Abstract
Therapeutic vaccination using T-cell receptor (TCR) peptides from V genes commonly expressed by potentially pathogenic T cells remains an approach of interest for treatment of multiple sclerosis (MS) and other autoimmune diseases. We developed a trivalent TCR vaccine containing complementarity determining region (CDR) 2 peptides from BV5S2, BV6S5 and BV13S1 emulsified in incomplete Freund's adjuvant that reliably induced high frequencies of TCR-specific T cells. To evaluate induction of regulatory T-cell subtypes, immunological and clinical parameters were followed in 23 treatment-naïve subjects with relapsing-remitting or progressive MS who received 12 monthly injections of the trivalent peptide vaccine over 1 year in an open-label study design. Prior to vaccination, subjects had reduced expression of forkhead box (Fox) P3 message and protein, and reduced recognition of the expressed TCR repertoire by TCR-reactive cells compared with healthy control donors. After three or four injections, most vaccinated MS subjects developed high frequencies of circulating interleukin (IL)-10-secreting T cells specific for the injected TCR peptides and significantly enhanced expression of FoxP3 by regulatory T cells present in both ‘native’ CD4+ CD25+ and ‘inducible’ CD4+ CD25− peripheral blood mononuclear cells (PBMC). At the end of the trial, PBMC from vaccinated MS subjects retained or further increased FoxP3 expression levels, exhibited significantly enhanced recognition of the TCR V gene repertoire apparently generated by perturbation of the TCR network, and significantly suppressed neuroantigen but not recall antigen responses. These findings demonstrate that therapeutic vaccination using only three commonly expressed BV gene determinants can induce an expanded immunoregulatory network in vivo that may optimally control complex autoreactive responses that characterize the inflammatory phase of MS.
Keywords: multiple sclerosis, T-cell receptor, vaccine, FoxP3 expression, immunoregulation
Introduction
Multiple sclerosis (MS) is a paralytic autoimmune demyelinating disease of the central nervous system (CNS). Potentially encephalitogenic T cells specific for myelin antigens, particularly myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG), are thought to contribute to disease progression during the inflammatory phase of MS.1,2 The emergence of pathogenic T cells in MS appears to be permitted as a result of reduced suppression mediated by interleukin (IL)-10-secreting T regulatory (Tr) 1 cells,3,4 natural CD4+ CD25+ regulatory T cells (Treg)5,6 and possibly CD8+ T suppressor cells.7 Thus, development of an immune-based vaccine strategy that can restore deficient suppressive mechanisms remains an important therapeutic goal.8
In previous studies, we pioneered the use of T-cell receptor (TCR) peptides in both the experimental autoimmune encephalomyelitis (EAE) animal model and human clinical studies to induce Treg that could target activated pathogenic T cells bearing commonly expressed V genes.9 Development of TCR peptide vaccination as a treatment for MS first required the identification of target TCR BV genes and the creation of a vaccine capable of reliably boosting TCR reactive T cells. Our meta-analysis of ∼1000 AV and BV genes from neuroantigen-specific T cells from ∼ 200 MS subjects revealed BV5S2, BV6S5 and BV13S1 as excellent candidate BV gene targets.10 On the basis of successful therapeutic vaccination in the EAE model using complementarity determining region (CDR) 2 peptides, we developed a trivalent TCR vaccine using these three CDR2 peptides emulsified in incomplete Freund's adjuvant (IFA).11 Several blinded studies using both single and multiple TCR peptides demonstrated that TCR vaccination could induce high frequencies of TCR-specific T cells concomitant with suggestions of clinical benefit.12,13 The recent formulation of the TCR tripeptides more reliably induced high frequencies of TCR-reactive T cells than formulations containing single or multiple TCR peptides in saline or single TCR peptides in IFA.11
Although induction of TCR-specific T cells in MS subjects can easily be assessed in peripheral blood mononuclear cells (PBMC) by proliferation using the limiting dilution assay (LDA)11 or by cytokine secretion, most notably secretion of IL-10,3,12 the mechanisms by which these cells regulate immune responses have been more difficult to identify. In previous trials we demonstrated significant reductions in MBP-specific T cells in vaccinated MS subjects12 but generally neuroantigen reactivity was low, could be directed at several different peptide determinants, and could naturally vary over the course of disease.14,15 Thus, it is important to explore additional avenues to ascertain the effects of TCR peptide therapy on potentially pathogenic autoreactive T cells, a quest that may be dictated by the operative regulatory mechanisms.
