Abstract
AIMS
To investigate the time course of the increase in 4β-hydroxycholesterol and carbamazepine plasma concentrations during treatment of paediatric patients with epilepsy.
METHODS
Eight paediatric patients with newly diagnosed epilepsy were studied. Blood samples were drawn before and after about 1, 2, 4, 8 and 16 weeks of carbamazepine treatment. The plasma concentrations of 4β-hydroxycholesterol were determined by gas chromatography–mass spectrometry and carbamazepine and its epoxide metabolite by high-performance liquid chromatography.
RESULTS
The basal plasma concentrations of 4β-hydroxycholesterol showed a large range of observed values between 18 and 99 ng ml−1. Carbamazepine treatment increased mean plasma 4β-hydroxycholesterol significantly already after 1 week of treatment (from 43 to 80 ng ml−1, P < 0.001). 4β-Hydroxycholesterol concentrations continued to increase until at least 8 weeks of treatment and the concentrations in the final samples (8–23 weeks of treatment) varied between 122 and 494 ng ml−1. Plasma concentrations of carbamazepine and its epoxide metabolite reached steady state at 1–2 weeks after last dose change.
CONCLUSIONS
Carbamazepine treatment of paediatric patients with epilepsy resulted in an induction of CYP3A4/5 and a concomitant increase in plasma 4β-hydroxycholesterol. Whereas the induction of CYP3A4/5 was apparently complete after 1–2 weeks, the increase in 4β-hydroxycholesterol continued for several weeks. Thus CYP3A4 activity is not the only determinant of the circulating level of 4β-hydroxycholesterol. Additional factors such as transport and storage or presence of another enzyme may thus be of importance.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
CYP3A4 converts cholesterol into 4β-hydroxycholesterol.
We have suggested that 4β-hydroxycholesterol could be used as a clinical marker for CYP3A4 activity aiding in dose adjustments.
The kinetics of 4β-hydroxycholesterol formation is not known, however, and must be determined in order to establish under what conditions 4β-hydroxycholesterol can be used as a CYP3A marker.
WHAT THIS STUDY ADDS
The concentration of 4β-hydroxycholesterol increases very slowly during CYP3A4/5 induction in paediatric patients.
Whereas induction of CYP3A4/5 was apparently complete within 1–2 weeks of carbamazepine treatment, plasma 4β-hydroxycholesterol levels continued to increase until at least 8 weeks of treatment.
Keywords: 4β-hydroxycholesterol, carbamazepine, CYP3A4, CYP3A5, epilepsy
Introduction
Carbamazepine (CBZ) is a common antiepileptic drug that induces certain members of the cytochrome P450 family [1]. The drug can induce its own metabolism, but also that of other drugs that are substrates of the affected enzymes [2]. These enzymes are involved in the disposition of endogenous compounds as well. We have recently shown that adult patients treated with CBZ have significantly increased plasma concentrations (eight- to 10-fold) of the oxysterol 4β-hydroxycholesterol [3]. We could show that 4β-hydroxycholesterol is formed from cholesterol by cytochrome P4503A4 (CYP3A4) (Figure 1). Treatment of patients with a weak inducer of CYP3A4, ursodeoxycholic acid, resulted in a small (50%) increase in plasma 4β-hydroxycholesterol concentration. In another study we have shown that treatment of patients with rifampicin (600 mg day−1 for 1 week) led to a 246% increase in plasma 4β-hydroxycholesterol concentration [4]. Investigation of the metabolism of 4β-hydroxycholesterol has revealed an unexpectedly slow elimination from the circulation, probably due to slow 7α-hydroxylation [5]. The mean half-life of injected deuterium-labelled 4β-hydroxycholesterol was 62 h, which is significantly longer than for other related oxysterols [6–8]. The physiological role of 4β-hydroxycholesterol is not known, but in vitro experiments have shown that it activates the nuclear receptor liver X receptor alpha (LXRα) [9]. Whether such activation also occurs in vivo is not known. We have earlier proposed 4β-hydroxycholesterol as a potential clinical marker for CYP3A4 activity [5]. Other markers for this enzyme activity are drugs such as quinine [10] or endogenous compounds such as the ratio of 6β-hydroxycortisol to cortisol in urine. These markers have some disadvantages. As cortisol secretion shows pronounced diurnal variation, the 6β-hydroxycortisol to cortisol ratio is usually determined in a 24-h urine collection. The disadvantage with the use of drugs as markers is that the subject has to take the drug and come repeatedly to the hospital to draw blood. These inconveniences would be overcome by using an endogenous compound as a marker. In the present study we have investigated the plasma concentration of 4β-hydroxycholesterol in children treated with CBZ for epilepsy to evaluate 4β-hydroxycholesterol as a potential marker for CYP3A activity. As it has been proposed that α-tocopherol is metabolized by CYP3A4 [11], this vitamin was also determined before and after administration of CBZ.
