Abstract
AIMS
To investigate the transfer of rac-tramadol and its rac-O-desmethyl metabolite into transitional milk, and assess unwanted effects in the breastfed infant.
METHODS
Tramadol HCl (100 mg six hourly) was administered to 75 breastfeeding mothers for postoperative analgesia on days 2–4 after Caesarian section. Milk and plasma samples were collected after administration of four or more doses. Rac-tramadol and rac-O-desmethyltramadol were measured by high performance liquid chromatography. Milk : plasma ratio (M : P) and infant doses were calculated by standard methods. The behavioural characteristics of the exposed breastfed infants and a matched control group of infants not exposed to tramadol were also studied.
RESULTS
At steady-state, mean (95% CI) M : P was 2.2 (2.0, 2.4) for rac-tramadol and 2.8 (2.5, 3.1) for rac-O-desmethyltramadol. The estimated absolute and relative infant doses were 112 (102, 122) μg kg−1 day−1 and 30 (28, 32) μg kg−1 day−1, and 2.24% (2.04, 2.44)% and 0.64% (0.59, 0.69)% for rac-tramadol and rac-O-desmethyltramadol, respectively. The exposed infants and control breastfed infants had similar characteristics, including Apgar scores at birth and Neurologic and Adaptive Capacity Scores.
CONCLUSIONS
The combined relative infant dose of 2.88% at steady-state was low. The similarity of NACS in exposed infants and controls suggests that there were no significant behavioural adverse effects. We conclude that short-term maternal use of tramadol during establishment of lactation is compatible with breastfeeding.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
There are presently no published data on tramadol transfer into breast milk or on its effects in the breastfed infant.
WHAT THIS STUDY ADDS
We have provided quantitative data on the absolute and relative infant doses of rac-tramadol and it rac-O-desmethyl metabolite for the breastfed infant.
We have also demonstrated a novel sparse sampling data collection method for investigating infant exposure via milk.
Keywords: infant dose, infant wellbeing, rac-O-desmethyltramadol, rac-tramadol, transitional milk
Introduction
Opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) are effective analgesics after Caesarian section and, except for repeated dosing of pethidine, their breast milk transfer and potential neonatal effects are considered acceptable as breast feeding is established. However, opioids can cause adverse reactions such as sedation, nausea and vomiting, while NSAIDs are contraindicated in women with peptic ulcer or gastro-oesophageal reflux disease, bleeding disorders, renal impairment and hypertension.
In nonobstetric populations, tramadol is now widely used in the treatment of pain of diverse origins [1] and is particularly useful for perioperative pain control [2]. Tramadol is effective against mild to moderate pain [3], and together with its active metabolite acts synergistically through μ-opioid and monoaminergic-mediated mechanisms [3, 4]. It is one of the most widely prescribed analgesics with more than 5 billion patient treatment days arising from 30 years of clinical experience [5]. Although tramadol can cause dizziness, sweating, dry mouth and postural hypotension in some patients, in our hospital it is frequently used after Caesarian section, particularly as a relatively strong oral analgesic for step-down analgesia from neuraxial or intravenous opioid techniques after 24 h postpartum. Maternal concern about the effect of a drug when breastfeeding is a reality and drug administration should ideally be subject to risk vs. benefit analysis [6]. The Manufacturer's Product Information on tramadol gives very limited data on the transfer of tramadol and its active O-desmethyl metabolite (M1) into breast milk, with unpublished information suggesting that 0.12% of a single 100 mg intravenous dose was found in milk collected over 16 h after dose [7].
This investigation was designed to provide detailed information about the breast milk transfer of tramadol and its pharmacologically active O-desmethyl metabolite during repeated tramadol administration for the management of postoperative pain following Caesarian section. The hypothesis tested was that the infant doses of tramadol and O-desmethyltramadol received in breast milk of early stage lactating women would be insufficient to cause concern about significant clinical effects in the neonate. Because we anticipated a limited milk supply in our early lactation patient group, we chose to use a sparse sampling study design.
