Abstract
AIMS
Selective serotonin reuptake inhibitors (SSRIs), such as paroxetine, are associated with an increased risk of bleeding disorders, probably due to decreased platelet serotonin levels. Polymorphisms in the serotonin transporter gene (5-HTT) may influence the risk of SSRI-induced bleedings. The aim of this study was to investigate whether and to what extent the serotonin transporter polymorphism increases the bleeding time in paroxetine users.
METHODS
A prospective study, using routinely collected hospital and pharmacy data, was conducted among 43 patients between 18 and 70 years old and on >4 weeks of paroxetine therapy. The genotype for the serotonin transporter (5-HTTLPR), trough paroxetine levels, platelet function analyser (PFA)-closure time (collagen/epinephrine) and a complete blood count were assessed.
RESULTS
No significant difference was seen between the SS, SL, LL genotypes of the serotonin transporter and the PFA-closure time. None of the covariates had a significant influence on the association between the serotonin transporter polymorphism and the PFA-closure time. Age and von Willebrand factor showed the largest contribution, but not significant. No difference was seen between the PFA-closure time and the frequency of bruising and spontaneous bleedings between patients with at least one S allele and with the LL genotype.
CONCLUSION
Our prospective study does not support the assumption that paroxetine can cause a prolonged PFA-closure time during paroxetine therapy due to a serotonin transporter polymorphism. Old age, use of platelet inhibitors and a history of gastrointestinal bleeding remain the focus for SSRI-induced bleeding complications.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
From case reports it has become clear that selective serotonin reuptake inhibitors (SSRIs) can cause bleeding disorders.
The causative mechanism is as yet unknown.
Several publications have described the relationship between the serotonin transporter genotype and the prevalence of certain diseases such as depression, but few have focused on the relationship with side-effects of antidepressive drugs such as SSRIs.
WHAT THIS STUDY ADDS
This study suggests that the association between SSRI therapy and prolonged bleeding time may not be related to the polymorphism of the serotonin transporter (5-HTTLPR) investigated.
Keywords: bleeding time, paroxetine, platelet aggregation, serotonin transporter polymorphism, SSRI
Introduction
The lifetime prevalence for major depression in the community ranges from 15 to 17%. Depressive disorders occur at any age, but typically develop between the 20s and 30s. The exact aetiology is unknown, but probably involves genetic factors, changes in neurotransmitter levels, altered neuroendocrine function and psychosocial factors. Expression of genetic polymorphisms for the serotonin transporter active in the brain and peripheral tissues may be triggered by life events and stress [1].
Pharmacotherapy of moderate to severe mood disorders mostly has a chronic character, especially in patients with more than one depressive episode. The most commonly used antidepressants are the selective serotonin reuptake inhibitors (SSRIs). SSRIs have a wide therapeutic margin and are relatively easy to administer, with little need for dose adjustments.
From case reports and observational studies it has become clear that treatment with SSRIs can lead to haemorrhagic complications [2–5]. In the observational case–control study of Abajo et al.[6], SSRI users had a relative risk of 3.0 (95% confidence interval 2.1, 4.4) for gastrointestinal haemorrhage. Movig et al.[2] have shown that users of serotonergic medication undergoing orthopaedic surgery lost on average 500 ml of blood more than non-SSRI users and were four times more likely to be given a perioperative blood transfusion.
The most likely mechanism underlying this increased bleeding risk in users of serotonergic antidepressants is inhibition of serotonin uptake in the platelet, which influences the functioning of the primary haemostasis. Serotonin is a strong vasoconstrictor and a relatively weak platelet activator. At rest, serotonin is stored in the platelets in so-called ‘dense bodies’, but after platelet activation it is released into the circulation, together with other aggregating factors such as adenosine diphosphate (ADP), adrenaline and collagen, and is thus a stimulus for aggregation [7].