Figure 1.
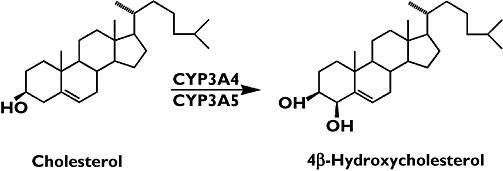
Conversion of cholesterol to 4β-hydroxycholesterol catalysed by cytochrome P450 3A4 and 3A5
Methods
Study subjects
The catchment area has around 100 000 inhabitants in the age group 0–18 years and 98% of the children with epilepsy are treated at our clinic. Ten consecutive children with newly diagnosed epilepsy and without previous treatment for epilepsy (except one boy) were included in the study. Six boys and two girls ranging in age from 1 year 4 months to 17 years 8 months completed the study. Two children could not complete the study due to neurodevelopmental disorders, which made it difficult to obtain the blood samples.
The diagnosis of epilepsy was based on the International League against Epilepsy classification. All patients had had at least one EEG recorded and six of them also a magnetic resonance imaging (MRI) or computed tomography (CT) scan. In all patients routine blood tests [haemoglobin, white blood cell count, platelet count, liver function tests (aspartate aminotransferase, alanine aminotransferase)] were performed. The levels of 4β-hydroxycholesterol were determined before treatment and after about 1, 2, 4, 8 and 16 weeks of treatment. At the same time blood for analysing fat-soluble vitamins was obtained.
Six children were of Swedish origin, one child originated from Syria and Lebanon (patient D) and one child from China (patient G).
Slow-release formulations of CBZ (either Trimonil retard or Hermolepsin retard) were used in all subjects in accordance with Swedish clinical practice.
Patient A
Male, 7 years 9 months. He was diagnosed with partial epilepsy similar to benign epilepsy of childhood (BECT) but did not fulfil the criteria completely as the centrotemporal spikes (Rolandic spikes) were not absolutely typical for BECT, whereas MRI scan and laboratory tests were normal. He was treated with CBZ in doses ranging from 5 to 10 mg kg−1 body weight. He had one additional seizure after the beginning of treatment, and he experienced no side-effects.
Patient B
Female, 15 years 9 months. She had had complex partial seizures (CPS) for 5 years. The EEG showed right-sided focal epileptiform discharges; no MRI/CT scan was performed. The laboratory tests were normal. She was treated with CBZ in doses from 5 to 10 mg kg−1 body weight. She experienced drowsiness in the beginning of treatment.
Patient C
Male, 8 years 2 months. The boy had had diabetes mellitus prior to the onset of epilepsy. He was diagnosed with seizures (CPS) unrelated to hypoglycaemia. The EEG was within normal limits on several occasions. No MRI/CT scan was performed. Laboratory tests were normal. He was treated with CBZ in doses ranging from 5 to 10 mg kg−1 body weight. The medication had a mood-stabilizing effect. He experienced no side effects.
Patient D
Male, 11 years 1 month. The boy had had generalized tonic-clonic seizures (GTC) since 1 year before beginning of treatment. The EEG was within normal limits. The MRI scan showed a small lipoma on the left side close to the midline without clinical significance. The laboratory tests were normal. He was treated with CBZ in doses ranging from 5 to 23 mg kg−1 body weight. He developed a rash after a few weeks of medication, which was considered to be unrelated to the CBZ treatment.
