Abstract
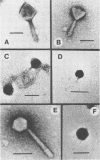
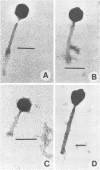
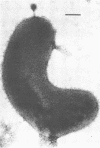
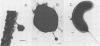
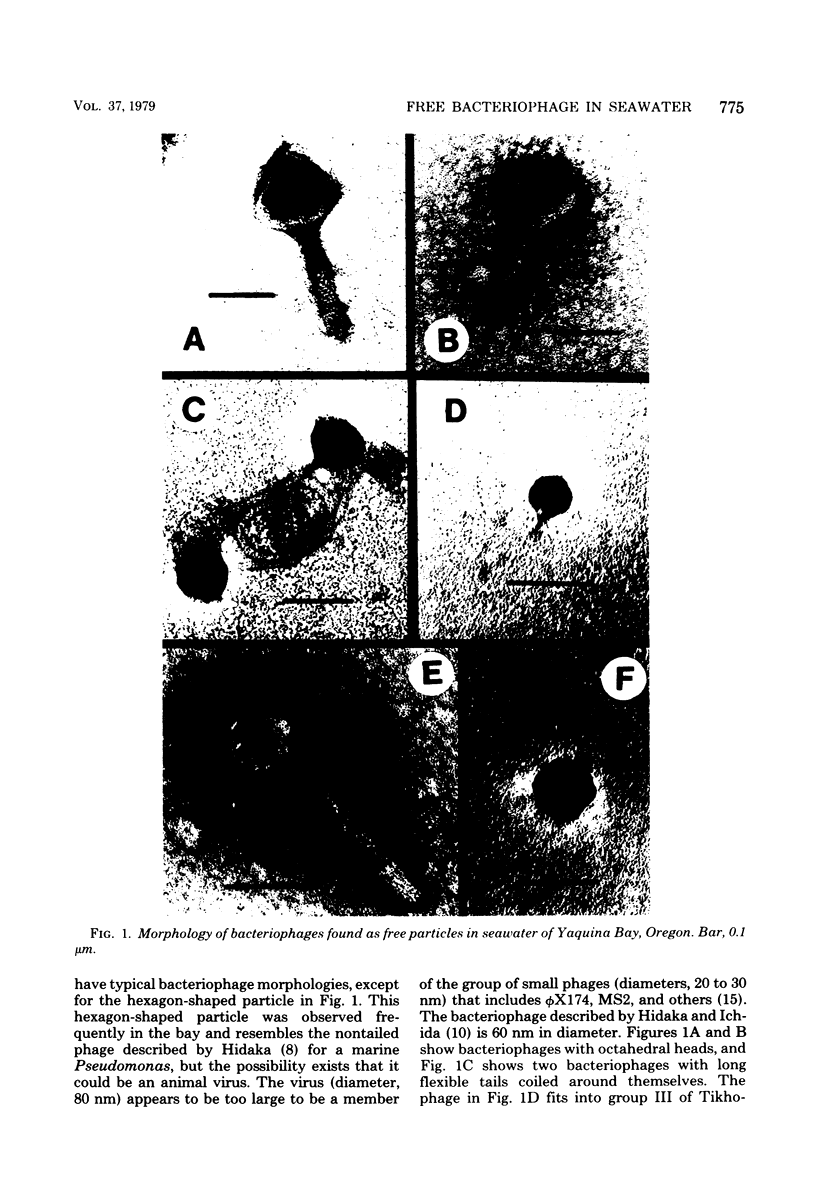
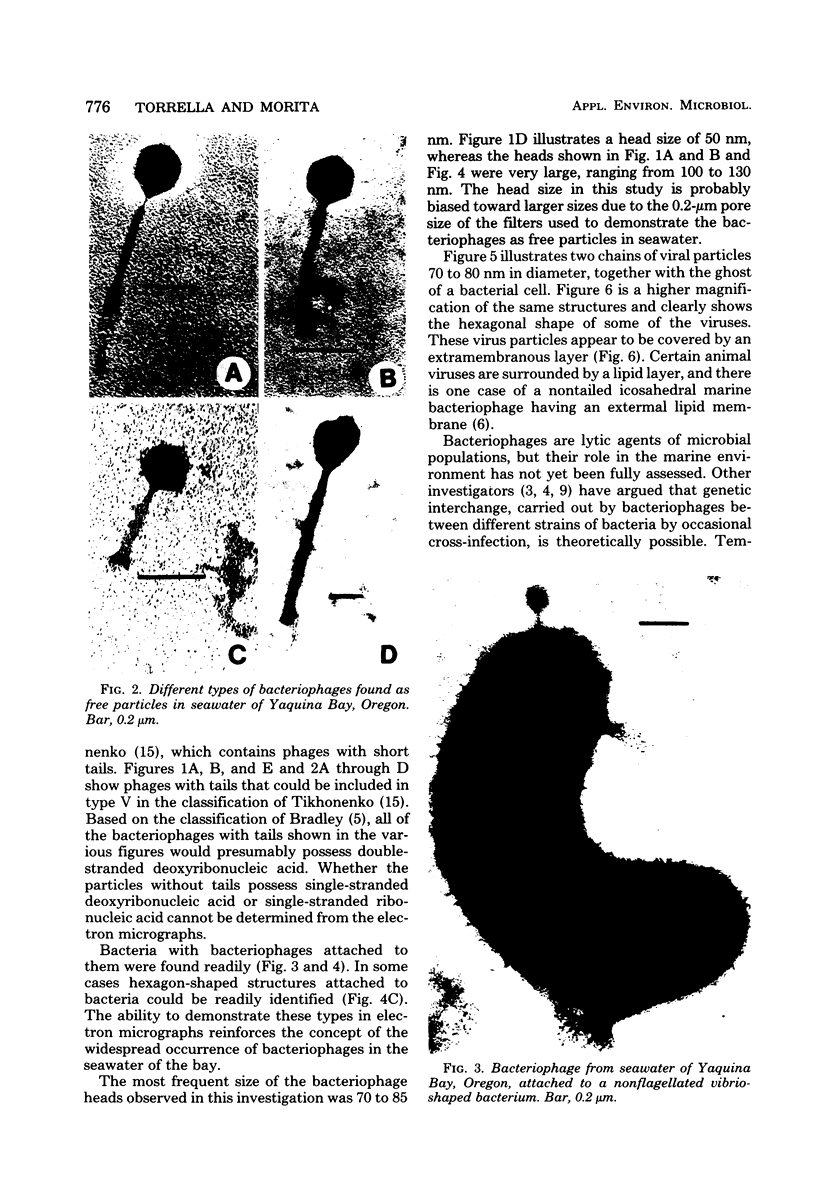
A variety of viral particles, the majority of them clearly identifiable as bacteriophages, were found in the seawater of Yaquina Bay, Oregon. These phages were obtained as free particles from the seawater without employing specific hosts for enrichments or further purification in the laboratory. A variety of electron micrographs showing different morphologies of phages as well as phage-bacterium interactions found in the seawater are presented. In the area where the bay received organic enrichment from seafood processing plants, a minimum of 10(4) phage particles per ml was estimated. Since the technique used was designed to concentrate particles 0.2 micrometer in diameter or larger it is assumed that the actual number of phage particles is higher than 10(4) particles per ml. The implications of the presence of such phage concentrations in bays and estuaries with a certain level of eutrophication are of obvious importance in considering the microbial ecology of these environments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Baross J. A., Liston J., Morita R. Y. Ecological relationship between Vibrio parahaemolyticus and agar-digesting vibrios as evidenced by bacteriophage susceptibility patterns. Appl Environ Microbiol. 1978 Sep;36(3):500–505. doi: 10.1128/aem.36.3.500-505.