Abstract
CD4+ CD25high regulatory T cells (Tregs) of patients with relapsing-remitting (RR) multiple sclerosis (MS), in contrast to those of patients with secondary progressive (SP) MS, show a reduced suppressive function. In this study, we analysed forkhead box P3 (FOXP3) at the single-cell level in MS patients and controls (healthy individuals and patients with other neurological diseases) by means of intracellular flow cytometry. Our data revealed a reduced number of peripheral blood CD4+ CD25high FOXP3+ T cells and lower FOXP3 protein expression per cell in RR-MS patients than in SP-MS patients and control individuals, which was correlated with the suppressive capacity of Tregs in these patients. Interestingly, interferon (IFN)-β-treated RR-MS patients showed restored numbers of FOXP3+ Tregs. Furthermore, a higher percentage of CD4+ CD25high FOXP3+ Tregs in RR-MS patients, as compared with controls and SP-MS patients, expressed CD103 and CD49d, adhesion molecules involved in T-cell recruitment towards inflamed tissues. This was consistent with a significantly increased number of CD27+ CD25high CD4+ T cells in the cerebrospinal fluid (CSF), as compared with peripheral blood, in RR-MS patients. Taken together, these data show aberrant FOXP3 expression at the single-cell level correlated with Treg dysfunction in RR-MS patients. Our results also suggest that Tregs accumulate in the CSF of RR-MS patients, in an attempt to down-regulate local inflammation in the central nervous system.
Keywords: autoimmunity, multiple sclerosis, tolerance, regulatory T cells, FOXP3
Introduction
CD4+ CD25+ regulatory T cells are a subpopulation of suppressor T cells that play an important role in down-modulating the activation and effector function of potential auto-aggressive T cells.1,2 Loss of Tregs in animal models has demonstrated their importance in maintaining self tolerance, as these animals develop a variety of autoimmune manifestations. The interleukin (IL)-2 receptor α-chain (CD25), a marker commonly used to quantify Tregs, is not sufficiently specific as it is also expressed by activated T cells. Measurement of FOXP3 expression on T cells isolated directly ex vivo allows a more accurate investigation of the Treg frequency in healthy individuals and patients with immune disorders. The gene encoding the transcription factor FOXP3 was discovered as a master gene in the development of Tregs of mice.3,4 Loss-of-function mutations in the FOXP3 gene lead to the development of a severe lymphoproliferative disease and autoimmune manifestations in mice (scurfy model) and in patients with the immuno-dysregulatory, polyendocrinopathy, enteritis X-linked (IPEX) syndrome.5
We and others have reported that Tregs are functionally impaired in patients with relapsing-remitting (RR) multiple sclerosis (MS), an inflammatory disease of the central nervous system (CNS).6–8 In contrast, patients with secondary progressive MS (SP-MS) have a normal Treg function, suggesting a possible restoration of Treg function in the later disease stages.8 In the early disease phase of MS, it is thought that myelin-reactive T cells, potentially activated in the periphery by molecular mimicry, are reactivated in the CNS and start an immune cascade, attracting other immune cells. This local inflammation can eventually lead to damage to the myelin sheath, oligodendrocytes and axons, resulting in the typical neurological symptoms seen in MS patients.9,10 One hypothesis is that pathogenic myelin-reactive T cells in MS patients escape from peripheral tolerance as a result of disturbed immunoregulation. Therefore, it is important to further characterize the observed regulatory T-cell dysfunction in RR-MS patients. In addition to in vitro suppressive activity, FOXP3 expression has also been evaluated in MS patients. FOXP3 levels were measured either by reverse transcription–polymerase chain reaction (RT-PCR) (mRNA) or by western blotting (protein), and these reports demonstrated lower FOXP3 expression in the CD4+ CD25+ T-cell populations of RR-MS patients as compared with healthy controls (HCs) and SP-MS patients.8,11,12 However, as these methods investigate FOXP3 expression in bulk CD25+ populations, it is still unclear whether the reduced FOXP3 mRNA expression in RR-MS patients is a reflection of a reduced number of FOXP3+ CD25+ CD4+ Tregs or decreased FOXP3 expression at the Treg cell level.
Recently, a number of reports have indicated that Tregs can be divided into different subsets based on the expression of adhesion molecules.13–16 Adhesion molecules play an important role in the trafficking of Tregs (and conventional T cells) between the lymphoid and blood circulation systems and also in the retention of T cells at sites of inflammation and/or infection. For example, l-selection (CD62L) is a lymph node homing receptor, whereas integrin αE+ (CD103), integrin α4+[CD49d, or very late antigen-4 (VLA-4)] and the hyalorunate receptor (CD44) are involved in T-cell migration into inflamed tissues.14,17–20 A report by Soilu-Hänninen et al. showed increased expression of CD44 and CD49d in the total T-cell population during MS relapses,21 but until now no information has been available about the expression of adhesion molecules on Tregs in patients with MS. Whether Tregs migrate into the CNS of MS patients is not clear, but results from the animal model, experimental autoimmune encephalomyelitis (EAE), have demonstrated an accumulation of Tregs in CNS lesions.22,23
To explore the reduced FOXP3 expression in MS patients, we measured FOXP3 at the single-cell level in the peripheral blood mononuclear cells (PBMC) of a large panel of MS patients and controls by means of flow cytometry. In addition, we analysed the migration of human Tregs towards the CNS by measuring the expression of adhesion molecules on blood circulating Tregs, and we compared Treg frequencies in paired cerebrospinal fluid (CSF) and peripheral blood samples of MS patients and controls.
Materials and methods
Blood and CSF samples
Peripheral blood samples were obtained from 70 patients with clinically definite MS, 18 patients with other neurological diseases (ONDs), 10 patients with rheumatoid arthritis (RA), five patients with systemic lupus erythematosis (SLE) and 40 HCs. The characteristics of patients and controls are listed in Table 1. All MS patients fulfilled the McDonald criteria,24 and were diagnosed as having either RR-MS (n = 55) or SP-MS (n = 15). All RR-MS patients were in clinical remission at the time of blood collection. Fifty MS patients were untreated (no immunomodulatory drugs), whereas the other 20 MS patients were under treatment with interferon (IFN)-β1a (Rebif™; Serono, Geneva, Switzerland) or methotrexate at the time of the blood samplings. Other patients were diagnosed following criteria described elsewhere.25–27 The OND group consisted of 16 patients with non-inflammatory neurological diseases (NINDs) and two patients with other inflammatory neurological diseases (OINDs).
Table 1.
