Abstract
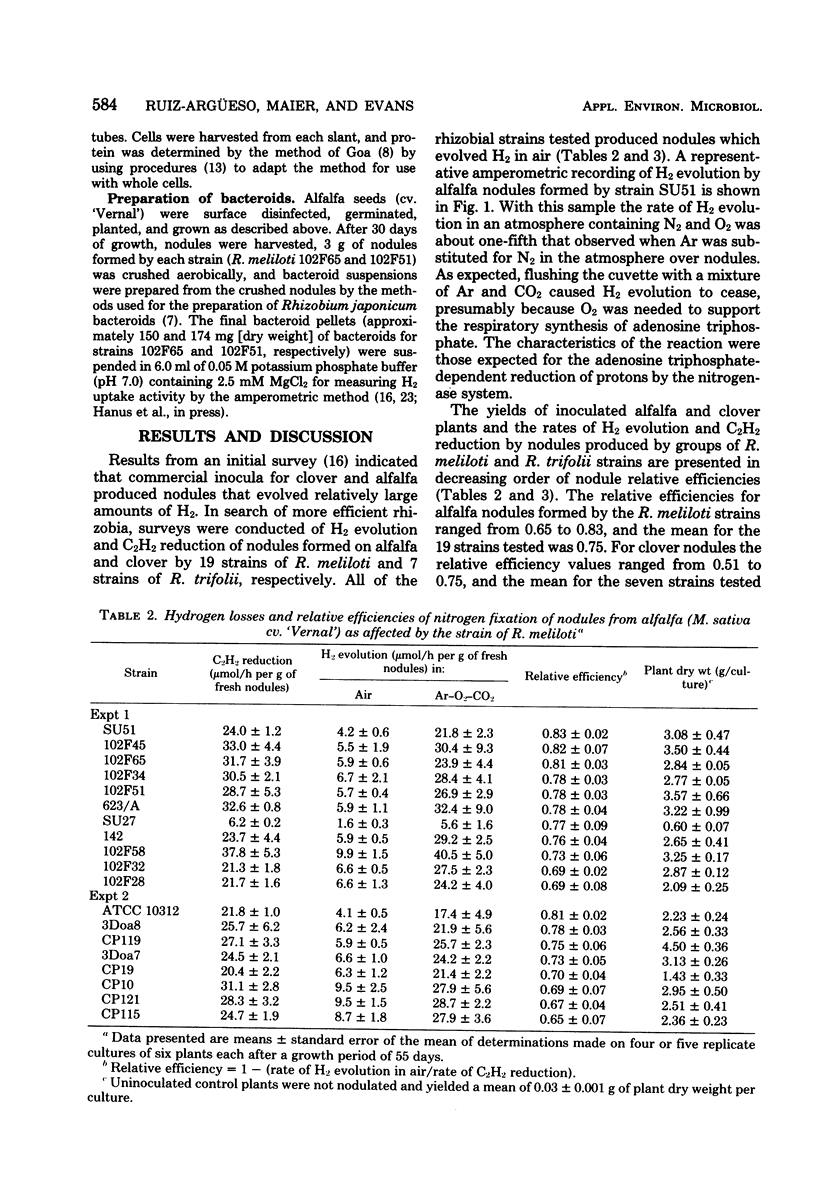
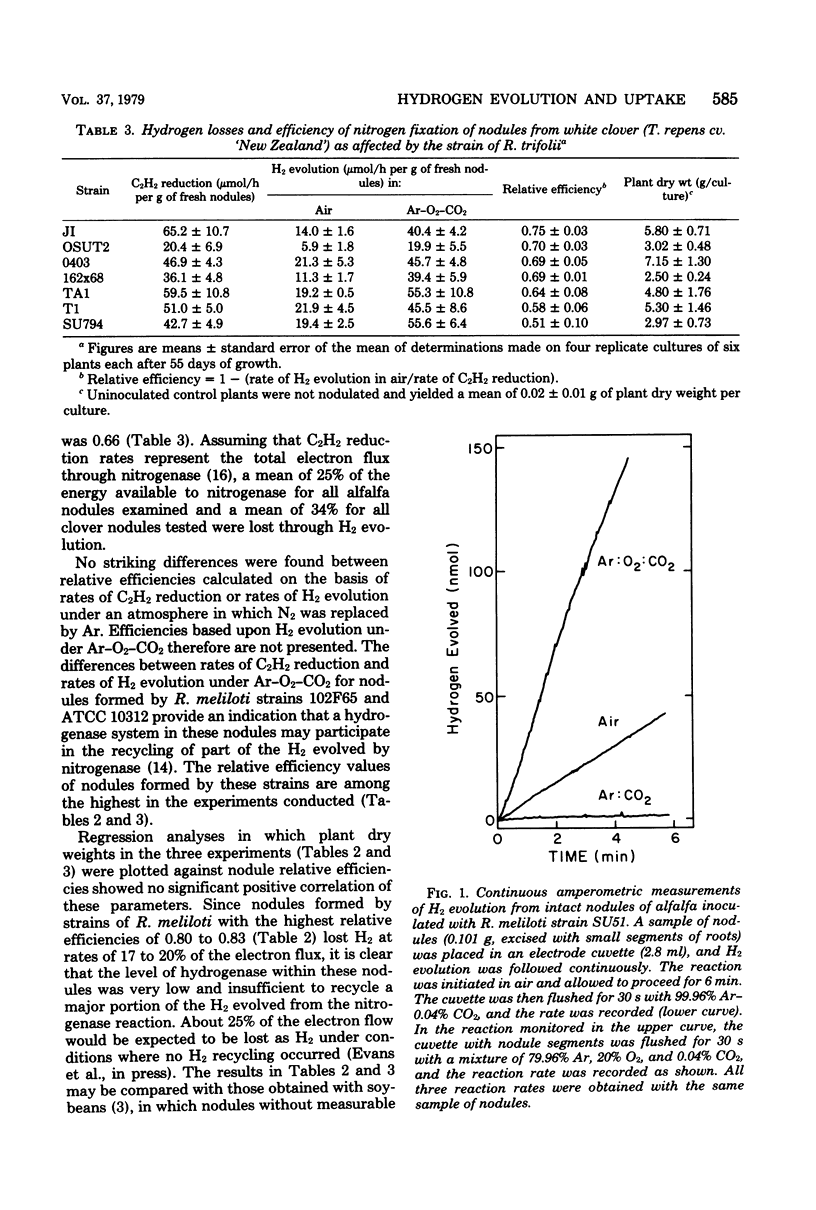
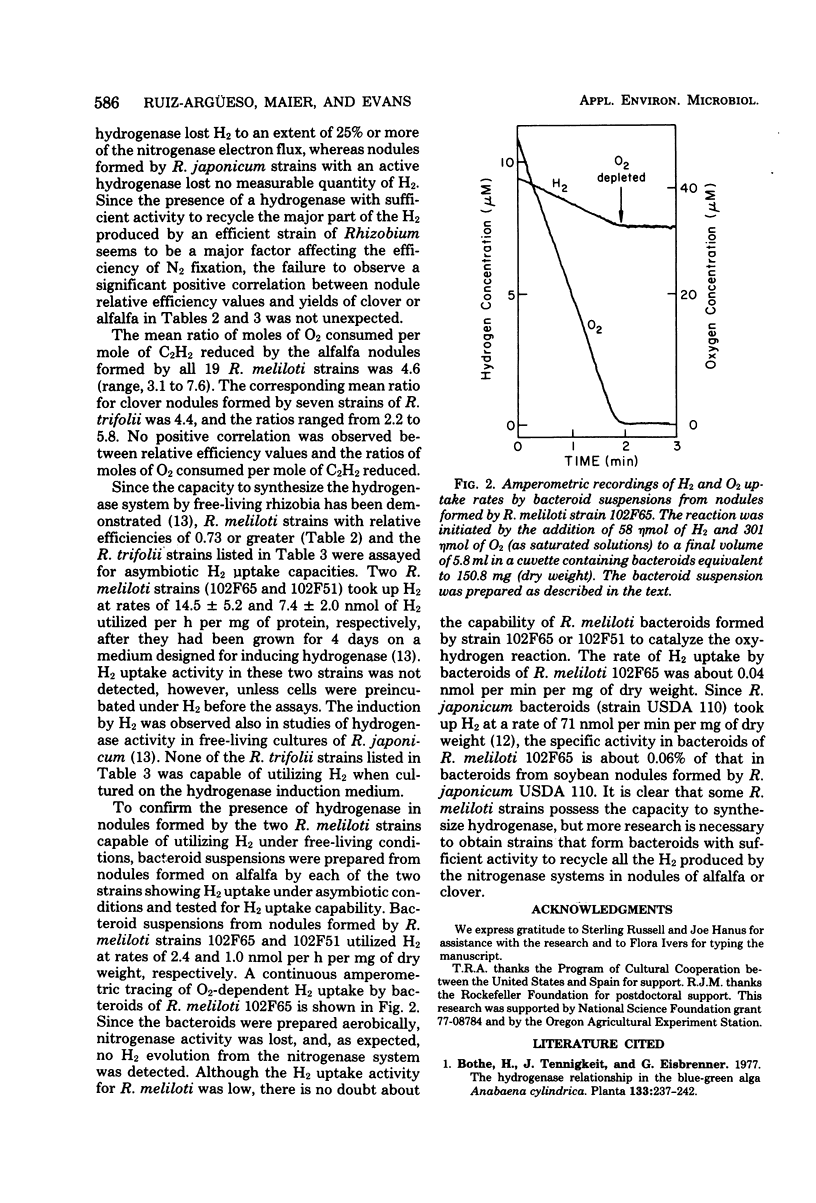
A series of Rhizobium meliloti and Rhizobium trifolii strains were used as inocula for alfalfa and clover, respectively, grown under bacteriologically controlled conditions. Replicate samples of nodules formed by each strain were assayed for rates of H2 evolution in air, rates of H2 evolution under Ar and O2, and rates of C2H2 reduction. Nodules formed by all strains of R. meliloti and R. trifolii on their respective hosts lost at least 17% of the electron flow through nitrogenase as evolved H2. The mean loss from alfalfa nodules formed by 19 R. meliloti strains was 25%, and the mean loss from clover nodules formed by seven R. trifolii strains was 35%. R. meliloti and R. trifolii strains also were cultured under conditions that were previously established for derepression of hydrogenase synthesis. Only strains 102F65 and 102F51 of R. meliloti showed measurable activity under free-living conditions. Bacteroids from nodules formed by the two strains showing hydrogenase activity under free-living conditions also oxidized H2 at low rates. The specific activity of hydrogenase in bacteroids formed by either strain 102F65 or strain 102F51 of R. meliloti was less than 0.1% of the specific activity of the hydrogenase system in bacteroids formed by H2 uptake-positive Rhizobium japonicum USDA 110, which has been investigated