Patient E
Male, 1 year 4 months. Initially he had focal febrile seizures, later nonfebrile GTC. He did not become seizure free on medication, and treatment has changed after the study period was completed. The EEG, MRI scan and laboratory tests were all normal. He was treated with CBZ in doses ranging from 7 to 30 mg kg−1 body weight.
Patient F
Male, 17 years 3 months. He had had a history of 5 months with GTC. The EEG showed left-sided focal epileptiform discharge. MRI scan and laboratory tests were within normal limits. He was treated with CBZ in doses ranging from 5 to 10 mg kg−1 body weight. He became seizure free, but because of experienced excessive tiredness and decreased ability to concentrate at school, the dose was reduced to 8 mg kg−1 body weight.
Patient G
Female, 11 years 3 months. She was adopted by Swedish parents at the age of 7 months. She had a 1-year history of CPS. The EEG showed spikes in the centreo-temporal region. The MRI scan and laboratory tests were within normal limits. The doses of CBZ ranged from 5 to 10 mg kg−1 body weight. Initially she experienced drowsiness.
Patient H
Male, 10 years 1 month. He had had a cerebral infarction on the left side during the neonatal period and seizures treated with phenobarbital. At 6 months old he had developed infantile spasm, which was treated with Vigabatrin until 18 months of age. Since then he had had normal psychomotor development and had been healthy until the present history of GTC. The EEG showed abundant left-sided epileptiform activity. The doses of CBZ ranged from 5 to 10 mg kg−1 body weight. Initially he experienced excessive tiredness.
Determination of CYP3A5 genotype
A venous blood sample (5 ml) was obtained from each patient and DNA was prepared using the QIAamp DNA Blood Midi Kit (Qiagen Ltd, Crawley, UK). All samples were analysed for the single nucleotide polymorphism A6986G (CYP3A5*3) as described before [12]. When the CYP3A5*3 allele was not detected, the allele was designated CYP3A5*1.
Plasma analyses
4β-Hydroxycholesterol was determined by isotope-dilution gas chromatography-mass spectrometry using a deuterium-labelled internal standard as described before [3]. The within-day coefficient of variation (CV) was 4.6% (at 28 ng ml−1) and the between-day CV was 8.3% (at 28 ng ml−1). 4β-Hydroxycholesterol 1 ng ml−1 corresponds to 2.5 nmol l−1.
Retinol, α- and γ-tochopherol were determined by high-performance liquid chromatography (HPLC) according to Catignani and Bieri [13]. A fluorescence detector was attached in series with the UV-detector for determination of γ-tocopherol. The CVs for retinol, α-tocopherol and γ-tocopherol were 7.2% (at 2.4 μmol l−1), 8.5% (at 29 μmol l−1) and 5.5% (at 2.2 μmol l−1), respectively.
Cholesterol and high-density lipoprotein (HDL)-cholesterol were determined using commercial enzymatic methods, Cholesterol Oxidase Phenol 4-Aminoantipyrine Peroxidase (CHOD-PAPP and HDL-C plus 2nd generation (Roche Diagnostics, Mannheim, Germany) run on a Roche/Hitachi Modular instrument. The CV was 1.3% (at 5.0 mmol l−1) for cholesterol and 1.7% (at 1.3 mmol l−1) for HDL-cholesterol.
Carbamazepine and carbamazepine-10,11-epoxide were determined in plasma by HPLC as described previously [14].
Statistical calculations were carried out using the software Statistica version 7.1 (StatSoft Inc., Tulsa, OK, USA; http://www.statsoft.com).
All carers of the subjects gave written, informed consent to participate, and the study was approved by the local research ethics committee at Karolinska Institute.
Results
CYP3A5 genotype
All patients were genotyped for the CYP3A5*3 allele and all except one were homozygous for this allele. Patient G was heterozygous (CYP3A5*1/*3).
Carbamazepine and carbamazepine-10,11-epoxide
The patients received CBZ in a dose of approximately 5 mg kg−1 day−1 during the first week, after which the dose was increased to10 mg kg−1 day−1 as a final dose. The concentration of CBZ in plasma rose to 10–30 μmol l−1 in all subjects within 1 week of treatment, as can be seen for the individual patients in Figure 2. The plasma concentration of the metabolite carbamazepine-10,11-epoxide, formed by CYP3A4/5, reached steady-state levels after about 1 week (Figure 2), indicating that induction of CYP3A4/5 was complete within this period of time.