Characteristics of study subjects
| Subjects | No. | Gender1 (M/F) | Age (years) [mean (range)] | Untreated/ treated2 | EDSS [median (range)] |
|---|---|---|---|---|---|
| MS patients | |||||
| RR-MS | 55 | 14/41 | 41.9 (18–59) | 40/15 | 2.0 (0–4) |
| SP-MS | 15 | 4/11 | 55.5 (45–69) | 10/5 | 6.5 (4–9) |
| Control subjects | |||||
| ONDs | 18 | 8/10 | 47.7 (27–74) | 18/0 | NA |
| RA | 10 | 3/7 | 67.0 (55–85) | 5/5 | NA |
| SLE | 5 | 1/4 | 36.2 (20–52) | 5/0 | NA |
| HCs | 40 | 12/28 | 38.9 (20–58) | 40/0 | NA |
Gender: male (M)/female (F).
Immunomodulatory treatment within 3 months of blood sampling. Fifteen RR-MS patients and three SP-MS patients were treated with interferon-β; two SP-MS patients were treated with methotrexate; RA patients were treated with disease-modifying antirheumatic drugs. Untreated: no immunomodulatory drugs.
EDSS, expanded disability status score; HCs, healthy controls; MS, multiple sclerosis; NA, not applicable; ONDs, other neurological diseases; RA, rheumatoid arthritis; RR-MS, relapsing-remitting MS; SLE, systemic lupus erythematosus; SP-MS, secondary progressive MS.
Paired CSF samples were collected by lumbar puncture from a subgroup of MS (n = 12; nine RR-MS and three SP-MS patients, all untreated) and OND (n = 11) patients. The CSF was put immediately one ice and handled within 1 hr for flow cytometric analysis. CSF samples contaminated with red blood cells were excluded from further analyses. This study was approved by the local Medical Ethical Committee and informed consent was obtained from all study subjects.
Flow cytometric analysis
Mononuclear cells were separated from whole blood (PBMC) by means of Ficoll density gradient centrifugation (Histopaque; Sigma Diagnostics, St Louis, MO). CSF cells were collected by centrifugation for 12 min at 250 g.
For surface marker analyses, PBMC or CSF cells were incubated for 30 min at 4° with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or peridinin chlorophyll protein (PerCP)-conjugated monoclonal antibodies (mAbs). The following mAbs were used: anti-human CD3 (clone SK7), CD4 (clone SK3), CD8 (clone SK1), CD25 (clone 2A3) (all from BD Biosciences, Erembodegem, Belgium), CD27 (clone 9F4), CD44 (clone NKI-P2), CD49d (clone BU49), CD62L (clone SK11), CD103 (clone 2G5) (all from Immunotools, Friesoythe, Germany) and the glucocorticoid-induced tumour necrosis factor (TNF) receptor family-related gene (GITR; clone 110416; R&D Systems, Abingdon, UK). For intracellular staining of FOXP3 and/or cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), cell surface staining was first completed and cells were subsequently fixed and permeabilized according to the FOXP3 staining buffer set protocol (eBioscience, San Diego, CA) before anti-human FOXP3 (clone PCH101; eBioscience) and/or anti-human CTLA-4 (clone BNI3; Immunotech, Marseille, France) was added for 30 min at 4°. Samples were analysed on a FACSCalibur flow cytometer (BD Biosciences). The percentage of positive cells and the mean fluorescence intensity (MFI) arbitrary units (AU) for a specific marker were calculated using CellQuest software (BD Biosciences).
Treg suppression assay
CD4+ T cells were pre-enriched from whole blood by means of the RosetteSep™ CD4+ T-cell Enrichment Cocktail (StemCell Technologies, Grenoble, France) as described previously.8 Next, cells were incubated for 30 min with CD25-PE- and CD4-PerCP-labelled antibodies (BD Biosciences) at 4°. CD4+ CD25high T cells were sorted as the top 2% of CD4+ T cells (showing the brightest expression of CD25) using a FACSaria™ (BD Biosciences). In parallel, CD4+ CD25– T cells were isolated as responder T cells. The purity of fluorescence-activated cell sorter (FACS)-sorted cell fractions was routinely 97–99% for each T-cell fraction. For assessment of Treg suppressive capacity, Tregs and 5,6-carboxy fluorescein diacetate succinimidyl ester (CFSE) (Invitrogen, Merelbeke, Belgium)-labelled CD4+ CD25– T cells were cocultured at different ratios, as described previously.8,28 Cell cultures were stimulated with 2 µg/ml anti-CD3 Ab (clone 2G3). After 5 days, cells were harvested and the CFSE signal of gated CD4+ T cells was analysed by flow cytometry. The suppressive capacity of Tregs towards responder T cells in coculture was expressed as the relative inhibition of the percentage of CFSElow responder cells.
Statistical analyses
All statistical analyses were performed using prism software version 4·0 (Graphpad, San Diego, CA). For comparisons of Treg parameters between patients and controls, Student's t-tests were applied. CD27+ CD25high CD4+ T-cell frequencies in paired CSF and blood samples of patients and controls were compared by paired Student's t-tests. Correlations between parameters were evaluated using Spearman's correlation tests. Differences were considered significant when P < 0·05. Regarding the IFN-β-treated patients, a treatment responder was defined as any patient free of relapse during the 12-month period of IFN-β therapy preceding the sample collection.
Results
Analysis of FOXP3 expression at the single-cell level in MS patients and controls
FOXP3 expression was measured in freshly isolated PBMC from 70 patients with MS, 40 HCs and 18 patients with ONDs by means of flow cytometry. FOXP3 was predominantly expressed by CD4+ T cells (range 1·7–10·9%) and was also expressed by a small fraction of CD8+ T cells (0·1–1·6%), but was not expressed by B cells or monocytes for both patients and controls (Fig. 1 and data not shown). A significantly reduced frequency of FOXP3+ CD25+ CD4+ T cells was observed in untreated RR-MS patients (3·5 ± 1·0% of CD4+ T cells) as compared with HCs (5·4 ± 0·3%; P < 0·01) and patients with ONDs (5·4 ± 0·4%; p < 0·01) (Figs 1 and 2a). Interestingly, SP-MS patients showed a normal frequency of FOXP3+ CD25+ CD4+ T cells (4·8 ± 0·4%; P > 0·05 as compared with HCs and patients with ONDs; P < 0·05 as compared with RR-MS patients; Figs 1 and 2a). A reduced number of FOXP3+ cells in the blood of RR-MS patients was also observed in the total CD4+ T-cell population (Fig. 1 and data not shown).
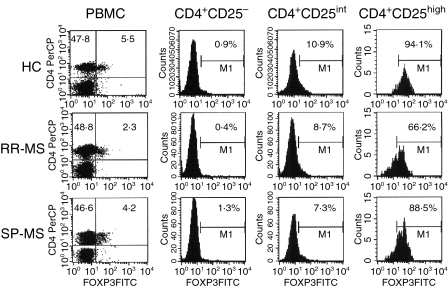
Figure 1.