Methods
Study protocol and data collection
The study was approved by the Ethics Committee of the Women's and Children's Health Service at King Edward Memorial Hospital for Women, Subiaco, Western Australia and all participants gave written informed consent. We studied mothers taking prescribed oral tramadol, on the second to fourth postpartum day after Caesarian section. Pilot data indicated that women were likely to be taking 50 or 100 mg four to six times daily. Women who had significant renal dysfunction, who had opted not to breast feed, or who consented but were then unable to provide an adequate sample of breast milk for analysis, were excluded.
Samples of transitional milk [8] produced on days 2–4 postpartum, and plasma, were taken over one dose interval, usually following the 4th consecutive 6-hourly oral tramadol dose when both analytes of interest can be expected to be at >93% of steady-state concentrations. Milk (three samples per patient) was collected in approximately equal aliquots from both breasts (mixed samples of 2–3 ml), using either manual expression or an electric breast pump. At the same time as one of these samples was taken, a single blood sample (5 ml, heparinized) was also collected by venepuncture. The sample collection times for each patient were determined according to a randomized schedule of 1 h blocks over the dose interval, to ensure an even distribution of both milk and plasma samples across the dose interval. In 57% of patients, where the study period corresponded with the last tramadol dose, one of the milk and/or blood samples was collected in the period between 6 and 14 h after dose.
Details of maternal age, weight, concomitant analgesic therapy on the study day, and tramadol doses and the dates/times of doses were recorded. The infants (n = 75) of the tramadol-treated mothers, and a comparator group of infants (n = 75) of mothers who received other analgesics, were studied. Infant sex, gestational age, and weight were recorded. Apgar scores at 1 and 5 min after birth were recorded from the medical record. On the same day as that of sampling, the wellbeing of all infants was assessed by one of three of the authors (RG, TC or KS), using a standardized clinical physical assessment, including an assessment of alertness (Neurologic and Adaptive Capacity Score; NACS) [9].
Materials
Authentic rac-tramadol, and ketamine were obtained from Sigma Chemical Co, St Louis, MO, USA, and rac-O-desmethytramadol from Grunenthal GmbH, Aachen, Germany. All other solvents and chemicals were of analytical grade.
Measurement of rac-tramadol and rac-O-desmethyltramadol by high performance liquid chromatography (HPLC)
Milk samples (1 ml) were spiked with internal standard (ketamine 400 ng) alkalinized with 0.4 ml of 5 M NaOH and extracted into 8 ml of hexane : ethyl acetate (80 : 20) by shaking for 10 min. After centrifugation at 1500 g for 10 min, supernatant (7 ml) was back extracted into 3 ml of 0.1 M HCl by shaking for 5 min, followed by centrifugation as above. The HCl phase was then alkalinized with 0.4 ml of 5 M NaOH, and re-extracted into 8 ml hexane : ethyl acetate (80 : 20). After centrifugation the upper organic layer (7.5 ml) was aspirated, and evaporated to dryness at 45°C under nitrogen. Residues were reconstituted in 0.1 ml of the HPLC mobile phase and 0.08 ml aliquots were injected onto the column. Separations were performed on a Lichrospher™ RP Select B C8 column (5 μm, 250 mm × 4 mm id; E Merck GmbH, Damstadt, Germany), with a mobile phase of 14% v/v acetonitrile in 20 mM K2HPO4, 0.1% v/v triethylamine (pH 