Figure 2.
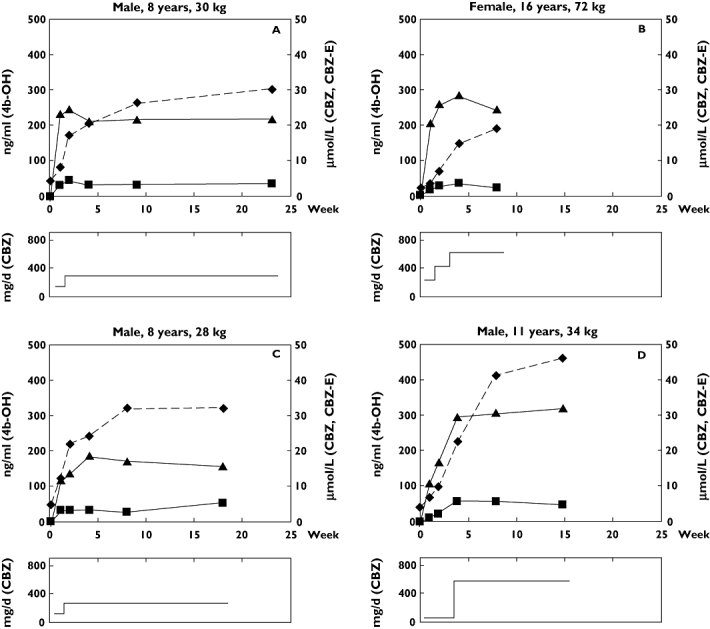
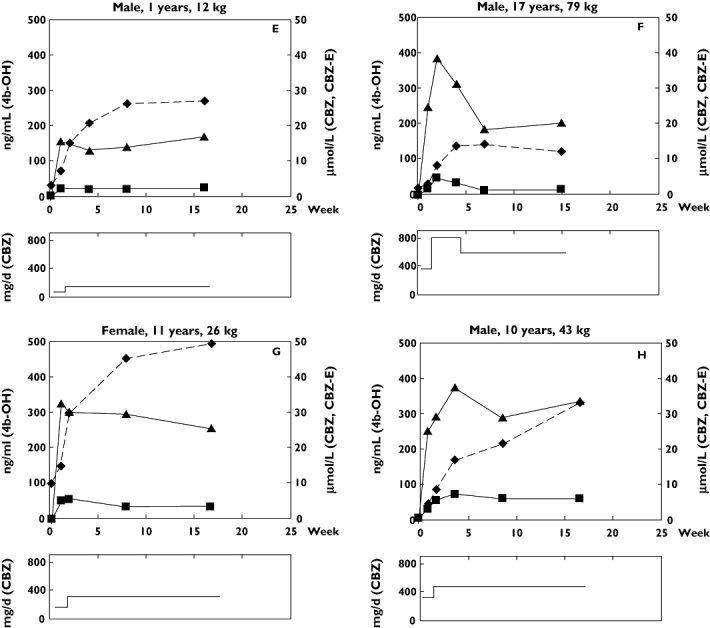
(a) Upper panels: Concentrations of 4β-hydroxycholesterol (4b-OH) (diamonds and dashed line), carbamazepine (CBZ, filled triangles) and carbamazepine-10,11-epoxide (CBZ-E, filled squares) for patients A, B, C and D. Lower panels: Daily dose (mg day−1) of carbamazepine (CBZ) for patients A, B, C and D (b) Upper panels: Concentrations of 4β-hydroxycholesterol (4b-OH) (dashed line), carbamazepine (CBZ, filled triangles) and carbamazepine-10,11-epoxide (CBZ-E, filled squares) for patients E, F, G and H. Lower panels: Daily dose (mg day−1) of carbamazepine (CBZ) for patients E, F, G and H
Cholesterol and HDL-cholesterol
Plasma cholesterol and HDL-cholesterol concentrations in samples taken before CBZ treatment and after completion of the study were unchanged (Table 1).