Intracellular forkhead box P3 (FOXP3) expression in peripheral blood mononuclear cells (PBMC) isolated from healthy controls (HCs) and multiple sclerosis (MS) patients. PBMC of HCs and MS patients were surface-stained with anti-CD4 and anti-CD25 antibodies. After permeabilization, cells were labelled with anti-human FOXP3 antibody. Cells were analysed by means of flow cytometry. The CD25 signal was subdivided into negative, intermediate (int) and high levels. The CD25high CD4+ T-cell population was defined as the top 2% of CD4+ T cells showing the brightest CD25 expression. The figure shows costainings of FOXP3 and CD4 (dot plots) or FOXP3 expression of CD4+ CD25–, CD4+ CD25int and CD4+ CD25high T cells (histograms). The cut-offs for positive and negative signals were set based on isotype-matched control antibody staining. Numbers in each histogram indicate the percentage of FOXP3-positive cells. Data are shown for one HC, one relapsing-remitting multiple sclerosis (RR-MS) patient and one secondary progressive multiple sclerosis (SP-MS) patient, and are illustrative for all study subjects, as indicated in Figure 2.
Figure 2.
Frequency of CD4+ CD25+ FOXP3+ T cells and forkhead box P3 (FOXP3) expression at the cellular level in multiple sclerosis (MS) patients and control subjects. Peripheral blood mononuclear cells (PBMC) of 40 healthy controls (HCs), 18 patients with other neurological diseases (ONDs), 55 patients with relapsing-remitting multiple sclerosis (RR-MS) (40 untreated and 15 IFN-β-treated), 15 patients with secondary progressive multiple sclerosis (SP-MS), 10 patients with rheumatoid arthritis (RA) and five patients with systemic lupus erythematosis (SLE) were stained for FOXP3, CD25 and CD4 as described in Fig. 1. (a) The percentage of FOXP3+ CD25+ CD4+ T cells relative to the total number of CD4+ T cells. (b) Percentage of FOXP3+ cells within the CD4+ CD25–, CD4+ CD25int and CD4+ CD25high T-cell populations. (c) For five untreated RR-MS patients and three HCs, FOXP3+ CD25+ CD4+ T-cell frequencies were measured at different time-points (0, 6 and 12 months after initial measurement). (d) Mean fluorescence intensity (MFI) of FOXP3 from CD4+ CD25high FOXP3+ T cells in patients and controls. Indicated values (shown as box and whiskers) represent MFI signals obtained from FOXP3 stainings. MFI values of cells stained by isotype-matched control antibody (background MFI) were subtracted from FOXP3 MFI values. *P < 0·05; **P < 0·01 as compared with the indicated study populations. (e) FOXP3 MFI of CD4+ CD25high FOXP3+ T cells was plotted against percentages of FOXP3+ cells within the CD4+ CD25high T-cell populations of 55 MS patients, 18 OND patients and 40 HCs. r and P values were calculated using Spearman's correlation test.
As expected, the FOXP3 expression correlated with the CD25 expression of CD4+ T cells, i.e. the CD25high CD4+ T cells showed the brightest expression of FOXP3. A fraction of CD25int CD4+ T cells also expressed FOXP3, whereas CD25– CD4+ T cells were mostly FOXP3-negative (Fig. 1). We therefore further analysed the Treg frequency in these different CD25 subpopulations of MS patients and controls. A reduced proportion of FOXP3+ cells in the CD25high CD4+ T-cell population (defined as the top 2% of CD25+ cells) in RR-MS patients (53·1 ± 3·3%) was seen as compared with HCs (85·0 ± 1·6%; P < 0·01), patients with ONDs (82·0 ± 3·5; P < 0·01) and SP-MS patients (71·0 ± 3·1%; P < 0·05) (Figs 1 and 2b). In contrast, no differences in FOXP3 expression were detected between, respectively, CD25int or CD25– CD4+ T cells of patients and controls (Figs 1 and 2b).
For five RR-MS patients and three HCs, we assessed the Treg frequency at three different time-points. The number of FOXP3+ CD4+ T cells in MS patients and controls did not change significantly in a 1-year period (Fig. 2c).
To obtain information about FOXP3 expression levels per cell, the FOXP3 MFI of CD4+ CD25high FOXP3+ T cells was determined in MS patients and controls. A decreased level of FOXP3 was seen in RR-MS patients (MFI: 28·7 ± 3·4) as compared with HCs (41·4 ± 2·6; P < 0·05) and OND patients (44·4 ± 2·5; P < 0·01) (Fig. 2d). There was a trend towards lower FOXP3 expression in SP-MS patients, but this difference was not statistically significant (29·7 ± 4·1; P = 0·07 compared with HCs and patients with ONDs) (Fig. 2d). No differences were seen in FOXP3 frequency or MFI between OIND and NIND patients (data not shown). Overall, there was a positive correlation between the percentage of FOXP3+ cells within the CD4+ CD25high population and the FOXP3 MFI of CD4+ CD25high FOXP3+ T cells (Fig. 2e; total population: r = 0·59, P < 0·0001; HCs: r = 0·32, P < 0·05; RR-MS patients: r = 0·47, P < 0·01; SP-MS patients: r = 0·82, P < 0·001; OND patients: r = 0·50, P < 0·05).
To evaluate whether changes in FOXP3 expression were also observed in patients with other autoimmune diseases, we measured intracellular FOXP3 expression in a small number of patients with RA (n = 10) and SLE (n = 5). Our preliminary data showed no significant differences in FOXP3 frequency (FOXP3+ CD25+ CD4+ T cells: RA patients: 5·0 ± 0·7%; SLE patients: 6·7 ± 2·0%) or expression level (MFI: RA patients: 39·9 ± 2·9; SLE patients: 47·1 ± 5·2) between these patient groups and HCs (Figs 2a and d). Remarkably, SLE patients consisted of two groups on the basis of high and low numbers of Tregs, which was in agreement with a recent report.29
In conclusion, RR-MS patients showed a reduced number of blood circulating CD4+ CD25high FOXP3+ regulatory T cells and decreased FOXP3 expression level per cell.