3) that was pumped at 0.9 ml min−1. Analytes were detected at 218 nm. Quantification of chromatograms was undertaken using Chemstation Software (Version 9, Agilent Technology, Waldbronn, Germany). For rac-tramadol, the assay intraday relative standard deviations (RSDs) were 5.9, 2.8, and 1.5% at 50 μg l−1, 500 μg l−1 and 2000 μg l−1, respectively (n = 5), while interday RSDs were 7.4, 7.2 and 5.7% at 50 μg l−1, 500 μg l−1 and 2000 μg l−1, respectively (n = 25). For rac-O-desmethyl tramadol, intraday RSDs were 6.3, 5.4 and 3.1% at 50 μg l−1, 250 μg l−1 and 500 μg l−1, respectively (n = 5), while interday RSDs were 7.4, 5.9, and 4.6% at 50 μg l−1, 250 μg l−1 and 500 μg l−1, respectively (n = 25). The limits of quantification and detection for rac-tramadol were 15 μg l−1 and 8 μg l−1, respectively, while for rac-O-desmethyltramadol they were 10 μg l−1 and 5 μg l−1, respectively. Plasma rac-tramadol and rac-O-desmethyltramadol were also measured as above. For rac-tramadol, intraday RSDs were 7.1 and 8.3%, while interday RSDs were 3.0 and 5.7% at 50 mg l−1 and 2000 mg l−1 respectively (n = 5). For rac-O-desmethyl tramadol, intraday RSDs were 6.2 and 6.9%, while interday RSDs were 5.7 and 4.5% at 25 mg l−1 and 500 mg l−1, respectively (n =5).
Data analysis
Milk to plasma ratios (M : P) were calculated from the steady-state rac-tramadol or rac-O-desmethyltramadol concentration measurements in the paired milk and plasma samples (n = 73). Average steady-state rac-tramadol and rac-O-desmethyltramadol concentrations in milk over a 6 h dose interval (Cavg) were calculated as the mean (95% CI) of 165 measurements made in 55 patients. Absolute infant dose (μg kg−1 day−1) was calculated as the product of the milk Cavg and an average infant milk intake of 0.15 l kg −1day−1[10]. Relative infant dose was calculated as absolute infant dose/maternal dose (μg kg−1 day−1) and expressed as a percentage [10]. The molar ratio of rac-O-desmethyltramadol : rac-tramadol in plasma was calculated to give an indication of the cytochrome P450 2D6 (CYP2D6) status of the participants. Data have been summarized as mean (95% CI) or median (range, or 25th and 75th percentiles) as appropriate. Statistical analyses were performed using SigmaStat Ver 3.5 (SPSS Inc, Chicago, IL, USA).
Results
Ninety-five women were enrolled over an 18-month period. One woman did not meet the inclusion criteria, two women withdrew consent, seven ceased medication on the study day because it was not required or had caused unacceptable side-effects, and 10 were unable to provide samples of breast milk for analysis. The median (IQR) age of the remaining 75 participants was 29 (25, 32) years and their median weight was 75 (67, 91) kg. The usual prescribed dose of tramadol was 100 mg, although eight women received 50 mg for their first and/or second doses. On the study day, the median (range) number of consecutive doses that each woman had received was 4 (3, 9), while the mean (95% CI) dose interval across all doses was 6.2 (5.9, 6.4) h.
The characteristics of the infants of the women who received tramadol and of a matched control group of infants from mothers who received alternate analgesics are summarized in Table 1. Both groups of infants were similar with respect to gestational age, birth weight, Apgar scores at 1 and 5 min after birth, and their NACS scores on days 2–4 after birth. On the study day, 49% of the tramadol patients and 100% of the controls also took other opiate analgesics (mostly oxycodone) while 61% of tramadol patients and 58% of controls also took NSAIDS (mostly diclofenac).
Table 1.
