Abstract
AIMS
Adherence to antihypertensive medication is essential for adequate long-term control of blood pressure (BP). This study investigated different methods of measuring adherence in hypertensive patients.
METHODS
Patients were included if BP was insufficiently controlled on monotherapy. After a placebo period patients were treated with trandolapril 2 mg/verapamil SR 180 mg (TV). BP was determined using a mercury sphygmomanometer and ambulatory BP monitoring. Adherence was measured by capsule counting, electronic registration of pill-box openings and by measuring serum bromide concentrations. Potassium bromide was added to each TV capsule.
RESULTS
Thirty patients participated in the study. Treatment with TV significantly lowered office BP and ambulatory BP.
Results for electronic monitoring and adherence based on bromide measurements were comparable. Adherence was slightly higher when assessed by capsule counting.
CONCLUSIONS
Measuring serum bromide concentrations may be suitable for assessment of adherence to drug therapy giving comparable results to electronic monitoring. Using capsule counting, electronic monitoring and measurement of bromide concentrations, nonadherent patients were identified.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Insufficient drug adherence is an important reason for inadequate blood pressure control.
Currently, methods that measure drug adherence objectively are lacking. Objective methods are needed to help improve blood pressure control and outcome in hypertensive patients.
WHAT THIS STUDY ADDS
Potassium bromide added to antihypertensive drugs can be used to monitor drug adherence in individual patients.
However, although this method is objective, it is rather time-, cost- and work-consuming.
Keywords: bromide, drug adherence, hypertension
Introduction
Hypertension is one of the most important risk-factors for cardiovascular morbidity and mortality. In general, high blood pressure (BP) responds well to drug treatment. However, it is often challenging for patients to take antihypertensive drugs for many years, especially if drug intake is associated with side-effects while the increased BP itself is asymptomatic.
Adherence to a medication regimen, defined as the extent to which patients take prescribed medications [1], is a major factor determining the success of hypertension treatment. In chronic conditions, including hypertension, adherence to the prescribed drug regimen is often low. Adherence is difficult to measure and although several methods have been described and tested [1], objective methods to measure drug-adherence are needed. We have previously documented that potassium bromide has pharmacokinetic properties that may make it a useful marker to estimate drug intake [2].
The aim of the present study was to examine whether the ‘bromide-method’ can be considered to be at least of similar quality as electronic monitoring (MEMS).
Methods
Patients were included if their diastolic BP (DBP) was ≥95 mmHg and/or systolic (SBP) ≥160 mmHg despite at least 4 weeks of monotherapy. Patients were informed that the purpose of the study was to see if BP could be adequately lowered with a combination of two antihypertensive drugs. They were not informed that the study was actually designed to assess adherence to drug therapy, as this would possibly affect study outcome. The study was approved by the Hospital Ethics Committee and written informed consent was obtained.
Antihypertensive medication was stopped. Patients received a placebo for 4 weeks and then treatment with the combination of trandolapril 2 mg and verapamil SR 180 mg (TV) once daily was started and continued for 20 weeks. To each TV capsule 30 mg potassium bromide was added. Office blood pressure (OBP) was measured with a mercury sphygmomanometer every 2 weeks during the placebo period and at weeks 2, 4, 8, 12, 16 and 20 of the treatment period. Ambulatory BP monitoring was carried out 2 weeks after starting placebo and 8 and 16 weeks after starting drug treatment.
Adherence was measured using three methods: capsule counting, electronic monitoring, and measuring serum bromide concentrations. Adherence based on capsule counting was measured by dividing the number of capsules taken by the expected number of capsules taken Adherence was defined as ‘good’, when the drug intake was ≥80% based on capsule counting.
The second method for measuring adherence was electronic monitoring. Adherence was calculated as the number of times the container was opened divided by the number of days the capsules should have been taken. It was defined as ‘good’ when adherence was ≥80% based on electronic monitoring.
The third method for assessment of adherence was measuring serum bromide concentrations. Blood samples for bromide concentrations were taken at clinic visits throughout the study period: every 2 weeks during the placebo period and at weeks 2, 4, 8, 12, 16 and 20 of the treatment period.
Recently we studied the pharmacokinetic properties of bromide in a group of 24 healthy volunteers using different amounts of potassium bromide [2]. The mean increase in bromide concentrations in the eight volunteers taking 24 mg potassium bromide daily was used as the cut-off level for 80% adherence: an increase in serum bromide concentration equal to or above the cut-off level was defined as good adherence.
Statistical analysis
Results were analyzed using Student's t-test. Differences with a P value < 0.05 (two-sided) were considered statistically significant. Correlations were calculated by using the Pearson's coefficient of correlation. Results are presented as mean ± SD, unless indicated otherwise.
Results
Fourteen men and 16 women, participated in the study. Baseline characteristics are shown in Table 1. Two patients discontinued drug treatment, one patient in treatment week 2 because of a maculo-papular reaction and another patient at week 16 because of dramatic personal events not related to the study. No serious adverse effects were reported.
Table 1.