Treg FOXP3 expression is correlated with in vitro CD4+ CD25high T-cell-mediated suppression
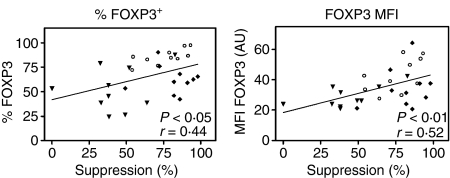
To evaluate whether FOXP3 expression was correlated with the in vitro suppressive capacity of Tregs, FACS-sorted CD4+ CD25high T cells (Tregs) and CD4+ CD25– T cells (Tresp) of 10 HCs, 10 RR-MS patients (untreated) and 10 SP-MS patients were set up in a coculture assay28, next to the flow cytometric FOXP3 analysis of the CD4+ CD25high T cells. First, we could confirm previous observations6,8 of reduced Treg function in RR-MS patients and normal suppression in SP-MS patients (Fig. 3). Furthermore, a clear positive correlation was observed between CD4+ CD25high T-cell-mediated suppression (at a Tresp:Treg ratio of 1 : 1) and FOXP3 expression levels (MFI) (Fig. 3; Spearman's r = 0·55; P < 0·01). In addition, we also observed a correlation between the CD4+ CD25high T-cell suppressive capacity and the number of FOXP3+ cells within this population (Fig. 3; Spearman's r = 0·44; P < 0·05).
Figure 3.
The suppressive capacity of CD4+ CD25high T cells in function of forkhead box P3 (FOXP3) expression. The suppressive capacity of CD4+ CD25high T cells from 10 patients with relapsing-remitting multiple sclerosis (RR-MS;  ), 10 patients with secondary progressive multiple sclerosis (SP-MS; ♦) and 10 healthy controls (HCs; ○) were plotted against the percentage of CD4+ CD25high T cells positive for FOXP3, and FOXP3 mean fluorescence intensity (MFI). Suppression values represent percentages of inhibition of proliferation (5,6-carboxy fluorescein diacetate succinimidyl ester (CFSE)) of CD4+ CD25– T cells (Tresp) by CD4+ CD25high T cells (Tregs) as measured in coculture experiments at a 1 : 1 (Tresp:Treg) ratio. r and P values were calculated using Spearman's correlation tests.
), 10 patients with secondary progressive multiple sclerosis (SP-MS; ♦) and 10 healthy controls (HCs; ○) were plotted against the percentage of CD4+ CD25high T cells positive for FOXP3, and FOXP3 mean fluorescence intensity (MFI). Suppression values represent percentages of inhibition of proliferation (5,6-carboxy fluorescein diacetate succinimidyl ester (CFSE)) of CD4+ CD25– T cells (Tresp) by CD4+ CD25high T cells (Tregs) as measured in coculture experiments at a 1 : 1 (Tresp:Treg) ratio. r and P values were calculated using Spearman's correlation tests.
These results indicate that a combination of a reduced number of FOXP3+ CD25high CD4+ T cells and reduced FOXP3 expression per cell in RR-MS patients contribute to the functional Treg defect in these patients.
Influence of IFN-β treatment on FOXP3 expression
A panel of 15 RR-MS patients treated with IFN-β were also tested for FOXP3 expression. Interestingly, these patients showed significantly higher frequencies of CD4+ CD25+ FOXP3+ T cells (5·3 ± 1·2%) than untreated RR-MS patients (3·5 ± 1·0%; P < 0·01; Fig. 2a). This higher number of CD4+ CD25+ FOXP3+ T cells was consistent with a significantly higher number of FOXP3+ cells in the CD4+ CD25high population of the IFN-β-treated patients (Fig. 2b). The mean FOXP3 expression levels (MFI: 40·6 ± 8·1) of IFN-β-treated patients were also higher than those of untreated patients (MFI: 28·7 ± 3·4), although the difference was not statistically significant (P > 0·05; Fig. 2d). We next divided patients into IFN-β responders (n = 11) and non-responders (n = 4), based on clinical data (see ‘Materials and methods’), to see if there was a correlation with treatment responsiveness. FOXP3 MFI values and the percentage of CD4+ CD25+ FOXP3+ T cells were slightly higher in responders (MFI: 44·3 ± 7·2; frequency: 5·4 ± 0·3%) than in non-responders (MFI: 40·1 ± 3·3; frequency: 4·8 ± 0·7%), although no significant differences were found. However, the difference in FOXP3 MFI between IFN-β responders and untreated RR-MS patients was significant (P < 0·05).
In conclusion, IFN-β treatment in vivo could potentially compensate for the reduced Treg numbers in RR-MS patients.
Phenotypic analysis of Tregs in MS patients and controls
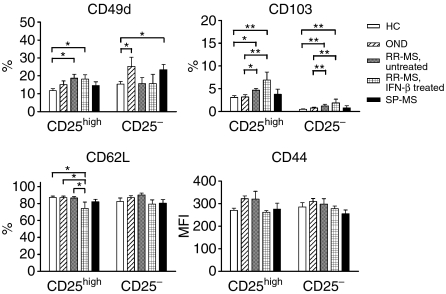
It has recently been shown that CD25high Tregs represent a heterogeneous population which can be divided into different Treg subsets based on the expression of adhesion molecules, which regulate T cell trafficking.13,14 Therefore, we determined the expression of l-selectin (CD62L), the hyalorunate receptor (CD44), integrin αE+ (CD103), integrin α4+ (CD49d) and Treg function-associated molecules (GITR and CTLA-4) on CD4+ CD25high T cells and their counterparts CD4+ CD25– T cells from MS patients (15 untreated and 10 IFN-β-treated RR-MS patients and 12 SP-MS patients) and control subjects (20 HCs and 15 patients with OND) by means of flow cytometry. An overview of these results is shown in Fig. 4 and Table S1 (Supplementary material). GITR, CTLA-4 (intracellular) and CD103 were mainly expressed by CD4+ CD25high T cells in both MS patients and controls. All CD4+ CD25high and CD4+ CD25– T cells expressed CD44 (100% for all individuals tested; data not shown) and were CD62Lhigh, whereas high expression levels of CD49d (CD49dhigh) were found in a smaller subset of both cell types in patients and controls (Supplementary material, Table S1). In a comparison of the expression profiles of CD4+ CD25high T cells of MS patients and controls, we observed a significantly higher number of cells positive for CD103 and CD49dhigh in RR-MS patients (untreated and IFN-β-treated) as compared with HCs and patients with OND. Also, significantly more CD4+ CD25– T cells of RR-MS patients were CD103+ compared with controls, although the number of CD4+ CD25– CD103+ T cells in these patients was still very small. No significant correlation (P > 0·05) was observed between the percentage of CD103+ or CD49dhigh cells and the percentage of FOXP3+ cells or FOXP3 MFI within the CD4+ CD25high T-cell population of MS patients (data not shown). Regarding the CTLA-4 and GITR expression of peripheral CD4+ CD25high T cells, we observed no differences between MS patients and controls, which was in agreement with our previous observations.8
Figure 4.