Characteristics of the infants of mothers receiving tramadol and comparator group mothers (n = 75 in each group unless otherwise specified)
| Parameter | Tramadol | Control |
|---|---|---|
| Sex ratio (M : F) | 1.08 | 0.89 |
| Gestational age (weeks) | 38.7 (38.2, 39.2)* | 39.3 (39, 39.6)* |
| Birth weight (kg) | 3.4 (3.3–3.5)* | 3.4 (3.3–3.5)* |
| Apgar score 1 min | 9 (9, 10)† | 9 (9, 10)† |
| Apgar score 5 min | 9 (9, 10)† | 9 (9, 10)† |
| Assessment day post birth | 4 (3, 4)† | 3 (2, 4)† |
| Neurologic and Adaptive Capacity Score (total) | 36 (33, 38)†‡ | 37 (33, 40)†‡ |
| Neurologic score component | 28 (26, 29)†‡ | 27 (26, 30)†‡ |
| Adaptive capacity score component | 9 (8, 10)†‡ | 10 (8, 10)†‡ |
mean (95% CI),
median (IQR),
n = 67 (data for four infants lost, while four infants were not tested because they were in the Special Care Nursery for prematurity (3) or ventilation (1)). Neurologic and Adaptive Capacity Score range 0–40, composed of neurologic score range 0–30 and adaptive capacity score range 0–10.
The milk and plasma concentration measurements of rac-tramadol and rac-O-desmethyltramadol are shown in Figure 1. The M : P values for analytes are summarized in Table 2. Steady-state milk Cavg (for a 6 h dose interval) and calculated mean absolute and relative infant doses for rac-tramadol and rac-O-desmethyltramadol are also summarized in Table 2. The mean relative infant dose was 2.24% for rac-tramadol and 0.64% for rac-O-desmethyltramadol.
Figure 1.
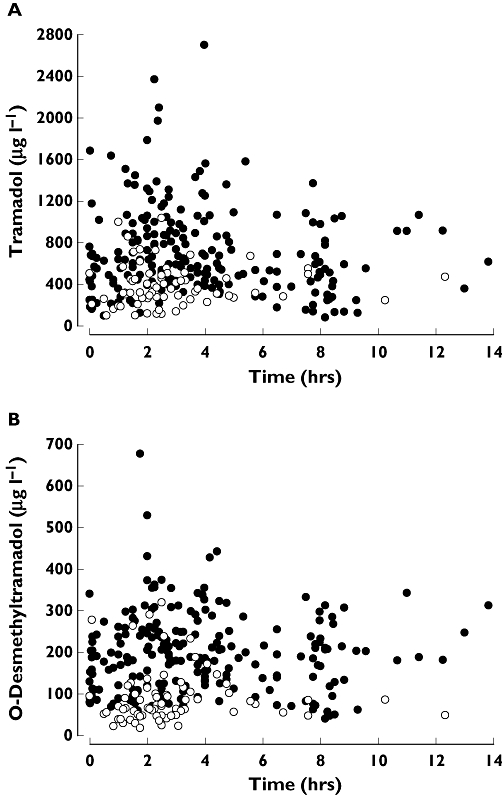
Milk (•) and plasma (○) concentration–time observations for rac-tramadol (A) and rac-O-desmethyltramadol (B) following the fourth or later 100 mg oral dose of tramadol HCl in 75 mothers
Table 2.
M : P values, average concentrations in milk, absolute infant doses and relative infant doses for rac-tramadol and rac-O-desmethyltramadol calculated from the steady-state data*
| Parameter | Number of: patients/data points | rac-Tramadol | rac-O-Desmethyltramadol |
|---|---|---|---|
| M : P | 73/73 | 2.2 (2.0, 2.4) | 2.8 (2.5, 3.1) |
| Milk Cavg (μg l−1) | 55/165 | 748 (681, 815) | 203 (188, 217) |
| Absolute infant dose (μg kg−1 day−1) | 55/165 | 112 (102, 122) | 30 (28, 32) |
| Relative infant dose | 55/165 (%)† | 2.24 (2.04, 2.44) | 0.64 (0.59, 0.69)‡ |
mean (95% CI),
assuming 100 mg tramadol HCl administered 6 hourly to a 70 kg mother (5020 μg kg −1day−1 as tramadol base),
as tramadol molar equivalents. M : P, milk to plasma ratio.