Table 1.
Mean plasma concentrations (±SD) of total cholesterol (mmol l−1) and high-density lipoprotein (HDL)-cholesterol (mmol l−1) in eight patients before and after 8–23 weeks of carbamazepine treatment
| Before treatment | During treatment | P-value | |
|---|---|---|---|
| Cholesterol | 3.72 ± 0.76 | 4.28 ± 0.71 | NS |
| HDL-cholesterol | 1.47 ± 0.48 | 1.56 ± 0.39 | NS |
NS, Not significant.
4β-Hydroxycholesterol
The basal plasma concentration of 4β-hydroxycholesterol showed a large range of observed values between 18 and 99 ng ml−1. CBZ treatment resulted in a significant increase in plasma 4β-hydroxycholesterol concentrations already after 1 week (P = 0.0009) (Figure 2). The concentration of 4β-hydroxycholesterol increased with time during CBZ treatment and levelled off at about 8 weeks when the concentration was almost eightfold higher than the basal level (Table 2). The concentration of 4β-hydroxycholesterol was not statistically different at 15–23 weeks compared with 7–9 weeks of treatment (P = 0.50). The 4β-hydroxycholesterol concentration curves vs. time during CBZ treatment are shown for all eight patients in Figure 3.
Table 2.
Mean plasma concentrations (±SD) of 4β-hydroxycholesterol, retinol, α- and γ-tocopherol before and after treatment for 7–9 weeks and 15–23 weeks with carbamazepine in patients with epilepsy
| Before treatment (n = 8) | Weeks of treatment 7–9 weeks (P-value*) (n = 8) | 15–23 weeks (P-value*) (n = 7) | |
|---|---|---|---|
| 4β-Hydroxycholesterol (ng ml−1) | 42.7 ± 25.4 | 296 ± 104 (0.00006) | 321 ± 125 (0.0005) |
| Retinol (μmol l−1) | 1.09 ± 0.30 | 1.39 ± 0.38 (0.029) | 1.43 ± 0.46 (NS) |
| α-Tocopherol (μmol l−1) | 17.9 ± 4.87 | 21.3 ± 4.01 (0.036) | 21.0 ± 6.95 (NS) |
| γ-Tocopherol (μmol l−1) | 1.51 ± 0.61 | 1.69 ± 0.57 (NS) | 1.61 ± 0.95 (NS) |
Compared with ‘Before treatment’ (T-test for dependent samples). NS, Not significant.
Figure 3.
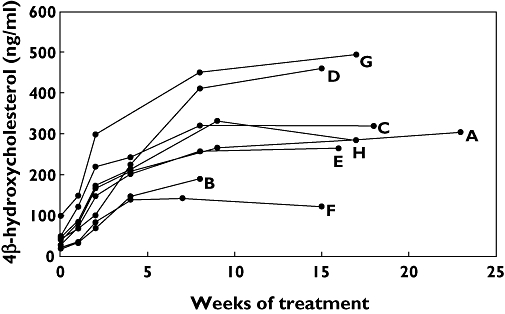
Plasma concentration of 4β-hydroxycholesterol (ng ml−1) in patients A–H as a function of time of treatment with carbamazepine
Fat-soluble vitamins in plasma
The fat-soluble vitamins, retinol, α-tocopherol and γ-tocopherol were determined in plasma from the eight patients before and after 8–23 weeks of CBZ treatment. The concentrations of retinol and α-tocopherol both increased significantly after 8 weeks of treatment, whereas the change in γ-tocopherol concentration was not significant (Table 2). There were no statistically significant changes in concentrations between 8 weeks and 15–23 weeks of treatment for any of the vitamins.
Discussion
We have earlier proposed that the oxysterol 4β-hydroxycholesterol may be a potential clinical marker for CYP3A4/5 activity. In a recent investigation we were able to show that endogenous 4β-hydroxycholesterol and the exogenous drug quinine gave similar results when approximately 450 healthy subjects were screened for CYP3A4/5 activity [23]. In that study we also showed that the plasma concentration of 4β-hydroxycholesterol was related to the number of active CYP3A5*1 alleles. This functional allele is frequent in Africans but rare in Whites [12]. In the present study of children we have found only one subject with one active CYP3A5*1 allele and we have therefore not been able to analyse its effect on 4β-hydroxycholesterol concentrations.