Expression of adhesion molecules on regulatory T cells (Tregs) of multiple sclerosis (MS) patients and controls. CD4+ CD25high and CD4+ CD25– T cells within the peripheral blood mononuclear cell (PBMC) population of 20 healthy controls (HCs), 15 patients with other neurological diseases (ONDs), 15 untreated and 10 interferon (IFN)-β-treated relapsing-remitting multiple sclerosis (RR-MS) patients and 12 secondary progressive multiple sclerosis (SP-MS) patients were analysed for expression of the indicated markers [percentage positive cells or mean fluorescence intensity (MFI)] by means of flow cytometry. *P < 0·05; **P < 0·01 compared with the corresponding population.
Remarkably, whereas no differences in CD44 expression levels or numbers of CD62Lhigh CD4+ CD25high T cells of untreated RR-MS and SP-MS patients could be detected as compared with controls, IFN-β-treated RR-MS patients showed a significantly lower number of CD4+ CD25high T cells that were CD62Lhigh than untreated RR-MS patients (P < 0·05) and controls (P < 0·05).
In the analysis of the same set of markers on FOXP3+ and FOXP3– CD4+ T cells in a small cohort of MS patients and control subjects, the same significant differences were detected in percentages of CD103+ and CD49dhigh cells within CD4+ FOXP3+ T cells of RR-MS patients compared with control individuals (Table S1).
Taken together, the detection of a higher percentage of CD103+ and VLA-4+ T cells in the Treg population in RR-MS patients could reflect an increased migration capacity of Tregs towards inflammatory lesions in the CNS.
Treg numbers are increased in the CSF compared with peripheral blood in RR-MS patients
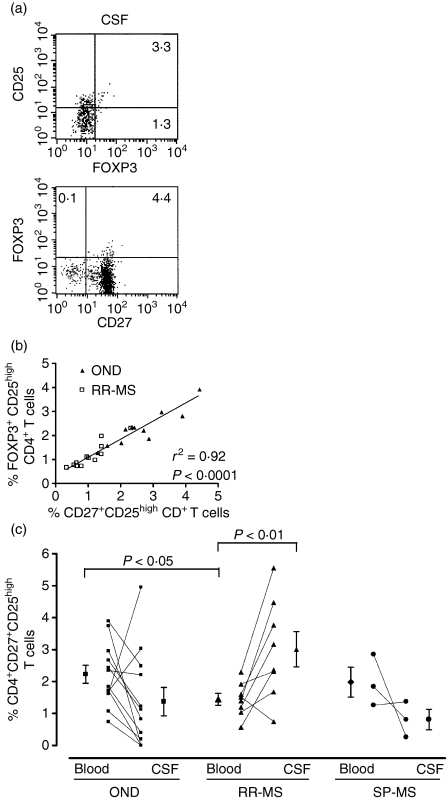
To evaluate a possible migration of Tregs towards the CNS in MS patients, we quantified Treg numbers in paired CSF and blood samples in MS patients (n = 11; eight RR-MS patients and three SP-MS patients, all untreated) and patients with ONDs (n = 12; 10 NIND patients and two OIND patients). Interestingly, we could detect CD4+ CD25+ FOXP3+ T cells in the cerebrospinal fluid of RR-MS patients (n = 2; Fig. 5a). However, because of the low number of cells collected and the requirements of the intracellular staining protocol, it was not feasible to analyse all the CSF samples for FOXP3 expression. In an alternative approach, we focused on the frequency of CD27+ CD25high CD4+ T cells, as a previous study showed that Tregs and effector T cells could be distinguished near the site of inflammation in patients with juvenile idiopathic arthritis (JIA) based on CD27 expression.30 In agreement with that report, we demonstrated that the majority of CD4+ FOXP3+ T cells in both the peripheral blood (data not shown) and CSF (Fig. 5a) of MS patients was positive for CD27. To further verify our approach, we compared percentages of CD27+ CD25high and FOXP3+ CD25high CD4+ T cells (within the total CD4+ T-cell population) in PBMC isolated from OND (n = 11) and RR-MS (n = 12) patients. Percentages of CD27+ CD25high and FOXP3+ CD25high CD4+ T cells were strongly comparable in all individuals tested (P < 0·0001; linear regression analysis r2 = 0·92; Fig. 5b). These results indicate that quantification of CD27+ CD25high CD4+ T cells accurately reflects the number of FOXP3+ CD25high CD4+ T cells and therefore can be used to measure CD25high Treg frequencies in CSF samples.
Figure 5.
Analysis of regulatory T cell (Treg) frequency in the cerebrospinal fluid (CSF) of patients with multiple sclerosis (MS) and patients with other neurological diseases (ONDs). (a) Flow cytometric analysis of forkhead box P3 (FOXP3), CD25 and CD4 on cells isolated from the CSF of relapsing-remitting multiple sclerosis (RR-MS) patients. The dot plots shown are representative for data obtained from two RR-MS patients. (b) Percentages of CD27+ CD25high and FOXP3+ CD25high CD4+ T cells (within the total CD4+ T-cell population) were determined by flow cytometric analysis of peripheral blood mononuclear cells (PBMC) isolated from 11 OND and 12 RR-MS patients. The cut-off value for high staining of CD25 was set at a constant value (102) to allow comparison between the T-cell subsets. The percentages of CD27+ CD25high and FOXP3+ CD25high CD4+ T cells were significantly correlated (P < 0·0001) in the PBMC of the same individuals as measured by linear regression analysis (r2 = 0·92). (c) Frequency of CD4+ CD27+ CD25high T cells in paired peripheral blood and CSF samples of 12 OND, eight RR-MS and three SP-MS patients.
Interestingly, we observed a significantly higher number of CD27+ CD25high CD4+ T cells in the CSF of RR-MS patients (3·0 ± 0·6%) compared with numbers in paired blood samples (1·4 ± 0·2%; P < 0·01; Fig. 5c). For patients with OND and SP-MS, the frequencies of CD27+ CD25high CD4+ T cells in the CSF (OND patients: 2·2 ± 0·3%; SP-MS patients: 2·0 ± 0·5%) and in blood (OND patients: 1·4 ± 0·4%; SP-MS patients: 0·8 ± 0·3%) were not significantly different (Fig. 5c). Numbers of CD27+ CD25high CD4+ T cells in the peripheral blood were lower in RR-MS patients than in OND patients (Figs 5b and c), further supporting our initial observations of a reduced frequency of FOXP3+ T cells in RR-MS patients.
Taken together, these results indicate that Tregs of RR-MS patients, but not of SP-MS or OND patients, accumulate in the CSF.