SSRIs are antagonists of the serotonin transporter and the transporter is necessary to transport serotonin into the platelet. Since platelets themselves do not produce serotonin but are dependent on blood uptake, blockade of the serotonin transporter with a SSRI leads to a lower concentration of serotonin in the platelet. Meijer et al.[8] have examined the relationship between the degree of serotonin uptake inhibition from an antidepressant and risk of haemorrhage in a case–control study. They found that the risk of an abnormal bleeding event was largest for fluoxetine, sertraline, clomipramine and paroxetine; these drugs have the strongest affinity for the serotonin transporter and the lowest dissociation constant [2]. In the study of Hergovich et al.[7], serotonin concentration in the thrombocyte decreased by 83% (P < 0/01) after 14 days of paroxetine use (20 mg day−1). After 14 days the platelet function analyser (PFA)-closure time was increased by 31% (P < 0.05).
It has been shown that the serotonin transporter gene is polymorphic. The polymorphism that is considered most relevant is an insertion/deletion polymorphism in the promoter region of the gene, which encodes for the serotonin transporter (5-HTTLPR or SERTPR) [9–12]. The 5-HTTLPR gene has two variant alleles: a short (S) and a long (L) allele. The S allele has been associated with a nearly 50% reduction in expression of the serotonin transporter protein, vulnerability for mood disorders, inadequate response to SSRIs and side-effects [13, 14]. In Whites, the genotype frequencies are approximately 25% SS, 47% SL and 28% LL [12].
Until now it has been unclear whether the functioning of the primary haemostasis during paroxetine therapy is influenced by the serotonin transporter polymorphism. Theoretically, paroxetine users with one or more S alleles for the serotonin transporter have fewer transporters functioning on the platelet and could therefore have less serotonin stored in platelets. This could put them at higher risk for bleeding complications. The aim of this study was to investigate whether, and to what extent, a serotonin transporter polymorphism increases the bleeding time in users of paroxetine.
Methods
Setting, patients and procedure
The Medical Ethics Committee of the Maxima Medical Centre in Veldhoven, the Netherlands, approved the study protocol, and written informed consent was obtained from all the patients. Patients were recruted by means of an advertisement in a regional daily newspaper. Patients were eligible if they had been using paroxetine for at least 4 weeks and were aged between 18 and 70 years. Exclusion criteria were: thrombocytopenia (<150 × 109 U l−1), liver or kidney function impairment (by earlier diagnosis), recent (<1 month) surgical intervention, pregnancy, lactation and the use of clinically relevant interacting medication [oral anticoagulants, thrombocyte aggregation inhibitors and nonsteroidal anti-inflammatory drugs (NSAIDs)].
A structured questionnaire was administered by the researcher to detail (besides the above-mentioned covariates) the blood type and the usage of over-the-counter drugs (especially painkillers such as NSAIDs).
Blood was drawn for genotyping the serotonin transporter gene, measurement of the closure time by the platelet function analyser (PFA-100®), through serum samples of paroxetine and complete blood count [haemoglobin (Hb), leucocyte count + differentiation, thrombocyte count and von Willebrand factor (vWF)].
The following covariates were studied as possible confounders: current usage of alcohol, smoking, mild spontaneous bleeding events (nose, eye), bruising (purpura or epistaxis), dosage and duration of paroxetine treatment and indication for paroxetine treatment.
Outcome measures
The primary end-point of this study was bleeding time defined by measuring the PFA-closure time with the platelet function analyser (PFA-100®).
The PFA-100® is an in vitro test where venous ceased citrate blood under vacuum is drawn trough a small opening in a membrane. The membrane is coated with an agonist: collagen/epinephrine (COL/EPI) or collagen/ADP (COL/ADP) to activate the platelets. A stable platelet plug forms and occludes the opening; the time taken for the blood to stop passing trough the opening is registered as the PFA-closure time. The study of Hergovich et al.[7] showed a prolonged PFA closure time of 31% (but only with the agonist COL/EPI). For that reason, COL/ADP was left out of consideration in this research. The COL/EPI was carried out directly (or at least within 4 h after blood drawn) in duplo.
Secondary end-points were the occurrence of spontaneous bleedings and bruising. They were recorded according to the structured questionnaire to detail the occurrence of mild spontaneous bleeding events and bruising (purpura or epistaxis). The questions were answered by ‘yes’ or ‘no’ and and a description of where the bleeding events took place (nose, eye).
The other haematological parameters (Hb, leucocyte count + differentiation, thrombocyte count and vWF) followed the same day. Blood type was stipulated (if unknown by the patient) if the vWF was <60% because with blood type O a lower threshold is approved.
