Abstract
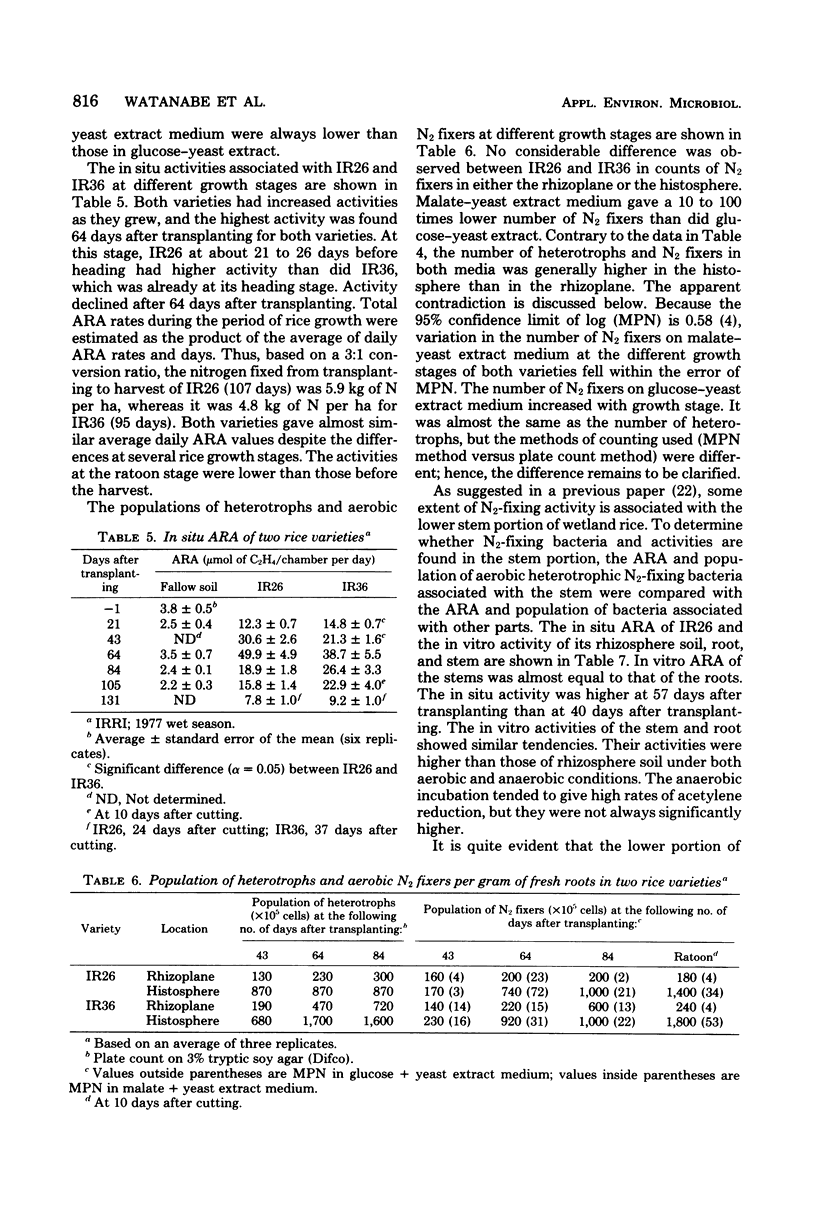
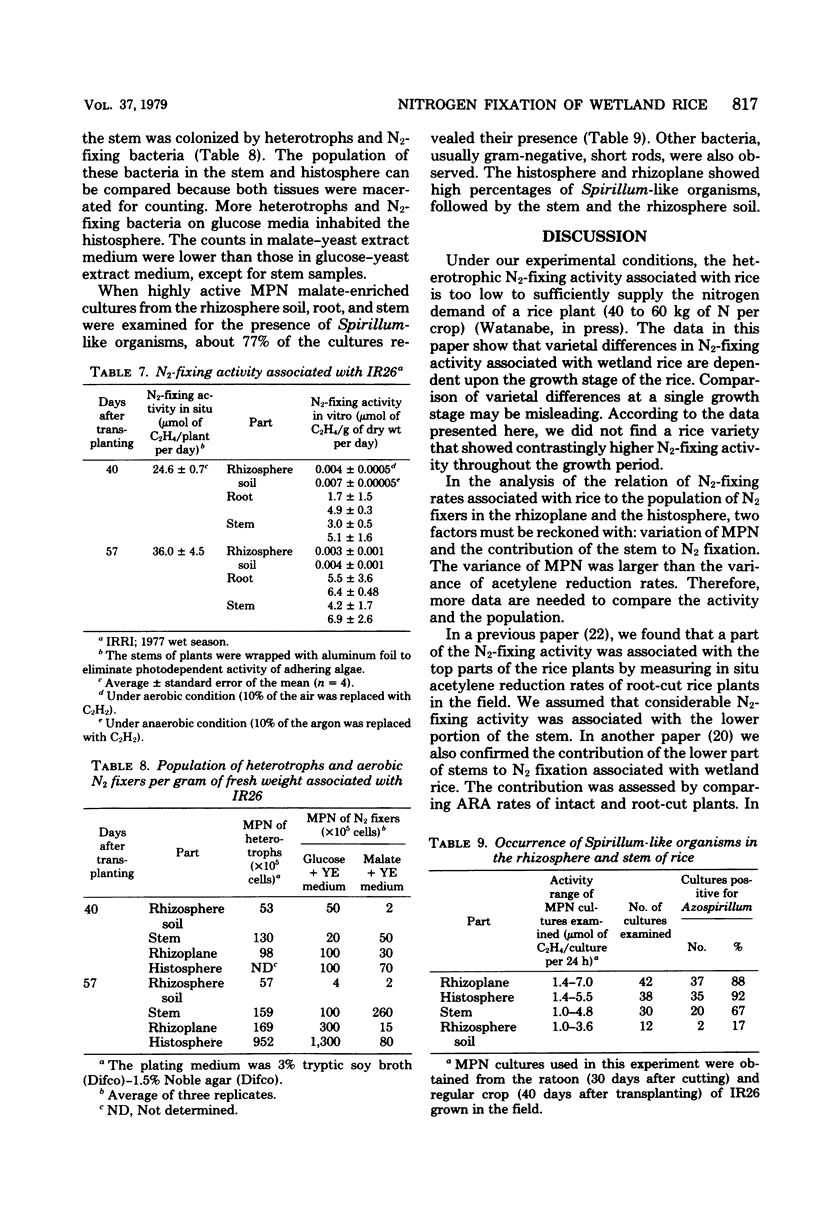
Nitrogen-fixing activity associated with different wetland rice varieties was measured at various growth stages by an in situ acetylene reduction method after the activities of blue-green algae (cyanobacteria) in the flood water and on the lower portion of the rice stem were eliminated. Nitrogen-fixing activities associated with rice varieties differed with plant growth stages. The activities increased with plant age, and the maximum was about at heading stage. The nitrogen fixed during the whole cropping period was estimated at 5.9 kg of N per ha for variety IR26 (7 days) and 4.8 kg of N per ha for variety IR36 (95 days). The population of aerobic heterotrophic N2-fixing bacteria associated with rice roots and stems was determined by the most-probable-number method, using semisolid glucose-yeast extract and semisolid malate-yeast extract media. The addition of yeast extract to the glucose medium increased the number and activity of aerobic heterotrophic N2-fixing bacteria. The glucose-yeast extract medium gave higher counts of aerobic N2-fixing bacteria associated with rice roots than did the malate-yeast extract medium, on which Spirillum-like bacteria were usually observed. The lower portion of the rice stem was also inhabited by N2-fixing bacteria and was an active site of N2 fixation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber L. E., Evans H. J. Characterization of a nitrogen-fixing bacterial strain from the roots of Digitaria sanguinalis. Can J Microbiol. 1976 Feb;22(2):254–260. doi: 10.1139/m76-034. [DOI] [PubMed] [Google Scholar]
- Barber L. E., Tjepkema J. D., Russell S. A., Evans H. J. Acetylene reduction (nitrogen fixation) associated with corn inoculated with Spirillum. Appl Environ Microbiol. 1976 Jul;32(1):108–113. doi: 10.1128/aem.32.1.108-113.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCHRAN W. G. Estimation of bacterial densities by means of the "most probable number". Biometrics. 1950 Jun;6(2):105–116. [PubMed] [Google Scholar]