A probit plot of the molar ratio of plasma rac-tramadol : rac-O-desmethyltramadol (range 0.7–33.6) for the 73 patients with data showed a break point at a ratio of around 10, with nine mothers (12.3%) identified as having a putative CYP2D6 poor metabolizer phenotype (graph not shown). None of the women was taking other drugs that are known inhibitors of CYP2D6.
Discussion
Despite the use of tramadol for labour pain and its extensive postoperative clinical use over the last 10 years or so, there are limited data on its pharmacokinetics in both mothers and their neonates. A recent study has shown that following intramuscular administration of tramadol (100–250 mg) for pain relief during labour, both the parent drug and O-desmethyltramadol readily crossed the placenta (cord : maternal ratios of around 0.94 : 1), while in the first 12 h postpartum the elimination t1/2 values in mothers and their neonates, respectively, were 7.2 and 7 h for tramadol and 5.5 and 8.5 h for O-desmethyltramadol [11]. The authors interpreted their findings to indicate that the neonate has the complete capacity for metabolic formation of O-desmethyltramadol, but that immaturity of neonatal renal function delayed its excretion. In addition, based on Apgar scores or NACS performance in the neonates, they reported no drug-related adverse effects. Others have also reported that CYP2D6-mediated O-demethylation of tramadol is present in early neonatal life and matures by about 12–16 weeks of life [12]. There is one report of opioid withdrawal in a neonate who was exposed to maternal use of tramadol during pregnancy [13].
To our knowledge, this is the first time that a sparse sampling strategy similar to that used in population pharmacokinetic studies has been used for this type of study. This involved collecting three milk samples and one plasma sample from each of 73 breastfeeding mothers with samples distributed across a dose interval at steady-state. The data show that rac-tramadol and its active metabolite can readily transfer from maternal plasma to milk with mean steady-state M : Ps of 2.2 and 2.8, respectively. However, M : P is merely an indicator of transfer capacity and does not of itself have any value in predicting infant exposure [14]. Absolute and relative infant doses estimated from drug concentration in milk and average milk intake are generally the preferred predictors of infant exposure to drugs in milk [10].
Given that in almost all cases, we measured drug concentrations after the fourth or later oral tramadol doses, both rac-tramadol and rac-O-desmethyltramadol can be expected to be at >93% of steady-state concentration. At steady-state, the absolute infant dose of 112 μg kg−1 day−1 for rac-tramadol via milk can be compared with analgesic doses used for postoperative pain in children. One study in children aged 2–8 years used a single intravenous dose of 1000 μg kg−1[15], while another in children aged 1–6 years used a single rectal dose of 1000–2500 μg kg−1[16]. Although these are not perfect comparator groups, even when the additional 30 μg kg−1 day−1 from rac-O-desmethyltramadol is included, maximum infant exposure in our study is around 14% of a child therapeutic dose. A level of not greater than 10% of the infant therapeutic dose has been suggested as being ‘safe’ for breastfeeding [17]. The relative infant dose at steady-state was well below the notional 10% level of concern [10], and similar or less than relative infant doses for other commonly used opioid analgesics [14]. Hence the potential for adverse effects in the exposed breastfed infants in our study should be very low.
It should be noted that our method of calculating infant dose at steady-state using milk Cavg (mean and 95% CI) measured in a sparse data set collected from a large population over a dose interval is novel. Such calculations are usually derived from measurements of milk Cmax or Cavg in individuals or small groups of patients. Moreover, the sparse dataset population design can be applied without the need for population pharmacokinetic modelling.
We addressed the possibility of adverse behavioural drug-related effects in the infants by including a comparator group in our study. The comparator and tramadol-exposed infants had similar demographic characteristics, including Apgar scores at birth. Although there is no ideal instrument for the assessment of drug effects in the neonate, the NACS has been used to assess drug effects in many previous studies. Scores for the two tramadol-exposed and control groups were similar (both the median and the IQR). It should also be noted that infants from the comparator group were also exposed to maternal analgesics (opioids and NSAIDs). Within these limitations, our findings suggest that exposure to tramadol via milk did not result in additional drug-related clinically adverse effects.