Determinant
The determinant was the polymorphism of the insertion/deletion polymorphism in the promoter region of the gene, which encodes for the serotonin transporter (5-HTTLPR or SERTPR). Leucocyte DNA was extracted from whole blood. Patients were genotyped for 5-HTT promoter region s/l variation (5-HTTLPR). The polymerase chain reaction (PCR) amplification method and primers were according to the method described in detail by Caspi et al.[15]. This PCR reaction resulted in a 419 and 375 bp long fragment for the ‘L’ and ‘S’ allele, respectively (Figure 1).
Figure 1.
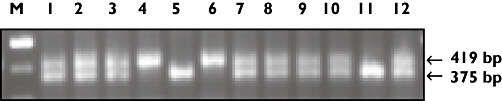
Genotyping the serotonin transporter (5-HTTLPR). *The S-allele [375 base pairs (bp)] has a lower transcription activity (up to 50%) and therefore fewer amino acids are formed. As a result a protein with a lower molecular weight arises. The L allele consists of 419 bp. M, Marker; 1 t/m 12 displays the genotypes of 12 patients
Data analyses and statistics
The power calculation of the sample size was based on the study by Hergovich [7]. Paroxetine users with a SS genotype were expected to have a closure time 20 s longer than the average closure time by COL/EPI (116 + 20 s = 136 s). With a power of 80% and ? of 0.05 this means 12 persons for each genotype have to be included, i.e. a minimum of 36 patients.
All records were transferred to a spreadsheet (Excel) for analysis. Statistical analysis was done with SPSS 12.0 for Windows (SPSS Inc., Chicago, IL, USA). The relationship between PFA-closure time, serotonin transporter genotype and the influence of potential confounding effects of, for example: age, paroxetine concentration and blood count was examined using anova. Those potential confounding factors that were significantly (P < 0.05) associated with PFA-closure time were included as covariate in the analysis. Furthermore, Student's t-test was carried out to examine the difference in PFA-closure time between patients with an S allele and those with an l allele. A P-value of 0.05 or less was regarded as significant.
Results
Between September and November 2006, 52 patients were screened for inclusion. All included patients were White. After selection, 43 patients were eligible for inclusion. Excluded were two because of age >70 years, one for liver function impairments, three for NSAID usage and three for use of platelet aggregation inhibitors. Table 1 reflects the patient characteristics according to genotype.
Table 1.
PatIent characterIstIcs
| LL | SL | SS | Total | |
|---|---|---|---|---|
| Number of patients | 19 (44%) | 18 (42%) | 6 (14%) | 43 (100%) |
| Age (years)* | 47.4 (6.7) | 50.7 (13.5) | 44.4 (15.0) | 48.4 (11.1) |
| Sex (no. female) | 15 (79%) | 12 (67%) | 5 (84%) | 32 (74%) |
| Usage of alcohol (yes) | 8 (42%) | 9 (50%) | 2 (33%) | 19 (44%) |
| Smoking (yes) | 9 (47%) | 7 (39%) | 2 (33%) | 18 (42%) |
| Diagnosis depression | 9 (47%) | 5 (28%) | 1 (17%) | 15 (35%) |
| Diagnosis panic disorder | 0 (0%) | 2 (11%) | 1 (17%) | 3 (7%) |
| Diagnosis depression/panic disorder | 2 (11%) | 1 (6%) | 1 (17%) | 4 (9%) |
| Other diagnosis | 8 (42%) | 10 (56%) | 3 (49%) | 36 (84%) |
| Paroxetine dosage (mg)* | 21.1 (6.5) | 21.1 (7.4) | 23.3 (7.5) | 21.0 (6.9) |
| Paroxetine Css (mg l−1)* | 44.2 (57.8) | 36.9 (42.8) | 63.5 (21.9) | 43.9 (48.0) |
| Blood type O | 5 (56%) | 6 (50%) | 5 (83%) | 16 (59%) |
| Haemoglobin (mmol l−1)* | 8.7 (0.8) | 9.0 (0.7) | 8.3 (0.6) | 8.7 (0.7) |
| Leucocyte count (x 109 l−1)* | 7.1 (1.4) | 6.6 (1.5) | 6.8 (2.3) | 6.8 (1.6) |
| Leuco + diff out of spec | 6 (32%) | 3 (17%) | 2 (33%) | 16 (59%) |
| Thrombocyte count (x 109 l−1)* | 306.4 (44.6) | 307.0 (62.4) | 295.5 (27.7) | 305.2 (50.4) |
| vWF (%)* | 89.4 (17.6) | 99.7 (18.0) | 94.5 (25.7) | 94.6 (19.1) |
Continuous variables displayed as mean ± standard deviation. vWF, Von Willebrand factor; S, short allele; L, long allele.