previously. R. meliloti and R. trifolii strains tested possessed insufficient hydrogenase to recycle a substantial proportion of the H2 evolved from the nitrogenase reaction in nodules of their hosts. Additional research is needed, therefore, to develop strains of R. meliloti and R. trifolii that possess an adequate H2-recycling system.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter K. R., Jennings N. T., Hanus J., Evans H. J. Hydrogen evolution and uptake by nodules of soybeans inoculated with different strains of Rhizobium japonicum. Can J Microbiol. 1978 Mar;24(3):307–311. doi: 10.1139/m78-051. [DOI] [PubMed] [Google Scholar]
- Dixon R. O. Hydrogenase in pea root nodule bacterioids. Arch Mikrobiol. 1968;62(3):272–283. doi: 10.1007/BF00413898. [DOI] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOA J. A micro biuret method for protein determination; determination of total protein in cerebrospinal fluid. Scand J Clin Lab Invest. 1953;5(3):218–222. doi: 10.3109/00365515309094189. [DOI] [PubMed] [Google Scholar]
- HOCH G. E., SCHNEIDER K. C., BURRIS R. H. Hydrogen evolution and exchange, and conversion of N2O to N2 by soybean root nodules. Biochim Biophys Acta. 1960 Jan 15;37:273–279. doi: 10.1016/0006-3002(60)90234-1. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Havelka U. D. Nitrogen fixation research: a key to world food? Science. 