Discussion
The transcriptional repressor FOXP3 plays a key role in the development and function of naturally occurring CD4+ CD25+ Tregs. Analyses of FOXP3 mRNA and protein expression using, respectively, quantitative RT-PCR or western blotting indicated reduced FOXP3 levels in the CD25 Treg population of RR-MS patients but provided no information about FOXP3 expression at the single-cell level. This report is the first to demonstrate lower numbers of circulating CD4+ CD25high FOXP3+ T cells and decreased FOXP3 expression per cell in RR-MS patients compared with SP-MS patients and controls.
In our study, FOXP3 expression of CD4+ CD25+ T cells was measured on freshly isolated PBMC in a large panel of patients and different controls by means of flow cytometry. The observation of a lower number of CD4+ CD25high FOXP3+ T cells and a reduced FOXP3 expression level in RR-MS patients compared with HCs and SP-MS patients is consistent with our current and previous functional analyses of Tregs in these patients. We and others reported a reduced suppressor function of CD4+ CD25high T cells isolated from RR-MS patients and a normal Treg function in SP-MS patients.6–8 We now extend these results by demonstrating a correlation between the suppressive capacity of Tregs and FOXP3 expression at the cellular level. This is consistent with our earlier report of an association between FOXP3 mRNA levels and Treg suppression.8 Moreover, for the majority of patients and controls, there was a positive correlation between FOXP3+ cells within their CD4+ CD25high T-cell population and FOXP3 levels. This means that the blood circulating Tregs in some RR-MS patients, in addition to being reduced in number, also expressed low levels of FOXP3 per cell. These data further support a potential Treg dysfunction in a subset of RR-MS patients as a result of alterations in FOXP3 expression.
Several mechanisms may account for the reduced FOXP3 expression and Treg frequency in RR-MS patients. First, we and others showed that the number of CD4+ CD25high T cells is not significantly different in MS patients and HCs.6–8 Here, we found that the frequency of FOXP3+ cells within the CD4+ CD25high T cells of RR-MS patients was reduced, suggesting that a subgroup of the CD25high Treg cells may have completely or partly lost their FOXP3 expression. Oh and coworkers recently reported that patients with human T-lymphotrophic virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP), an inflammatory neurological disease, have a reduced expression of FOXP3 in PBMC.31 Their data indicated that this reduction was mediated by HTLV-1 tax proviral DNA load in these patients. Viral infections have also been associated with MS pathology, although an unequivocal role for a specific virus has not been demonstrated to date.10
Alternatively, it could be that the reduced FOXP3 expression is associated with FOXP3 gene alterations within our MS population. For type 1 diabetes, another T-cell-mediated autoimmune disorder, a functional FOXP3 polymorphism has been associated with disease susceptibility in a Japanese population.32 In MS, no FOXP3 polymorphism studies have yet been conducted. Our preliminary data show that FOXP3 levels can be up-regulated in the CD4+ T cells of RR-MS patients after T-cell receptor stimulation (KV, unpublished data), indicating that FOXP3 expression can be regulated in these patients. Nevertheless, polymorphisms within the FOXP3 gene could have contributed to altered FOXP3 expression, transcript stability, protein turnover or function.
In addition, suboptimal thymic development or peripheral homeostasis of Tregs could have accounted for the lower Treg numbers. Indeed, several reports indicate an age-inappropriate reduction in thymic output or altered T-cell homeostasis in a subset of MS patients.33–35 A detailed analysis of parameters related to Treg thymic output in MS patients is necessary to investigate this possibility.
It could also be that the balance between auto-aggressive T cells and Tregs is disturbed in RR-MS patients, resulting in more activated T cells in the CD25high CD4+ T-cell population. It has recently been shown in a mouse model that decreased FOXP3 expression can cause defective Treg function and Treg conversion into effector T cells, which would contribute to immune disorders observed in these animals.36 Results obtained by Viglietta et al. also support this possibility, as these investigators showed a lower cloning efficiency of CD4+ CD25high T cells with suppressor function in RR-MS patients compared with HCs.6
Finally, the possibility cannot be excluded that Tregs actively migrate to the site of inflammation in RR-MS patients, causing a preferential shift towards the CNS compartment. To investigate this possibility, we first measured the expression of a set of adhesion molecules (CD62L, CD44, CD103 and CD49d) and subsequently quantified Tregs in the CSF of MS patients and controls. We found that a significantly higher percentage of CD4+ CD25high FOXP3+ Tregs expressed CD103 and VLA-4 in RR-MS than in HCs and SP-MS patients. In mice, it has been demonstrated that CD103+ CD4+ T cells, regardless of their CD25 expression, represent a subpopulation of effector/memory Tregs, which can actively migrate to inflammatory lesions induced in these animal models.14 In this regard, we should also draw attention to our observation of higher numbers of CD25– CD103+ T cells in RR-MS patients. In an EAE study, an accumulation of CD103+ FOXP3+ T cells was detected in CNS lesions.23 A similar role for CD103 on human Tregs as that shown for animal-derived Tregs is therefore possible.37 In addition, it is known that VLA-4 plays a central role in endothelial transmigration of T cells into the inflamed CNS.18 Our observations therefore suggest that a number of peripheral Tregs in RR-MS patients have an increased capacity to migrate into the inflamed CNS lesions in RR-MS patients. Indeed, we observed FOXP3+ CD4+ T cells in the CSF of MS patients and OND. However, because of technical restrictions we could not analyse FOXP3 expression for all CSF/blood samples. Therefore, to allow accurate comparison of Treg numbers among the different tissue samples and subjects, we focused on the CD4+ T cells with high expression of CD25 and CD27. The number of these CD27+ CD25high CD4+ T cells corresponded to the frequency of FOXP3+ CD25high CD4+ T cells, indicating that this gating strategy excluded a possible contamination of activated cells in our Treg quantification. We have to mention that regulatory FOXP3+ CD25int CD4+ T cells could be missed using this approach. Nevertheless, our analysis showed that Tregs with a similar phenotype (i.e. high CD25 and CD27 expression) were increased in the CSF as compared with the peripheral blood in RR-MS patients, but not in OND or SP-MS patients. This was in agreement with recent observations by Feger et al., who quantified CD25high CD45RO+ T cells in the CSF and blood of MS patients.38 Functional analysis of CSF-derived CD4+ CD25high T cells of MS patients would be very informative. Unfortunately, because of the low frequency of these cells in the CSF, it is not possible to perform Treg/Tresp coculture experiments. It can be speculated that Tregs in the CSF of MS patients have a lower suppressive capacity, as these Tregs could represent the same population of cells (i.e. with lower FOXP3 expression levels) as those in the peripheral blood. However, it is possible that the most potent Tregs are present within the MS lesions, as has been shown in inflammatory lesions in EAE animals22 and in the synovial fluid of patients with RA.39 Korn et al.23 recently showed, in an EAE model, that CNS-accumulated Tregs could inhibit the proliferation of peripheral blood-derived naïve and myelin oligodendrocyte glycoprotein (MOG)-reactive T cells but not the proliferation of (inflamed) CNS tissue-derived MOG-reactive T effector cells. Their data underline a role for the local inflammatory cytokine environment in determining the in situ outcome of the Treg suppressive capacity. In our study, no correlation between CD103 or VLA-4 expression and FOXP3 levels in CD4+ CD25high T cells of MS patients was detected, suggesting that CD103 and CD49d are not exclusively expressed on Tregs with the strongest suppressive capacity. The identification of surface markers related to the in vitro suppressor function is needed to clarify this issue.
