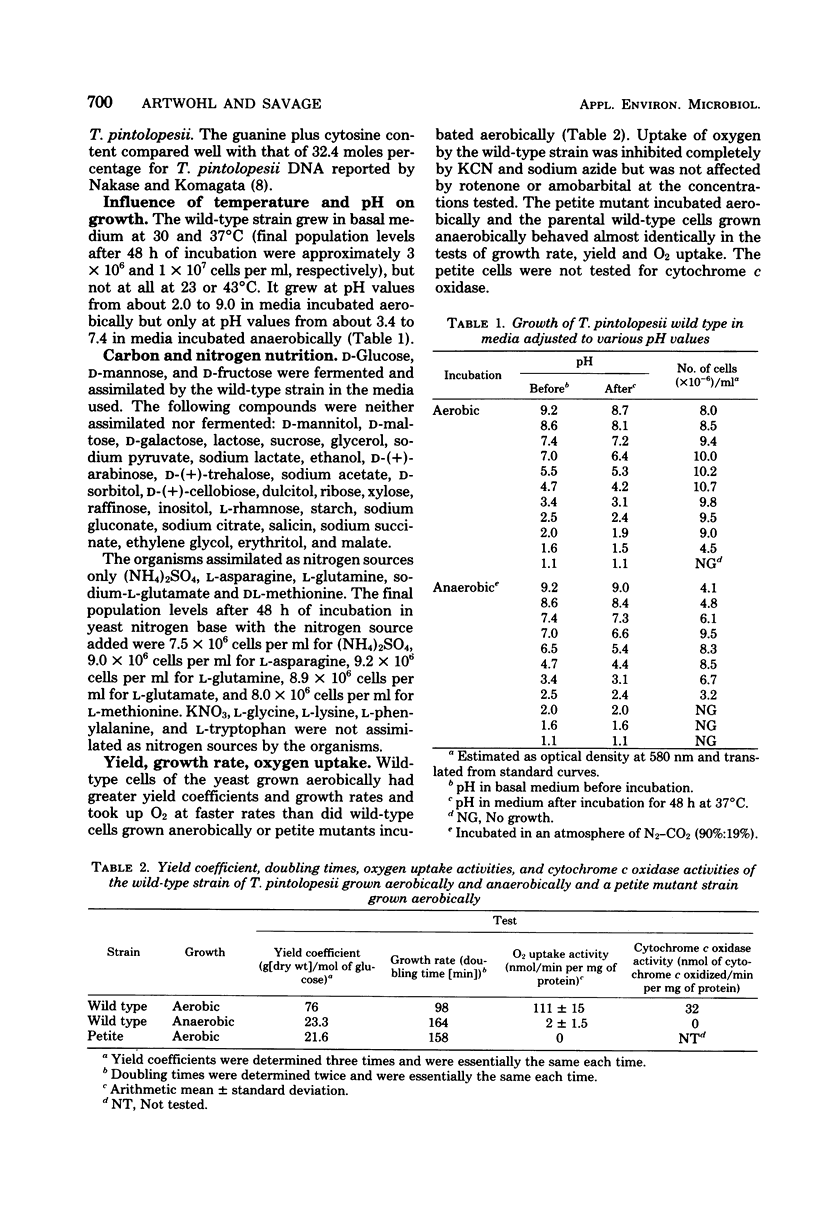
Abstract
Torulopsis pintolopesii is an indigenous yeast that colonizes the secreting epithelia in the stomachs of mice and rats. A wild-type strain of this microbe was isolated and identified. To attempt to learn characteristics of the yeast that are advantageous to it in colonizing its natural habitat in vivo, we examined some aspects of its nutrition and energy-yielding metabolism and some environmental conditions that influence its growth in vitro. The yeast appeared to be limited in the compounds it can utilize as carbon and nitrogen sources. It grew best at 37°C and did not grow at 23 or 43°C. It grew optimally at neutral pH but could grow aerobically at pH values as low as 2.0 and anaerobically at pH values as low as 3.4. As assessed by measurements of growth rates and yield coefficients, it grew better aerobically than anaerobically. When grown aerobically, it had a cyanide-sensitive system for taking up O2 and tested positively for cytochrome c oxidase activity. A petite mutant strain isolated from the wild-type strain had a growth rate and yield coefficient when incubated aerobically that were essentially the same as those of the wild-type parent grown anaerobically. Likewise similar to the wild-type parent grown anaerobically, the petite strain, though incubated aerobically, did not take up O2. Yeast-free mice associated with either the wild-type or the petite mutant strain were colonized at essentially the same rates and to similar final population levels by both strains. The yeast's capacity to respire may be of little advantage to it in its natural environment. By contrast, its abilities to grow best at 37°C and to grow at low pH values are undoubtedly advantageous characteristics in this respect. The limitations in its carbon and nitrogen nutrition are difficult to evaluate as ecological factors in its colonization of the natural habitat.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hernandez E., Johnson M. J. Energy supply and cell yield in aerobically growth microorganisms. J Bacteriol. 1967 Oct;94(4):996–1001. doi: 10.1128/jb.94.4.996-1001.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocková-Kratochvílová Comparative taxonomy of the genus Torulopsis Berlese. J Gen Microbiol. 1973 Dec;79(2):239–256. doi: 10.1099/00221287-79-2-239. [DOI] [PubMed] [Google Scholar]
- Kunstyr I., Peters K., Gärtner K. Investigations on the function of the rat forestomach. Lab Anim Sci. 1976 Apr;26(2 Pt 50):166–170. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PARLE J. N. Yeasts isolated from the mammalian alimentary tract. J Gen Microbiol. 1957 Oct;17(2):363–367. doi: 10.1099/00221287-17-2-363. [DOI] [PubMed] [Google Scholar]
- SCHAEDLER R. W., DUBOS R., COSTELLO R. THE DEVELOPMENT OF THE BACTERIAL FLORA IN THE GASTROINTESTINAL TRACT OF MICE. J Exp Med. 1965 Jul 1;122:59–66. doi: 10.1084/jem.122.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Savage D. C., Dubos R. J. Localization of indigenous yeast in the murine stomach. J Bacteriol. 1967 Dec;94(6):1811–1816. doi: 10.1128/jb.94.6.1811-1816.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C., Dubos R., Schaedler R. W. The gastrointestinal epithelium and its autochthonous bacterial flora. J Exp Med. 1968 Jan 1;127(1):67–76. doi: 10.1084/jem.127.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage D. C. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Microbial interference between indigenous yeast and lactobacilli in the rodent stomach. J Bacteriol. 1969 Jun;98(3):1278–1283. doi: 10.1128/jb.98.3.1278-1283.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Savage D. C. Indigenous microorganisms prevent reduction in cecal size induced by Salmonella typhimurium in vaccinated gnotobiotic mice. Infect Immun. 1976 Jan;13(1):172–179. doi: 10.1128/iai.13.1.172-179.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannock G. W., Savage D. C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974 Mar;9(3):591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN UDEN N. The occurrence of Candida and other yeasts in the intestinal tracts of animals. Ann N Y Acad Sci. 1960 Aug 27;89:59–68. doi: 10.1111/j.1749-6632.1960.tb20130.x. [DOI] [PubMed] [Google Scholar]