- Dobereiner J., Marriel I. E., Nery M. Ecological distribution of Spirillum lipoferum Beijerinck. Can J Microbiol. 1976 Oct;22(10):1464–1473. doi: 10.1139/m76-217. [DOI] [PubMed] [Google Scholar]
- Lee K. K., Watanabe I. Problems of the acetylene reduction technique applied to water-saturated paddy soils. Appl Environ Microbiol. 1977 Dec;34(6):654–660. doi: 10.1128/aem.34.6.654-660.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson A. D., Barber L. E., Tjepkema J., Russell S. A., Powelson R., Evans H. J. Nitrogen fixation associated with grasses in Oregon. Can J Microbiol. 1976 Apr;22(4):523–530. doi: 10.1139/m76-078. [DOI] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Carbon and ammonia metabolism of Spirillum lipoferum. J Bacteriol. 1976 Nov;128(2):592–597. doi: 10.1128/jb.128.2.592-597.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okon Y., Albrecht S. L., Burris R. H. Methods for Growing Spirillum lipoferum and for Counting It in Pure Culture and in Association with Plants. Appl Environ Microbiol. 1977 Jan;33(1):85–88. doi: 10.1128/aem.33.1.85-88.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen W. L., Chakrabarty K., Klucas R. V., Vidaver A. K. Nitrogen fixation (acetylene reduction) associated with roots of winter wheat and sorghum in Nebraska. Appl Environ Microbiol. 1978 Jan;35(1):129–135. doi: 10.1128/aem.35.1.129-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkema J., Van Berkum P. Acetylene reduction by soil cores of maize and sorghum in Brazil. Appl Environ Microbiol. 1977 Mar;33(3):626–629. doi: 10.1128/aem.33.3.626-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bülow J. F., Döbereiner J. Potential for nitrogen fixation in maize genotypes in Brazil. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2389–2393. doi: 10.1073/pnas.72.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I., Cabrera D. R. Nitrogen fixation associated with the rice plant grown in water culture. Appl Environ Microbiol. 1979 Mar;37(3):373–378. doi: 10.1128/aem.37.3.373-378.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