In the light of our own and other findings, we suggest the following hypothesis concerning the role of Tregs in MS pathology. In the early phase of the disease, a defect in Treg function caused by either genetic alterations or reduced thymic Treg output could contribute to suboptimal peripheral tolerance, leading to an escape of auto-aggressive T cells. As a feedback mechanism, circulating Tregs could up-regulate tissue retention molecules and preferentially migrate to the inflamed CNS lesions. Whether these Tregs are capable of influencing local inflammation will depend on the functional capacity of these Tregs, the in situ Treg/Teffector balance, the costimulatory and cytokine environment (e.g. IL-6, TNF-α and IL-17) and possibly the presence of other (induced) regulatory immune cells (e.g. CD8+ Tregs). The outcome of this response could be associated with phases of relapse and remission during the disease course. In the more progressive disease stage, neurodegenerative processes may dominate inflammatory pathological features and peripheral T-cell-dependent inflammatory processes could play a minor role,40,41 which may have led to a normalization of the peripheral Treg/Teff balance. The detection of a normal Treg frequency in the peripheral blood and CSF of SP-MS patients is consistent with this idea. Some SP-MS patients showed relatively low Treg FOXP3 expression (MFI) which was not correlated with their high Treg suppressive capacity. However, this could have been partly compensated by a high number of FOXP3+ cells within the CD25high population in these patients. In addition, the possibility cannot be excluded that other factors besides FOXP3 [e.g. suppressive cytokines such as transforming growth factor (TGF)-β] could also have contributed to the observed high Treg suppressive capacity in these SP-MS patients.
A disturbance of FOXP3 expression and/or Treg function could be associated with a general susceptibility for autoimmune disease development, although we must emphasize that this represents only one of the many risk factors for autoimmunity, as genetic background and environmental factors also have to be taken into account. Our preliminary observation of normal FOXP3 levels in RA patients and a subset of SLE patients, in agreement with recent studies,29,42 supports this conclusion.
From a therapeutic point of view, it would be challenging to restore Treg dysfunction in RR-MS patients in the early disease stage. In this regard, it was interesting to observe that RR-MS patients treated with IFN-β had increased FOXP3 levels compared with untreated patients. In addition to other effects such as suppression of T-cell proliferation or a shift in T helper type 1 (Th1) towards a Th2 cytokine profile,43 our data suggest that IFN-β treatment may also affect regulatory T-cell function in MS patients. Consistent with this observation, we8 and others44 showed increased Treg suppression in RR-MS patients treated with IFN-β. Whether there was a direct correlation between a beneficial clinical outcome mediated by IFN-β in MS patients and increased FOXP3 levels has yet to be established. The exact mechanism by which IFN-β increases Treg numbers is unknown. Our preliminary data demonstrate no induction of FOXP3 upon short-term in vitro incubation of Tregs with IFN-β (KV, unpublished data). It was recently shown that plasmacytoid dendritic cells in RR-MS patients, in contrast to those in healthy donors, were impaired in the induction of CD4+ FOXP3+ T cells.45 Therefore, it could be that IFN-β has an indirect effect on Treg induction via modulation of dendritic cells, as reported previously.46 Of note, CD4+ CD25high T cells in IFN-β-treated RR-MS patients in our study included a higher number of CD62L-negative cells. It has been demonstrated that anti-TNF-α therapy in RA patients induces a distinct regulatory T-cell population lacking CD62L expression.15 Whether the CD62L– CD4+ CD25high FOXP3+ T cells in IFN-β-treated RR-MS patients also represent a different subset of Tregs should be investigated in future studies.
Taken together, the results obtained here show differences in numbers of circulating FOXP3+ CD4+ CD25+ T cells in the peripheral blood of RR-MS patients and HCs. In addition, lower FOXP3 expression at the cellular level was observed, further supporting a Treg dysfunction in RR-MS patients. Our results indicate that Tregs accumulate in the CSF of RR-MS patients, probably in an attempt to down-regulate local inflammation in the CNS of these patients.
Acknowledgments
This study was supported by research funding from the Belgian Charchot Foundation and the Hasselt University and Transnational University Limburg. The authors thank Dr D. Van Hoof and Dr P. Van Paassen for providing RA and SLE blood samples. We acknowledge A. Bogaers, Bertine Timmermans and Riny Wieers for blood collections. We are grateful to Hanne Jongen for excellent technical assistance. We also thank all patients and control subjects involved in this study for participation in blood and CSF collections.
Abbreviations
- CSF
cerebrospinal fluid
- FOXP3
forkhead box P3
- GITR
glucocorticoid-induced tumour necrosis factor receptor family-related gene
- HC
healthy control
- RR-MS
relapsing-remitting multiple sclerosis
- SP-MS
secondary progressive multiple sclerosis
- Treg
CD4+ CD25high regulatory T cell
- VLA-4
very late antigen-4
Supplementary Material
The following supplementary material is available for this article online:
Table S1. Overview of the phenotype of regulatory and non-regulatory T cells of healthy controls (HCs), patients with other neurological diseases (ONDs) and multiple sclerosis (MS) patients.