Baseline characteristics of patients (mean ± SD are given)
| Mean | (± SD) | |
|---|---|---|
| Age (years) | 53 | (± 10) |
| Height (cm) | 171 | (± 7) |
| Body weight (kg) | 82 | (± 13) |
| BMI (kg m−2) | 27.9 | (± 4.6) |
| Heart rate (beats min−1) | 76 | (± 12) |
| Blood pressure (mmHg) | Office SBP | Office DBP |
| At start of placebo period | 151 (± 13) | 101 (± 4) |
| At week 2 of placebo period | 159 (± 15) | 105 (± 5) |
| At week 4 of placebo period | 158 (± 15) | 105 (± 5) |
DBP, diastolic blood pressure; SBP, systolic blood pressure.
The results for measuring adherence by the three methods are shown in Table 2. Based on capsule counting almost all patients showed good adherence (intake ≥80% of expected) over the whole treatment period. One patient returned the container 2 weeks after the last visit and was considered to be nonadherent. According to electronic monitoring adherence was ‘good’ (at least 80% of expected openings of the drug container) for all patients except for two patients during the second treatment period (between 12 weeks and 20 weeks of treatment). Two other patients were considered to be nonadherent because recordings were missing after the last visit.
Table 2.
Assessment of adherence by three different methods
| Method | n | Number adherent at 12 weeks of treatment period (%) | n | Number adherent at 20 weeks of treatment period (%) |
|---|---|---|---|---|
| Capsule counting* | ||||
| ‘Good’ adherence | 29 | 29 (100) | 28 | 27 (96) |
| Electronic monitoring† | ||||
| ‘Good’ adherence | 29 | 29 (100) | 28 | 24 (86) |
| Serum bromide concentration | ||||
| ‘Good’ adherence | 29 | 27 (93) | 28 | 26 (93) |
n, number of patients. For all methods: one patient withdrew at week 2 of treatment period, one patient at week 16.
One patient returned container 2 weeks after last visit, this patient was considered to be nonadherent.
For two patients track cap recording was missing at last visit (week 20). These patients were considered to be nonadherent. Also two patients were found to have an adherence rate less than 80%.
The bromide concentration increased from 0.06 ± 0.01 mmol l−1 at baseline to 0.26 ± 0.06 mmol l−1 (mean of weeks 12, 16 and 20). The individual changes in bromide concentration during treatment are shown in Figure 1. The mean ± SEM change in bromide concentration after 12, 16 and 20 weeks was 0.21 ± 0.01 mmol l−1. This change was comparable with the mean increase in bromide in the eight volunteers taking 30 mg potassium bromide (0.21 ± 0.01 mmol l−1vs. 0.18 ± 0.02 mmol l−1, Figure 1) [2].
Figure 1.
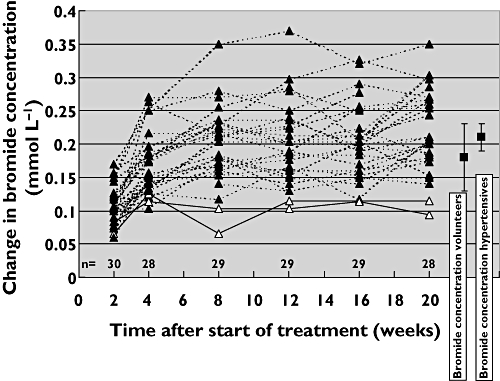
Changes in serum bromide concentrations in 30 patients treated with trandolapril/verapamil. The broken lines indicate patients with good adherence. The two patients with poor adherence are shown (solid lines, with open triangles). On the right side of the figure the mean changes in bromide concentration (± 2 SE) are shown for the volunteers (n = 8) and hypertensive patients (n = 29), both taking 30 mg potassium bromide
In our previous study we demonstrated that the increase in serum bromide concentrations associated with a defined dose of potassium bromide, negatively correlates with body weight [2]. This was confirmed in the current study where the coefficient of correlation between body weight and change in bromide concentration was −0.53.
The two patients who were categorized as ‘poor’ adherers appeared to have a high body-weight, 91 kg and 98 kg, respectively. Based on electronic monitoring the adherence of these patients was 82% and 102%, respectively, during treatment weeks 12–20. For all four patients who were found to be nonadherent based on electronic monitoring, adherence was good based on serum bromide measurements.
Treatment with TV for 20 weeks decreased OBP by 9.6/7.5 (± 11.4/6.4) mmHg (both P values < 0.05).
Compared with baseline (visit 2), there was also a significant decrease in systolic and diastolic ABPM. SBP decreased from 146.2 (± 13.9) to 135.4 (± 12.8) at week 8 and to 135.8 (± 11.6) mmHg at week 16. DBP decreased from 92.7 (± 8.4) to 85.0 (± 7.7) at week 8 and to 85.5 (± 8.0) mmHg at week 16.