Recently, there has been considerable interest in the pharmacogenetics of tramadol enantiomers, because of polymorphic metabolism to O-desmethyltramadol by CYP2D6. These studies show pharmacogenetic-related differences in both the disposition and/or pharmacodynamics of tramadol and its active metabolite [18–21]. Tramadol has also been suggested as a probe drug for CYP2D6 phenotyping [22]. Approximately 12.3% of our mothers (predominantly Caucasians) had a putative CYP2D6 poor metabolizer phenotype, which is comparable with the mean (95% CI) of 10.1 (6.8, 14.2)% reported by Pedersen et al. using the urinary ratio of (–)-O-desmethyltramadol : (+)-O-desmethyltramadol in a Danish population [22].
One limitation of our study could be that we chose to measure rac-tramadol and rac-O-desmethyltramadol, rather than their individual enantiomers. Nevertheless, given our finding of a low level of infant exposure to both rac-tramadol and rac-O-desmethyltramadol via breastmilk, differences in the disposition and pharmacodynamics of their enantiomers are unlikely to be an important influence on effects in exposed infants.
In summary, the combined absolute infant dose for rac-tramadol and its active metabolite at steady-state was about 14% of the child therapeutic dose for tramadol. In addition, the combined relative infant doses of 2.88% at steady-state, was also low, and there was no evidence for behavioural adverse effects in the exposed infants. We therefore suggest that short-term maternal use of tramadol in the post Caesarian section context is compatible with breastfeeding. However this does not obviate the need for a full individual risk-benefit analysis when tramadol is used during breastfeeding. We have also shown that a population sampling design with a sparse dataset can be used to estimate infant exposure to drugs without the need for population pharmacokinetic modelling.
Acknowledgments
This study was supported by a research grant from the Australian and New Zealand College of Anaesthetists. We wish to thank our research nurses, Tracy Bingham and Desiree Cavill, and the Department of Pharmacy at King Edward Memorial Hospital.
REFERENCES
- 1.Shipton EA. Tramadol-present and future. Anaesth Intensive Care. 2000;28:363–74. doi: 10.1177/0310057X0002800403. [DOI] [PubMed] [Google Scholar]
- 2.Scott LJ, Perry CM. Tramadol: a review of its use in perioperative pain. Drugs. 2000;60:139–76. doi: 10.2165/00003495-200060010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet. 2004;43:879–923. doi: 10.2165/00003088-200443130-00004. [DOI] [PubMed] [Google Scholar]
- 4.Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992;260:275–85. [PubMed] [Google Scholar]
- 5.Anonymous. Tramadol – IMS Chemical Kilochem Profile and IMS Midas 1994–2005. Norwalk, CT, USA: IMS Health Inc; 2007. [Google Scholar]
- 6.Hale TW, Ilett KF. Drug therapy and breastfeeding. From theory to clinical practice. 1. London: The Parthenon Publishing Group; 2002. pp. 1–94. [Google Scholar]
- 7.Anonymous. Tramal; MIMS Abbreviated Prescribing Information. MultiMedia Australia Pty Limited; 2007. E-MIMS. Ver 5.00.0248. [Google Scholar]
- 8.Lawrence RA, Lawrence RM. Biochemistry of human milk. In: Lawrence RA, Lawrence RM, editors. Breastfeeding; A Guide for the Medical Profession. 6. St Louis, USA: Mosby; 2005. pp. 105–70. [Google Scholar]