The PFA-closure time (COL/EPI) is considered normal within a closure time of 82–150 s. Six patients had a PFA-closure time >150 s. The average PFA-closure times in paroxetine users with the SS, SL and LL genotype of the serotonin transporter were 126.3 s (SD 38.3), 122.6 s (SD 33.8) and 122.5 s (SD 24.1), respectively (Table 2). The differences in PFA-closure times are not significant for the examined genotypes. None of the examined covariates had a significant contribution to the relation between serotonin transporter genotype and PFA-closure times. Age and vWF showed the largest contribution, but not significant. The paroxetine serum trough levels showed a large variation, but the cause was multifactorial and not relevant to this study. The duration of paroxetine therapy was measured in terms of categories: paroxetine therapy between 1 and 3 months, 3–12 months and >12 months. The majority had been on long-term (>12 months) paroxetine therapy (n = 36), some had been using paroxetine between 3 and 12 months (n = 6) and only one person had been using paroxetine between 1 and 3 months.
Table 2.
Influence of genotype on haematologIcal parameters
| Genotype | PFA closure time (s) + SD | Bruising (%) | Spontaneous bleeding events (%)* |
|---|---|---|---|
| LL (n = 19) | 122.5 (24.1) | 7 (36.8%) | 4 (21.1%) |
| SL (n = 18) | 122.6 (33.8) | 3 (16.7%) | 3 (16.7%) |
| SS (n = 6) | 126.3 (38.3) | 2 (33.3%) | 1 (16.7%) |
| SL + SS (n = 24) | 123.5 (34.1) | 5 (20.8%) | 4 (16.7%) |
Spontaneous bleeding events in nose and eyes. PFA, Platelet function analyser; S, short allele; L, long allele.
Furthermore, there were no significant differences in PFA-closure time, frequency of bruising and mild spontaneous bleeding events between patients with at least one S allele and patients with the LL genotype (Table 2).
Discussion
In this study no association was discovered between the serotonin transporter polymorphism and PFA-closure time in paroxetine users. This is the first study to investigate the relationship between the serotonin transporter polymorphism and bleeding time in paroxetine users.
In this study it was decided to use PFA-closure time as the primary end-point for measuring bleeding time. The current de facto‘gold standard’ for measuring bleeding time is turbidometric platelet aggregometry. Although this method is successful, it has several limitations, including poor reproducibility, high volume sampling and length of assay time, skilled technicians, and costs [16]. The platelet function analyser (PFA-100®) is a screening tool for primary haemostasis. It depends on platelet count and haematocrit and is variably sensitive to certain defects and deficiencies. It has been suggested that the PFA-closure time is more sensitive for the detection of von Willebrand's disease and possibly more sensitive for tracing thrombocytopathology [16]. Moreover, the PFA-100®, in contrast to aggregometry, is influenced by storage pool deficiency of serotonin in platelets and therefore suitable to use as a screening tool in this study [7].
Our study has several limitations, mainly in its limited power. The frequency of the genotypes for the serotonin transporter polymorphism are unequivocal in literature. Our power calculations are based on a frequency of SS 25%, SL 47% and LL 28% [12]. During our study another study [14] was published, showing a genotype frequency of SS 16%, SL 48% and LL 36%. The latter frequencies correspond very well to the results of our study: SS 14%, SL 42% and LL 44%. This may indicate that the power used in our study was limited and the sample size should have been 24 in each group, reflecting not a minimum of 36 patients but a minimum of 72 patients to investigate a relationship between the serotonin transporter polymorphism and bleeding time. However, 56% of the included patients have at least one S allele. If possessing an S allele could induce a prolongation of the PFA-closure time, this would have been visible in our population. Since no effect was seen, we think that having one S allele does not prolong the PFA-closure time, but since fewer patients with an S allele were found than expected from our power calculations (14% vs. 25%), no definite conclusions can be drawn from this study for patients having the SS genotype in terms of PFA-closure or bleeding time.