Discussion
Several methods have been developed for assessment of adherence [1]. Questionnaires like the Morisky medication adherence scale and the Hill-Bone medication adherence scale were developed to provide a simple method of assessing adherence [3, 4]. These methods were not used in the current study. Another method of assessing adherence is capsule counting. This method is known to be less reliable, because its results can easily be influenced by the patient who can discard capsules [5]. More recently electronic monitoring has been introduced, an accurate method of measuring adherence [1, 6–8]. A patient would consistently have to open and close the pill container without taking medication to circumvent this method. However, both methods do not prove the ingestion of drugs. To overcome this particular disadvantage, markers have been added to the drugs and the marker concentrations have been used to measure adherence. In the past low doses of digoxin, phenobarbital and bromide have been used [9–12]. Bromide is potentially suitable as a marker for adherence, because of its long half-life (about 12 days) and because it is not associated with side-effects in the small dosages used [2].
In this small study all three methods of measuring adherence appeared to give similar results. Not surprisingly capsule counting was associated with the highest level of adherence. By using electronic monitoring and measuring serum bromide concentrations nonadherent patients could be identified. Although measurement of bromide added to the antihypertensive drug confirms actual drug intake, there are also disadvantages. Multiple blood samplings are necessary and the method is quite time-, cost- and work-consuming. Another disadvantage is that the increase in bromide concentrations is influenced by body weight and it can only monitor intake of one drug or a fixed combination of drugs in one capsule at a time. The interindividual variation in the serum bromide concentrations was quite large (Figure 1). Measuring serum bromide concentrations seems, therefore, to be most useful as a method to follow-up adherence in an individual patient. Short-term variations in adherence (so called drug ‘holidays’, which may be clinically relevant because of rebound effects) can be assessed with electronic monitoring, but not with bromide because of its long half-life. Its main application would probably be as a research tool in situations in which actual drug ingestion over longer time periods has to be confirmed. The discrepancies in results in a few patients between electronic monitoring and bromide measurements warrant further research in a larger cohort of patients.
Measuring adherence with several methods is difficult to do in clinical practice. Compared with the ‘bromide-method’, electronic monitoring is as reliable and a less complex method of measuring adherence. However, in clinical trials adequate assessment of adherence is critical for proper evaluation of study outcomes. Therefore in research studies, the combination of several methods (e.g. electronic monitoring, addition of a marker together with capsule counting) may be helpful in avoiding the disadvantages inherent to each individual method.
Acknowledgments
We thank Wim Lemmens, statistical analyst.
REFERENCES
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2.Braam RL, van Uum SH, Russel FG, Swinkels DW, Thien T. Bromide as a marker to measure adherence to drug therapy. Eur J Clin Pharmacol. 2006;62:285–90. doi: 10.1007/s00228-006-0103-5. [DOI] [PubMed] [Google Scholar]
- 3.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone compliance to high blood pressure therapy scale. Prog Cardiovasc Nurs. 2000;15:90–6. doi: 10.1111/j.1751-7117.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 5.Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. Is this patient taking the treatment as prescribed? JAMA. 1993;269:2779–81. [PubMed] [Google Scholar]
- 6.Urquhart J. Role of patient compliance in clinical pharmacokinetics. A review of recent research. Clin Pharmacokinet. 1994;27:202–15. doi: 10.2165/00003088-199427030-00004. [DOI] [PubMed] [Google Scholar]
- 7.Burnier M, Santschi V, Favrat B, Brunner HR. Monitoring compliance in resistant hypertension: an important step in patient management. J Hypertens. 2003;21(Suppl. 2):S37–42. doi: 10.1097/00004872-200305002-00007. [DOI] [PubMed] [Google Scholar]
- 8.Baulmann J, Düsing R, Vetter H, Mengden Th. Therapy resistant hypertension – significance of electronic compliance monitoring. Dtsch Med Wochenschr. 2002;127:2379–82. doi: 10.1055/s-2002-35355. [DOI] [PubMed] [Google Scholar]
- 9.Roth HP, Caron HS, Bartholomew P. Measuring intake of a prescribed medication, a bottle count and a tracer technique compared. Clin Pharmacol Ther. 1970;11:228–37. doi: 10.1002/cpt1970112228. [DOI] [PubMed] [Google Scholar]
- 10.Pullar T, Kumar S, Tindall H, Feely M. Time to stop counting the tablets? Clin Pharmacol Ther. 1989;46:163–8. doi: 10.1038/clpt.1989.121. [DOI] [PubMed] [Google Scholar]
- 11.Mäenpää H, Javela K, Pikkarainen J, Mälkönen M, Heinonen OP, Manninen V. Minimal doses of digoxin: a new marker for compliance to medication. Eur Heart J. 1987;8(Suppl.):31–7. doi: 10.1093/eurheartj/8.suppl_i.31. [DOI] [PubMed] [Google Scholar]
- 12.Feely M, Cooke J, Price D, Singleton S, Mehta A, Bradford L, Carlvert R. Low-dose phenobarbitone as an indicator of compliance with drug therapy. Br J Clin Pharmacol. 1987;24:77–83. doi: 10.1111/j.1365-2125.1987.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


