Bevacizumab, a recombinant humanized monoclonal IgG1 antibody binding to vascular endothelial growth factor (VEGF), was the first angiogenesis inhibitor to be approved in combination with chemotherapy, as an anticancer treatment option in daily practice. Its anti-angiogenic activity leads to decreased tumour perfusion, vascular volume and the number of viable and progenitor cells [1]. Arterial hypertension (manageable with conventional antihypertensive drugs) can occur in up to 30% of cases (grade 3 hypertension up to 16%) [2]. A few cases of bevazicumab-induced reversible posterior leukoencephalopathy syndrome (RPLS) have been reported with various clinical symptoms such as lethargy, confusion or loss of vision [3–5]. We report another suspected case of RPLS associated with bevacizumab.
A 33-year-old Asiatic woman with a past medical history of breast cancer in 2003 received a three-cycle course of bevacizumab (15 mg kg−1, since May 2007) and also liposomal doxorubicin (30 mg m−2) over 9 weeks (last infusion in 22 June 2007). She had no prior arterial hypertension. During chemotherapy, her blood pressure remained within the usual range (100/70 mmHg). On day 18 after the last infusion, she presented to the emergency department with serious headache (since 10 days) associated suddenly with gastralgia, nausea and vomiting. The first diagnoses were gastro-oesophageal reflux and then carcinomatous meningitis. Clinical examination and laboratory assessments were normal. Cerebrospinal fluid was clear and acellular with an increase of protein concentration to 133 mg dl−1, ruling out a diagnosis of meningitis. Blood pressure was 150/100 mm Hg. Symptomatic treatment including metoclopramide, tramadol, omeprazole orally and NaCl perfusion was administered. However, her condition worsened and blood pressure increased to 170/80 mm Hg the day after. Two days later (13 July 2007), she fell into a reactive coma. Magnetic resonance imaging (MRI) of the brain showed extensive leukoencephalopathy in the subcortical region without effect on the lateral ventricle (Figure 1). Treatment including prednisone (60 mg, i.v. three times daily), infusion of furosemide (40 mg), nicardipine and mannitol (1 g kg−1) as a 20% solution for cerebral oedema was started for 3 days. The following day, the patient's neurological deficits and high blood pressure had completely resolved. An electroencephalogram ruled out encephalopathy or epilepsy. A new MRI performed 4 days later showed a marked improvement in fluid-attenuated inversion recovery high-intensity lesions and resolution of the leukoencephalopathy.
Figure 1.
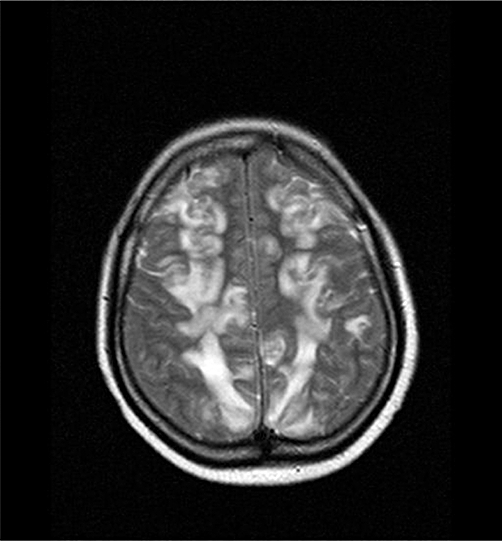
MRI scan of the brain with leucoencephalopathy. An axial T2 sequence image shows a subcortical high intensity lesion
Considering the physiological role of VEGF in regulating vasomotor tone, arterial hypertension remains the most prominent and ‘expected’ adverse effect of almost all angiogenesis inhibitors (monoclonal antibodies or VEGF tyrosine kinase inhibitors) [2]. Rixe et al. suggested that arterial hypertension should be a predictive factor of sunitinib activity in metastatic renal cell carcinoma [6]. RPLS has been also reported for sunitinib [7]. Nevertheless, the role of doxorubicin should be taken into account in our case since this drug has often been associated with RPLS and the association with bevacizumab could increase the risk of occurrence of this complication [8, 9].
RPLS remains a rare but serious adverse reaction of VEGF inhibitors. The warning symptoms could differ according to the patients and the prompt recognition of this syndrome will allow initiation of immediate treatment. Further studies are needed to investigate the opportunity of rechallenge of bevacizumab in patients showing an improvement of tumoral diseases with appropriate pressure monitoring.
REFERENCES
- 1.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eskens FA, Verweij J. The clinical toxicity profile of vascular endothelial growth factor (VEGF) and vascular endothelial growth factor receptor (VEGFR) targeting angiogenesis inhibitors: a review. Eur J Cancer. 2006;18:3127–39. doi: 10.1016/j.ejca.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Glusker P, Recht L, Lane B. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;9:980–1. doi: 10.1056/NEJMc052954. [DOI] [PubMed] [Google Scholar]
- 4.Oczan C, Wong SJ, Hari P. Reversible posterior leukoencephalopathy syndrome and bevacizumab. N Engl J Med. 2006;9:980–2. [PubMed] [Google Scholar]
- 5.Allen JA, Adlakha A, Bergethon PR. Reversible posterior leucoencephalopathy syndrome after bevacizumab/FOLFIRI regimen for metatstatic colon cancer. Arch Neurol. 2006;10:1475–8. doi: 10.1001/archneur.63.10.1475. [DOI] [PubMed] [Google Scholar]
- 6.Rixe O, Billemont B, Izzedine H. Hypertension as a predictive factor of sunitinib activity. Ann Oncol. 2007;6:1117. doi: 10.1093/annonc/mdm184. [DOI] [PubMed] [Google Scholar]
- 7.Martin G. reversible posterior leucoencephalopathy syndrome induced by sunitinib. J Clin Oncol. 2007;23:3559. doi: 10.1200/JCO.2007.12.8710. [DOI] [PubMed] [Google Scholar]
- 8.Haefner MD, Siciliano RD, Widmer LA, Vogel Wigger BM, Frick S. Reversible posterior leucoencephalopathy syndrome after treatment of diffuse large B-cell lymphoma. Onkologie. 2007;3:138–40. doi: 10.1159/000098706. [DOI] [PubMed] [Google Scholar]
- 9.Edwards MJ, Walker R, Vinnicombe S, Barlow C, MacCallum P, Foran JM. Reversible posterior leucoencephalopathy syndrome following CHOP chemotherapy for diffuse large B-cell lymphoma. Ann Oncol. 2001;9:1327–9. doi: 10.1023/a:1012248800195. [DOI] [PubMed] [Google Scholar]