- 9.Amiel-Tison C, Barrier G, Shnider SM, Levinson G, Hughes SC, Stefani SJ. A new neurologic and adaptive capacity scoring system for evaluating obstetric medications in full-term newborns. Anesthesiology. 1982;56:340–50. doi: 10.1097/00000542-198205000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Bennett PN. Use of the monographs on drugs. In: Bennett PN, editor. Drugs and human lactation. 2. Amsterdam: Elsevier; 1996. pp. 67–74. [Google Scholar]
- 11.Claahsen-van der Grinten HL, Verbruggen I, van den Berg PP, Sporken JM, Kollee LA. Different pharmacokinetics of tramadol in mothers treated for labour pain and in their neonates. Eur J Clin Pharmacol. 2005;61:523–9. doi: 10.1007/s00228-005-0955-0. [DOI] [PubMed] [Google Scholar]
- 12.Allegaert K, Van den Anker JN, Verbesselt R, de Hoon J, Vanhole C, Tibboel D, Devlieger H. O-demethylation of tramadol in the first months of life. Eur J Clin Pharmacol. 2005;61:837–42. doi: 10.1007/s00228-005-0045-3. [DOI] [PubMed] [Google Scholar]
- 13.Meyer FP, Rimasch H, Blaha B, Banditt P. Tramadol withdrawal in a neonate. Eur J Clin Pharmacol. 1997;53:159–60. doi: 10.1007/s002280050356. [DOI] [PubMed] [Google Scholar]
- 14.Ilett KF, Kristensen JH. Drug use and breastfeeding. Expert Opin Drug Saf. 2005;4:745–68. doi: 10.1517/14740338.4.4.745. [DOI] [PubMed] [Google Scholar]
- 15.Garrido MJ, Habre W, Rombout F, Troconiz IF. Population pharmacokinetic/pharmacodynamic modelling of the analgesic effects of tramadol in pediatrics. Pharm Res. 2006;23:2014–23. doi: 10.1007/s11095-006-9049-7. [DOI] [PubMed] [Google Scholar]
- 16.Zwaveling J, Bubbers S, van Meurs AH, van Schoemaker RCHI, Vermeij P, Burggraaf J. Pharmacokinetics of rectal tramadol in postoperative paediatric patients. Br J Anaesth. 2004;93:224–7. doi: 10.1093/bja/aeh178. [DOI] [PubMed] [Google Scholar]
- 17.Ito S. Drug therapy: drug therapy for breast-feeding women. N Engl J Med. 2000;343:118–26. doi: 10.1056/NEJM200007133430208. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Quetglas E, Azanza JR, Sadaba B, Munoz MJ, Gil I, Campanero MA. Pharmacokinetics of tramadol enantiomers and their respective phase I metabolites in relation to CYP2D6 phenotype. Pharmacol Res. 2007;55:122–30. doi: 10.1016/j.phrs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Fliegert F, Kurth B, Gohler K. The effects of tramadol on static and dynamic pupillometry in healthy subjects – the relationship between pharmacodynamics, pharmacokinetics and CYP2D6 metaboliser status. Eur J Clin Pharmacol. 2005;61:257–66. doi: 10.1007/s00228-005-0920-y. [DOI] [PubMed] [Google Scholar]
- 20.Gan SH, Ismail R, Wan Adnan WA, Wan Z. Correlation of tramadol pharmacokinetics and CYP2D6*10 genotype in Malaysian subjects. J Pharm Biomed Anal. 2002;30:189–95. doi: 10.1016/s0731-7085(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 21.Slanar O, Nobilis M, Kvetina J, Mikoviny R, Zima T, Idle JR, Perlik F. Miotic action of tramadol is determined by CYP2D6 genotype. Physiol Res. 2006;56:129–36. doi: 10.33549/physiolres.930872. [DOI] [PubMed] [Google Scholar]
- 22.Pedersen RS, Damkier P, Brosen K. Tramadol as a new probe for cytochrome P450 2D6 phenotyping: a population study. Clin Pharmacol Ther. 2005;77:458–67. doi: 10.1016/j.clpt.2005.01.014. [DOI] [PubMed] [Google Scholar]