We determined the time course of the increase in 4β-hydroxycholesterol concentrations after treatment of eight paediatric patients suffering from epilepsy with CBZ, a drug known to induce CYP3A4. Only eight subjects were studied, but it should be remembered that there are difficulties in performing research with venepuncture in paediatric populations. The small number of study subjects is obviously a limitation when it comes to generalizations of the observations of the present study, especially in extrapolating from a paediatric to an adult population. However, CBZ treatment resulted in an increase in plasma 4β-hydroxycholesterol concentrations in all patients (5–10-fold increase compared with the basal concentration). Unexpectedly, this increase was much slower than the increase in plasma CBZ or carbamazepine epoxide (which is formed by CYP3A4/5). CYP3A4/5 induction was essentially complete within 1–2 weeks, as judged by plasma concentrations of CBZ and its metabolite. This completion of induction within 1–2 weeks is in accord with our earlier observation [15]. In contrast, 4β-hydroxycholesterol continued to increase until at least 8 weeks after the initiation of treatment. We have shown earlier that the half-life of 4β-hydroxycholesterol is long compared with most other oxysterols [5] and that 4β-hydroxycholesterol is transported slowly over biological membranes [16]. However, these facts cannot explain the slow increase in plasma concentration following initiation of CBZ treatment. We have no explanation for this at present, but can only speculate that there may be a slow transfer of the oxysterol to other saturable compartments. In an earlier study, where deuterium-labelled cholesterol was administered to a healthy volunteer, we observed distribution into different compartments with different elimination characteristics [6]. We have no data on the distribution of 4β-hydroxycholesterol in different tissues after induction of CYP3A4/5, but such measurements would be valuable. It is not known whether 4β-hydroxycholesterol is transported between different compartments by active transport, but it should be remembered that drugs that induce CYP3A4 also induce transporters such as P-glycoprotein, ABCG1 and others [1, 4, 17, 18]. Such transporters could influence steady-state concentration and elimination kinetics if they participate in 4β-hydroxycholesterol transport. We cannot exclude the possibility that the slow increase in 4β-hydroxycholesterol concentration following CBZ treatment is due to one or more additional inducible enzymes capable of forming this metabolite from cholesterol. We have previously studied recombinant CYP1A2, CYP2C9, CYP2B6 and CYP2D6, but none of these enzymes was capable of converting cholesterol into 4β-hydroxycholesterol in vitro[3].
The magnitude of the increase in 4β-hydroxycholesterol (5–10-fold) is larger than usually observed for CYP3A markers following induction by CBZ. Urinary excretion of 6β-hydroxycortisol, a frequently used CYP3A marker, increased 3.5-fold in nine children treated with CBZ [19], and the 6β-hydroxycortisol:cortisol ratio was 2.6-fold higher in adult Chinese with epilepsy treated with CBZ compared with untreated controls [20]. The large increase in 4β-hydroxycholesterol concentration following CBZ treatment could theoretically be due to inhibition of its further metabolism or transport. We have previously shown, however, that the half-life of elimination of 4β-hydroxycholesterol from the circulation was approximately the same in a patient treated with CBZ (t1/2 = 52 h) compared with two untreated controls (t1/2 = 60 h and t1/2 = 64 h, respectively) [5]. Whether or not the increased levels of 4β-hydroxycholesterol could have any impact on the health of the treated subjects is not known. It has been reported that 4β-hydroxycholesterol activates LXRαin vitro[9], but there are no in vivo studies on the effects of 4β-hydroxycholesterol.
There is very large interindividual variation in hepatic expression of CYP3A4 (>50-fold) and a 20-fold variation in CYP3A4-mediated drug clearance, mainly attributed to genetic factors [21]. In the present study we have also noted a large variation in pretreatment levels of 4β-hydroxycholesterol. Seven of the eight subjects were homozygous for CYP3A5*3 and the eighth subject was a *1/*3 heterozygote; thus only one individual expressed the CYP3A5 enzyme. This could not have contributed to the variation in expression of CYP3A indicated by the variation in the plasma level of 4β-hydroxycholesterol. Whether the variation was due to genetic or environmental factors is difficult to judge, since several dietary factors may induce or inhibit CYP3A activity. The diets during the study were not recorded.