Second, a possible explanation for our findings is that the reduced intracellular rise of serotonin in the thrombocyte is compensated for by other aggregating factors, such as ADP and adrenaline.
Furthermore, the relative shortage of serotonin may correct itself during the first weeks or months after starting paroxetine therapy. In the study of Hergovich [7], a lengthening of 31% in the closure time was shown 2 weeks after the start of paroxetine therapy, but there are no data beyond this period. All patients in our study were using paroxetine for a much longer period of time, e.g. months (range 1 month up to >12 months).
Finally, we studied only one serotonin transporter gene; other genes encoding for the serotonin transporter have been disregarded, as well as genes that encode for tryptophan or serotonin receptors. Theoretically all these genes, separately or together, can make a contribution to a prolonged PFA-closure time during paroxetine usage.
None of the examined variables made a significant contribution to the relation between serotonin transporter genotype and PFA-closure time. In our study a longer PFA-closure time was observed in older patients and those with a lower level of vWF, although this difference was not significant. This corresponds to findings from the literature [4, 5]. Some subjects (n = 6) had a PFA-closure time >150 s. No relationship between the examined covariates could be found. We found that of the six persons with PFA-closure times >150 s, four had blood type O and in two the blood type was unknown. Blood type O is associated with lower levels of vWF than the other blood types. The primary haemostasis is influenced by deficiencies of vWF.
High-risk interventions such as surgery have been shown to expose the SSRI user to a clinically serious bleeding risk [17, 18]. The pharmacological mechanism is a decrease in intraplatelet serotonin concentrations. Furthermore, decreased platelet serotonin levels and a concurrent increase in plasma serotonin levels have been associated with surgical procedures. SSRIs may precipitate bleeding in patients with pre-existing haemostatic effects, e.g. in postoperative patients. Patients experiencing stress from surgical procedures are thought to be at higher risk for bleeding complications [2]. A recent study by Marczinski (unpublished data) has shown that desmopressin can reduce the perioperative blood loss in SSRI users undergoing orthopaedic surgery. In the present study, patients were seen at one point in time, not exposed to stress and in steady state according to their haemostasis. Therefore, an effect in bleeding time might not have been visible.
At a young age the compensatory mechanism for coagulation can still be sufficient, but as age increases this may be less compensated. Besides age, comorbidity and polypharmacy can play a role in distorted coagulation and prolonged bleeding time.
Conclusions
Our prospective study does not support the assumption that paroxetine causes a prolonged PFA-closure time during paroxetine therapy due to a serotonin transporter polymorphism. To our view, attention for risk of haemorrhage must continue to be paid to paroxetine users who are classified as high-risk patients (such as the elderly, history of gastrointestinal haemorrhage and users of NSAIDs and thrombocyte aggregation inhibitors) [2, 3, 5]. Moreover, more research is needed on the genetic profiling of SSRI users at risk for bleeding complications.
Acknowledgments
We thank Ronald Oosting, PhD (Department of Psychopharmacology, Utrecht Institute for Pharmaceutical Sciences, Utrecht, the Netherlands) for genotyping the serotonin transporter (5-HTTLPR), and S. Jackson and M. Naunton as English speakers for help during translation.