1975 May 9;188(4188):633–643. doi: 10.1126/science.188.4188.633. [DOI] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae R. E., Hanus J., Evans H. J. Properties of the hydrogenase system in Rhizobium japonicum bacteroids. Biochem Biophys Res Commun. 1978 Jan 30;80(2):384–390. doi: 10.1016/0006-291x(78)90688-5. [DOI] [PubMed] [Google Scholar]
- Schubert K. R., Engelke J. A., Russell S. A., Evans H. J. Hydrogen reactions of nodulated leguminous plants: I. Effect of rhizobial strain and plant age. Plant Physiol. 1977 Nov;60(5):651–654. doi: 10.1104/pp.60.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Evans H. J. Hydrogen evolution: A major factor affecting the efficiency of nitrogen fixation in nodulated symbionts. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1207–1211. doi: 10.1073/pnas.73.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert K. R., Jennings N. T., Evans H. J. Hydrogen Reactions of Nodulated Leguminous Plants: II. Effects on Dry Matter Accumulation and Nitrogen Fixation. Plant Physiol. 1978 Mar;61(3):398–401. doi: 10.1104/pp.61.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. A., Hill S., Yates M. G. Inhibition by acetylene of conventional hydrogenase in nitrogen-fixing bacteria. Nature. 1976 Jul 15;262(5565):209–210. doi: 10.1038/262209a0. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Luijk L. W., Packer L. An inducible hydrogenase in cyanobacteria enhances n2 fixation. FEBS Lett. 1977;78(1):49–52. doi: 10.1016/0014-5793(77)80270-6. [DOI] [PubMed] [Google Scholar]