In the year 2000 it was suggested that CYP3A4 might be responsible for the initial metabolism of α-tocopherol [11]. Later, the same authors showed that the P450 isoform 4F2 was a more likely candidate for the initial metabolic transformation of both α- and γ-tocopherol [22]. In the present study CYP3A4 was induced significantly, and if this P450 isoform were the most important enzyme for α-tocopherol metabolism, we would expect a decrease in α-tocopherol during the first few weeks of CBZ treatment. There was, however, no such decrease in α-tocopherol during CBZ treatment. On the contrary, a borderline significant increase in α-tocopherol concentration was seen (P = 0.036), arguing against the involvement of CYP3A4/5 in the metabolism of α-tocopherol in vivo in humans. A parallel increase in retinol was observed during CBZ treatment (P = 0.029), whereas the change in γ-tocopherol concentration was not statistically significant.
To conclude, 4β-hydroxycholesterol increased following initiation of treatment with CBZ, during which induction of CYP3A4/5 and other enzymes is known to occur. However, the magnitude and especially the time course of the observed effect cannot be explained solely by CYP3A4 induction, but effects on other enzymes and/or transporters offer other possibilities to explain this. Studies to characterize further the formation and metabolism of 4β-hydroxycholesterol are in progress.
Acknowledgments
This work was supported by grants from Magnus Bergvalls Stiftelse, Karolinska Institutet Research Foundations, Swedish Research Council, Medicine (3902), Insamlingsstiftelsen Barnens Sjukhus, Karolinska Universitetssjukhuset, Huddinge, Stiftelsen Margarethahemmet and ALF (SLL 560177).
REFERENCES
- 1.Oscarson M, Zanger UM, Rifki OF, Klein K, Eichelbaum M, Meyer UA. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin Pharmacol Ther. 2006;80:440–56. doi: 10.1016/j.clpt.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 2.Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61:246–55. doi: 10.1111/j.1365-2125.2005.02529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodin K, Bretillon L, Aden Y, Bertilsson L, Broomé U, Einarsson C, Diczfalusy U. Antiepileptic drugs increase plasma levels of 4β-hydroxycholesterol in humans. Evidence for involvement of cytochrome P450 3A4. J Biol Chem. 2001;276:38685–9. doi: 10.1074/jbc.M105127200. [DOI] [PubMed] [Google Scholar]
- 4.Marschall H-U, Wagner M, Zollner G, Fickert P, Diczfalusy U, Gumhold J, Silbert D, Fuchsbichler A, Benthin L, Grundström R, Gustafsson U, Sahlin S, Einarsson C, Trauner M. Complementary stimulation of hepatobiliary transport and detoxification systems by rifampicin and ursodeoxycholic acid in humans. Gastroenterology. 2005;129:476–85. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Bodin K, Andersson U, Rystedt E, Ellis E, Norlin M, Pikuleva I, Eggertsen G, Björkhem I, Diczfalusy U. Metabolism of 4β-hydroxycholesterol in humans. J Biol Chem. 2002;277:31534–40. doi: 10.1074/jbc.M201712200. [DOI] [PubMed] [Google Scholar]
- 6.Meaney S, Hassan M, Sakinis A, Lütjohann D, von Bergmann K, Wennmalm Å, Diczfalusy U, Björkhem I. Evidence that the major oxysterols in human circulation originate from distinct pools of cholesterol: a stable isotope study. J Lipid Res. 2001;42:70–8. [PubMed] [Google Scholar]
- 7.Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lütjohann D, Diczfalusy U, Björkhem I. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J Lipid Res. 1999;40:1417–25. [PubMed] [Google Scholar]