This material is available as part of the online article from http://www.blackwell-synergy.com
References
- 1.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–16. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 2.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 3.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 4.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–6. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 6.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas J, Hug A, Viehover A, et al. Reduced suppressive effect of CD4(+) CD25(high) regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 8.Venken K, Hellings N, Hensen K, et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FOXP3 expression. J Neurosci Res. 2006;83:1432–46. doi: 10.1002/jnr.20852. [DOI] [PubMed] [Google Scholar]
- 9.Hellings N, Raus J, Stinissen P. Insights into the immunopathogenesis of multiple sclerosis. Immunol Res. 2002;25:27–51. doi: 10.1385/IR:25:1:27. [DOI] [PubMed] [Google Scholar]
- 10.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 11.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 12.Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-I through activation of transcription factor Foxp3. Proc Natl Acad Sci USA. 2005;102:6449–54. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses 1-selectin and high levels of CCR7. J Immunol. 2002;169:2461–5. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 14.Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–13. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nadkarni S, Mauri C, Ehrenstein MR. Anti-TNF-alpha therapy induces a distinct regulatory T cell population in patients with rheumatoid arthritis via TGF-beta. J Exp Med. 2007;204:33–9. doi: 10.1084/jem.20061531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stassen M, Fondel S, Bopp T, et al. Human CD25+ regulatory T cells: two subsets defined by the integrins alpha 4 beta 7 or alpha 4 beta 1 confer distinct suppressive properties upon CD4+ T helper cells. Eur J Immunol. 2004;34:1303–11. doi: 10.1002/eji.200324656. [DOI] [PubMed] [Google Scholar]
- 17.Bradley LM, Watson SR, Swain SL. Entry of naive CD4 T cells into peripheral lymph nodes requires 1-selectin. J Exp Med. 1994;180:2401–6. doi: 10.1084/jem.180.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vajkoczy P, Laschinger M, Engelhardt B. Alpha4-integrin-VCAM-1 binding mediates G protein-independent capture of encephalitogenic T cell blasts to CNS white matter microvessels. J Clin Invest. 2001;108:557–65. doi: 10.1172/JCI12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not 1-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci USA. 1999;96:6896–901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–5. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- 21.Soilu-Hänninen M, Laaksonen M, Hanninen A. Hyaluronate receptor (CD44) and integrin alpha4 (CD49d) are up-regulated on T cells during MS relapses. J Neuroimmunol. 2005;166:189–92. doi: 10.1016/j.jneuroim.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 22.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–32. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 23.Korn T, Reddy J, Gao W, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–7. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 25.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 26.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 27.Fainardi E, Castellazzi M, Casetta I, Cultrera R, Vaghi L, Granieri E, Contini C. Intrathecal production of Chlamydia pneumoniae-specific high-affinity antibodies is significantly associated to a subset of multiple sclerosis patients with progressive forms. J Neurol Sci. 2004;217:181–8. doi: 10.1016/j.jns.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Venken K, Thewissen M, Hellings N, Somers V, Hensen K, Rummens JL, Stinissen P. A CFSE based assay for measuring CD4(+) CD25(+) regulatory T cell mediated suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Meth. 2007;322:1–11. doi: 10.1016/j.jim.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 30.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh U, Grant C, Griffith C, Fugo K, Takenouchi N, Jacobson S. Reduced Foxp3 protein expression is associated with inflammatory disease during human T lymphotropic virus type 1 infection. J Infect Dis. 2006;193:1557–66. doi: 10.1086/503874. [DOI] [PubMed] [Google Scholar]
- 32.Bassuny WM, Ihara K, Sasaki Y, Kuromaru R, Kohno H, Matsuura N, Hara T. A functional polymorphism in the promoter/enhancer region of the FOXP3/Scurfin gene associated with type 1 diabetes. Immunogenetics. 2003;55:149–56. doi: 10.1007/s00251-003-0559-8. [DOI] [PubMed] [Google Scholar]
- 33.Hug A, Korporal M, Schroder I, Haas J, Glatz K, Storch-Hagenlocher B, Wildemann B. Thymic export function and T cell homeostasis in patients with relapsing remitting multiple sclerosis. J Immunol. 2003;171:432–7. doi: 10.4049/jimmunol.171.1.432. [DOI] [PubMed] [Google Scholar]
- 34.Duszczyszyn DA, Beck JD, Antel J, Bar-Or A, Lapierre Y, Gadag V, Haegert DG. Altered naive CD4 and CD8 T cell homeostasis in patients with relapsing-remitting multiple sclerosis: thymic versus peripheral (non-thymic) mechanisms. Clin Exp Immunol. 2006;143:305–13. doi: 10.1111/j.1365-2249.2005.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thewissen M, Somers V, Venken K, Linsen L, Van Paassen P, Geusens P, Damoiseaux J, Stinissen P. Analyses of immunosenescent markers in patients with autoimmune disease. Clin Immunol. 2007;123:209–18. doi: 10.1016/j.clim.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 37.Allakhverdi Z, Fitzpatrick D, Boisvert A, Baba N, Bouguermouh S, Sarfati M, Delespesse G. Expression of CD103 identifies human regulatory T-cell subsets. J Allergy Clin Immunol. 2006;118:1342–9. doi: 10.1016/j.jaci.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 38.Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147:412–8. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao D, Malmstrom V, Baecher-Allan C, Hafler D, Klareskog L, Trollmo C. Isolation and functional characterization of regulatory CD25brightCD4+ T cells from the target organ of patients with rheumatoid arthritis. Eur J Immunol. 2003;33:215–23. doi: 10.1002/immu.200390024. [DOI] [PubMed] [Google Scholar]
- 40.Rovaris M, Confavreux C, Furlan R, Kappos L, Comi G, Filippi M. Secondary progressive multiple sclerosis: current knowledge and future challenges. Lancet Neurol. 2006;5:343–54. doi: 10.1016/S1474-4422(06)70410-0. [DOI] [PubMed] [Google Scholar]
- 41.Karni A, Abraham M, Monsonego A, Cai G, Freeman GJ, Hafler D, Khoury SJ, Weiner HL. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J Immunol. 2006;177:4196–202. doi: 10.4049/jimmunol.177.6.4196. [DOI] [PubMed] [Google Scholar]
- 42.Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253–61. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yong VW, Chabot S, Stuve O, Williams G. Interferon beta in the treatment of multiple sclerosis: mechanisms of action. Neurology. 1998;51:682–9. doi: 10.1212/wnl.51.3.682. [DOI] [PubMed] [Google Scholar]
- 44.de Andres C, de Aristimuno CLHV, Martinez-Gines ML, et al. Interferon beta-1a therapy enhances CD4+ regulatory T-cell function: an ex vivo and in vitro longitudinal study in relapsing-remitting multiple sclerosis. J Neuroimmunol. 2007;182:204–11. doi: 10.1016/j.jneuroim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Stasiolek M, Bayas A, Kruse N, Wieczarkowiecz A, Toyka KV, Gold R, Selmaj K. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain. 2006;129:1293–305. doi: 10.1093/brain/awl043. [DOI] [PubMed] [Google Scholar]
- 46.Huang YM, Stoyanova N, Jin YP, Teleshova N, Hussien Y, Xiao BG, Fredrikson S, Link H. Altered phenotype and function of blood dendritic cells in multiple sclerosis are modulated by IFN-beta and IL-10. Clin Exp Immunol. 2001;124:306–14. doi: 10.1046/j.1365-2249.2001.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]