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baross J. A., Liston J., Morita R. Y. Incidence of Vibrio parahaemolyticus bacteriophages and other Vibrio bacteriophages in marine samples. Appl Environ Microbiol. 1978 Sep;36(3):492–499. doi: 10.1128/aem.36.3.492-499.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota-Robles E., Espejo R. T., Haywood P. W. Ultrastructure of bacterial cells infected with bacteriophage PM2, a lipid-containing bacterial virus. J Virol. 1968 Jan;2(1):56–68. doi: 10.1128/jvi.2.1.56-68.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon T. S., Chan Y. S., Sun S. M., Chau W. S. Distribution of coliphages in Hong Kong sewage. Appl Microbiol. 1970 Aug;20(2):187–191. doi: 10.1128/am.20.2.187-191.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggins A. R., Sandine W. E. Incidence and properties of temperate bacteriophages induced from lactic streptococci. Appl Environ Microbiol. 1977 Jan;33(1):184–191. doi: 10.1128/aem.33.1.184-191.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott Y. Estimation of low numbers of Escherichia coli bacteriophage by use of the most probable number method. Appl Microbiol. 1966 Mar;14(2):141–144. doi: 10.1128/am.14.2.141-144.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza K. A., Ginoza H. S., Haight R. D. Isolation of a polyvalent bacteriophage for Escherichia coli, Klebsiella pneumoniae, and Aerobacter aerogenes. J Virol. 1972 May;9(5):851–856. doi: 10.1128/jvi.9.5.851-856.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]