- 8.Björkhem I, Lütjohann D, Diczfalusy U, Ståhle L, Ahlborg G, Wahren J. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res. 1998;39:1594–600. [PubMed] [Google Scholar]
- 9.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature. 1996;383:728–31. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 10.Mirghani RA, Ericsson O, Tybring G, Gustafsson LL, Bertilsson L. Quinine 3-hydroxylation as a biomarker reaction for the activity of CYP3A4 in man. Eur J Clin Pharmacol. 2003;59:23–8. doi: 10.1007/s00228-003-0575-5. [DOI] [PubMed] [Google Scholar]
- 11.Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun. 2000;277:531–4. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 12.Mirghani RA, Sayi J, Aklillu E, Allqvist A, Jande M, Wennerholm A, Ericksen J, Herben VMM, Jones BC, Gustafsson LL, Bertilsson L. CYP3A5 genotype has significant effect on quinine 3-hydroxylation in Tanzanians, who have lower total CYP3A activity than a Swedish population. Pharmacogenet Genomics. 2006;16:637–45. doi: 10.1097/01.fpc.0000230411.89973.1b. [DOI] [PubMed] [Google Scholar]
- 13.Catignani GL, Bieri JG. Simultaneous determination of retinol and α-tocopherol in serum or plasma by liquid chromatography. Clin Chem. 1983;29:708–12. [PubMed] [Google Scholar]
- 14.Tomson T, Svensson JO, Hilton-Brown P. Relationship of intraindividual dose to plasma concentration of carbamazepine: indication of dose-dependent induction of metabolism. Ther Drug Monit. 1989;11:533–9. [PubMed] [Google Scholar]
- 15.Bertilsson L, Höjer B, Tybring G, Osterloh J, Rane A. Autoinduction of carbamazepine metabolism in children by a stable isotope technique. Clin Pharmacol Ther. 1980;27:83–8. doi: 10.1038/clpt.1980.13. [DOI] [PubMed] [Google Scholar]
- 16.Meaney S, Bodin K, Diczfalusy U, Björkhem I. On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res. 2002;43:2130–5. doi: 10.1194/jlr.m200293-jlr200. [DOI] [PubMed] [Google Scholar]
- 17.Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–7. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- 18.Engel T, Kannenberg F, Fobker M, Nofer J-R, Bode G, Lueken A, Assman G, Seedorf U. Expression of ATP binding cassette-transporter ABCG1 prevents cell death by transporting cytotoxic 7β-hydroxycholesterol. FEBS Lett. 2007;581:1673–80. doi: 10.1016/j.febslet.2007.03.038. [DOI] [PubMed] [Google Scholar]
- 19.Moreland TA, Park BK, Rylance GW. Microsomal enzyme induction in children: the influence of carbamazepine treatment on antipyrine kinetics, 6β-hydroxycortisol excretion and plasma gamma-glutamyltranspeptidase activity. Br J Clin Pharmacol. 1982;14:861–5. doi: 10.1111/j.1365-2125.1982.tb02050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomlinson B, Young RP, Ng MC, Anderson PJ, Kay R, Critchley JA. Selective liver enzyme induction by carbamazepine and phenytoin in Chinese epileptics. Eur J Clin Pharmacol. 1996;50:411–5. doi: 10.1007/s002280050132. [DOI] [PubMed] [Google Scholar]
- 21.Eichelbaum M, Burk O. CYP3A genetics in drug metabolism. Nat Med. 2001;7:285–7. doi: 10.1038/85417. [DOI] [PubMed] [Google Scholar]
- 22.Sontag TJ, Parker RS. Cytochrome P450 ω-hydroxylase pathway of tocopherol catabolism. J Biol Chem. 2002;277:25290–6. doi: 10.1074/jbc.M201466200. [DOI] [PubMed] [Google Scholar]
- 23.Diczfalusy U, Miura J, Roh HK, Mirghani RA, Sayi J, Larsson H, Bodin KG, Allquist A, Jande M, Kim JW, Aklillu E, Gustafsson LL, Bertilsson L. 4B-Hydroxycholesterol is a new endogenous CYP3A marker-relationship to CYP3A5 genotype, quinine 3-hydroxylation and gender in Koreans, Swedes and Tanzanians. Pharmacogenet Genom; in press. [DOI] [PubMed]


