound examinations.
This study covered >100 000 pregnancies, and even if only a small proportion of the pregnancies were dispensed any particular drug, the absolute numbers will still be of importance. More detailed studies may be performed for individual drugs and drug groups of interest, whereas here only a few examples can be noted. In our study, about 1.2% of mothers used drugs for diabetes in connection with their pregnancies, 0.6% used antiepileptics and 5.2% used antiasthmatic drugs. Most of these probably had chronic illnesses prior to conception, which needed prolonged medication throughout pregnancy. Managing epilepsy during pregnancy is a major therapeutic challenge, as antiepileptic drugs have been associated with birth defects and impaired postnatal cognitive development. The pattern of birth defects varies with the type of antiepileptic drug [10]. Neural tube defects have been linked to the use of carbamazepine and valproic acid. There is scant information about the new antiepileptic drugs and possible teratogenicity.
The first trimester is critical for teratogenic effects. In our study, 1.5% of women were dispensed contraceptives during the first trimester, which may indicate that many were not aware they were pregnant until some weeks after conception and continued to use drugs as usual. The anti-acne preparation isotretinoin, a potent human teratogen, is of particular concern [11–13]. In our study, no women received isotretinoin during pregnancy and only a few were prescribed the drug 2–3 months prior to conception.
Psychotropic drugs have been under scrutiny for their teratogenic effects (e.g. in [14]), but also for complicating births and neonatal periods [11, 12]. In our study 0.9–1.1% of the women used anxiolytics, antidepressants or antipsychotics in the first trimester, dropping by about half in the last trimester.
Antibacterials for systemic use are frequently prescribed in pregnancy [15]. In our study, penicillins, which are considered safe during pregnancy, were the most prescribed anti-infective. However, tetracycline, fluoroquinolones and erythromycin, with possible teratogenic or toxic effects on the fetus, were dispensed to pregnant women during all three trimesters.
The identity of the father was known in 96% of the pregnancies, so we were able to study the prescriptions in a high percentage of fathers. Schirm et al. found that one-third of 63 000 Danish and Dutch fathers had received drugs in the 6 months before conception [6]. In our study, we found that one-quarter of all fathers were dispensed at least one prescription during the 3 months prior to conception. We observed that isotretinoin was prescribed to fathers closer to conception than to mothers.
In this registry-based study, we looked at prescription patterns for both fathers and mothers around the time of conception and pregnancy. We were able to include >100 000 pregnancies with information on both fathers and mothers. We have shown that a large proportion of women were dispensed drugs throughout pregnancy. In every trimesters of pregnancy, about 30% of women were dispensed a drug. The total drug exposure did not seem to diminish throughout pregnancy. More research is needed to explore whether medication of father shortly before conception has any teratogenic effect, since as many as one-quarter of fathers were dispensed drugs during the last 3 months prior to conception.
Competing interests
None to declare.
REFERENCES
- 1.Källén BAJ. Methodological issues in the epidemiological study of the teratogenicity of drugs. Congenit Anom (Kyoto) 2005;45:44–51. doi: 10.1111/j.1741-4520.2005.00062.x. [DOI] [PubMed] [Google Scholar]
- 2.Koren G. Ethical framework for observational studies of medicinal drug exposure in pregnancy. Teratology. 2002;65:191–5. doi: 10.1002/tera.10038. [DOI] [PubMed] [Google Scholar]
- 3.Koren G, Pastuszak A, Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128–37. doi: 10.1056/NEJM199804163381607. [DOI] [PubMed] [Google Scholar]
- 4.Bakker MK, Jentink J, Vroom F, van den Berg PB, de Walle HE, de Jong-van den Berg LT. Drug prescription patterns before, during and after pregnancy for chronic, occasional and pregnancy-related drugs in the Netherlands. Br J Obstet Gynaecol. 2006;113:559–68. doi: 10.1111/j.1471-0528.2006.00927.x. [DOI] [PubMed] [Google Scholar]
- 5.Trasler JM, Doerksen T. Teratogen update: paternal exposures-reproductive risks. Teratology. 1999;60:161–72. doi: 10.1002/(SICI)1096-9926(199909)60:3<161::AID-TERA12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 6.Schirm E, Pedersen L, Tobi H, Nielsen GL, Sørensen HT, de Jong-van den Berg LT. Drug use among fathers around time of conception: two register based surveys from Denmark and The Netherlands. Pharmacoepidemiol Drug Saf. 2004;13:609–13. doi: 10.1002/pds.959. [DOI] [PubMed] [Google Scholar]
- 7.Furu K, Strøm H, Rønning M, Skurtveit S, Engeland A, Tverdal A. The Norwegian prescription database (NorPD) – A new register for pharmacoepidemiologic research covering a whole nation. EuroDURG Ulster meeting. Pharmacoepidemiology Drug Saf. 2005;14(Suppl. 2):48. [Google Scholar]
- 8.WHO Collaborating Centre for Drug Statistics Methodology. ATC Classification Index with DDDs 2007. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2006. [Google Scholar]
- 9.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand. 2000;79:435–9. [PubMed] [Google Scholar]
- 10.Tomson T, Hiilesmaa V. Epilepsy in pregnancy. BMJ. 2007;335:769–73. doi: 10.1136/bmj.39266.473113.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. N Engl J Med. 2007;356:2684–92. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 12.Chambers CD, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchel AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354:579–87. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 13.Mortensen JT, Olsen J, Larsen H, Bendsen J, Obel C, Sørensen HT. Psychomotor development in children exposed in utero to benzodiazepines, antidepressants, neuroleptics, and anti-epileptics. Eur J Epidemiol. 2003;18:769–71. doi: 10.1023/a:1025306304635. [DOI] [PubMed] [Google Scholar]
- 14.Bar-Oz B, Einarson T, Einarson A, Boskovic R, O'Brien L, Malm H, Bérnard A, Koren G. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29:918–26. doi: 10.1016/j.clinthera.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Amann U, Egen-Lappe V, Strunz-Lehner C, Hasford J. Antibiotics in pregnancy: analysis of potential risks and determinants in a large German statutory sickness fund population. Pharmacoepidemiol Drug Saf. 2006;15:327–37. doi: 10.1002/pds.1225. [DOI] [PubMed] [Google Scholar]