REFERENCES
- 1.Ebmeier KP, Donaghey C, Steel JD. Recent developments and current controveries in depression. Lancet. 2006;367:153–67. doi: 10.1016/S0140-6736(06)67964-6. [DOI] [PubMed] [Google Scholar]
- 2.Movig KLL, Janssen MW, de Waal Malefijt J, Kabel PJ, Leufkens HG, Egberts AC. Relationship of serotonergic antidepressants and need for blood transfusion in orthopedic surgical patients. Arch Intern Med. 2003;163:2354–8. doi: 10.1001/archinte.163.19.2354. [DOI] [PubMed] [Google Scholar]
- 3.Dalton SO, Johansen C, Mellemkjaer L, Nørgård B, Sørensen HT, Olsen JH. Use of serotonin reuptake inhibitors and risk of upper gastrointestinal tract bleeding, a population based study. Arch Intern Med. 2003;163:59–64. doi: 10.1001/archinte.163.1.59. [DOI] [PubMed] [Google Scholar]
- 4.Ufkes JGR. Maagdarmbloedingen bij ouderen door antidepressiva. Pharm Weekbl. 2001;136:1630–1. [Google Scholar]
- 5.Walraven van C, Mamdani MM, Wells PS, Williams JI. Inhibition of serotonin reuptake by antidepressants and upper gastrointestinal bleeding in elderly patients: retrospective cohort study. BMJ. 2001;323:655–8. doi: 10.1136/bmj.323.7314.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Abajo FJ, Rodriguez LA, Montero D. Association between selective serotonin reuptake inhibitors and upper gastrointestinal bleeding: population based case–control study. BMJ. 1999;319:1106–9. doi: 10.1136/bmj.319.7217.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hergovich N, Aigner M, Eichler HG, Entlicher J, Drucker C, Jilma B. Paroxetine decreases platelet serotonin storage and platelet function in human beings. Clin Pharmacol Ther. 2000;68:435–42. doi: 10.1067/mcp.2000.110456. [DOI] [PubMed] [Google Scholar]
- 8.Meijer WEE, Heerdink ER, Nolen WA, Herings RMC, Egberts ACG. Association of bleeding with degree of serotonin uptake inhibition by antidepressants. Arch Intern Med. 2004;164:2367–70. doi: 10.1001/archinte.164.21.2367. [DOI] [PubMed] [Google Scholar]
- 9.Smith GS, Lotrich FE, Malhotra AK, Lee AT, Ma Y, Kramer E, Gregersen PK, Eidelberg D, Pollock BG. Effects of serotonin transporter promotor polymorfisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–34. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- 10.Murphy DL, Lerner A, Rudnick G, Lesch KP. Serotonin transporter: gene, genetic disorders, and pharmacogenetics. Mol Interv. 2004;4:109–23. doi: 10.1124/mi.4.2.8. [DOI] [PubMed] [Google Scholar]
- 11.Kunugi H, Hattori M, Kato T, Tatsumi M, Sakai T, Sasaki T, Hirose T, Nanko S. Serotonin transporter gene polymorphisms: ethnic difference and possible association with bipolar affective disorder. Mol Psychiatry. 1997;2:457–62. doi: 10.1038/sj.mp.4000334. [DOI] [PubMed] [Google Scholar]
- 12.Smits KM, Smits LJM, Schouten JSAG, Stelma FF, Nelemans P, Prins MH. Influence of SERTPR and Stin2 in the serotonin transporter gene on the effect of selective serotonin reuptake inhibitors in depression: a systematic review. Mol Psychiatry. 2004;9:433–41. doi: 10.1038/sj.mp.4001488. [DOI] [PubMed] [Google Scholar]
- 13.Murphy GM, Hollander SB, Rodrigues HE, Kremer C, Schatzberg AF. Effects of the serotonin transporter gene promoter polymorphism on mirtazapine and paroxetine efficacy and adverse events in geriatric major depression. Arch Gen Psychiatry. 2004;61:1163–9. doi: 10.1001/archpsyc.61.11.1163. [DOI] [PubMed] [Google Scholar]
- 14.Hariri AR, Brown SM. Images in neuroscience: serotonin. Am J Psychiatry. 2006;163:12. doi: 10.1176/appi.ajp.163.1.12. [DOI] [PubMed] [Google Scholar]
- 15.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Bralthwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 16.Hayward CP, Harrison P, Cattaneo M, Ortel TL, Rao AK. Platelet function analyzer (PFA) 100® closure time in the evaluation of platelet disorders and platelet function. J Thromb Haemost. 2006;4:312–9. doi: 10.1111/j.1538-7836.2006.01771.x. [DOI] [PubMed] [Google Scholar]
- 17.Serebruany VL. Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something? Am J Med. 2006;119:113–6. doi: 10.1016/j.amjmed.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Y, Tsoi K, Hunt RH. Selective serotonin reuptake inhibitors and risk of upper GI bleeding: confusion or confounding? Am J Med. 2006;119:719–27. doi: 10.1016/j.amjmed.2005.11.006. [DOI] [PubMed] [Google Scholar]