TCR-specific T cells that are expanded by vaccination in vivo may include a mixture of regulatory cell types, including T helper type 2 (Th2) (secreting IL-4), Th3 [secreting transforming growth factor (TGF)-β], and Tr1 (secreting IL-10) cells.9,13 Recently, we postulated that TCR-specific T cells may constitute a subset of CD4+ CD25+ Treg9,16 that have been widely characterized as controllers of autoimmune disease.17–19 TCR proteins are abundant in the thymus and their presence results in positive selection of CD4+ and CD8+ T cells specific for a variety of V gene determinants that have regulatory properties.9 A role for Treg in MS is attractive because of the recently described reduction in suppressive activity of CD4+ CD25+ T cells and the reduced expression of the master regulator gene, FoxP3, in MS patients.5,6 Recent evidence indicates that the regulatory effects of FoxP3 may represent a continuum rather than a binary on/off switch,19,20 with the implication that a reduction (as opposed to a lack) of FoxP3 expression in MS patients could result in enhanced autoreactivity which may exacerbate disease symptoms.
FoxP3 is a lineage specification factor that in combination with nuclear factor of activated T cells (NFAT) represses IL-2 production and up-regulates expression of cytotoxic T-lymphocyte antigen (CTLA)-4 and CD25, thus producing an anergic phenotype.21 Naturally selected CD4+ CD25+ FoxP3+ Treg specific for self antigens develop in the thymus at the double-positive stage.22 Upon activation, Treg can suppress the activation, proliferation, differentiation and effector functions of a wide variety of target cell types, including CD4+ and CD8+ T cells,23 B cells,24 natural killer (NK) cells25 and dendritic cells.26 However, regulation by activated Treg involves production of anti-inflammatory cytokines (e.g. TGF-β or IL-10) or cell–cell contact-dependent mechanisms that are not antigen specific or major histocompatibility complex (MHC) restricted.18,27 Thus, successful boosting of natural Treg would be expected to induce immunoregulation of target T cells that conceivably could include primary encephalitogenic T cells as well as bystander specificities.
There is now widespread acceptance of the idea that Treg are either ‘natural’ or ‘induced’. Natural Treg are part of the normal T-cell repertoire and are fully functional when isolated and tested ex vivo, whereas induced Treg may be generated from naïve precursors through immunization with nominal antigens.28,29 In mice, FoxP3+ Treg that possess protective activity against experimental autoimmune encephalomyelitis (EAE) can be induced by encephalitogenic neuroantigens30,31 as well as by non-disease-inducing antigens.32 In human cells, however, incubation with antigen induced only a small subset of CD4+ and CD8+ cells to transiently express FoxP3, and these T cells did not alter cell-surface phenotype or suppress Th1 cytokine expression, suggesting that induced FoxP3 did not activate a stable Treg lineage.33 In contrast, antigen-induced IL-10+ Treg were found to be FoxP3– and independent of the influence of ‘natural’ Treg.34
In the current study, we evaluated induction of TCR-specific Treg during the course of 12 monthly injections of TCR tripeptides in IFA, and followed the immunoregulatory effects of vaccination on responses of neuroantigen and recall antigen reactive T cells. Our results clearly demonstrated that TCR peptide vaccination could induce transiently high frequencies of IL-10-secreting TCR-reactive T cells, an increase in FoxP3 expression, expanded recognition of the expressed TCR repertoire, and suppression of neuroantigen but not recall antigen responses. These data demonstrate that relatively selective regulatory activity could be induced by repeated boosting with only three commonly expressed TCR peptides, a result that validates the use of this approach as an immunomodulatory therapy for MS.
Methods and subjects
Study design
This was an open-label single-arm study evaluating the effects of a mixture of three TCR CDR2 peptides on induction of TCR-specific T cells and response of PBMC to stimulation through the TCR. All recruited MS subjects received monthly intramuscular (i.m.) injections of NeuroVax™ (Orchestra Therapeutics, Carlsbad, CA) (IR902) which consisted of TCR CDR2 peptides from BV5S2 (IR901), BV6S5 (IR208) and BV13S1 (IR401) emulsified in IFA. Subjects were screened and enrolled at Oregon Health & Science University (OHSU) at the MS Center of Oregon. The study was approved by the Institutional Review Committees at OHSU. At the initial screening visit (week −;4), subjects signed an informed consent form and then were evaluated to determine if they met inclusion/exclusion criteria. Subjects meeting criteria returned 4 weeks later for the week 0 visit and received their first injection, followed by 12 additional injections, one every 4 weeks until week 48. Subjects returned at weeks 50, 52 and 54 for additional immunological and clinical assessments, after which time they exited the study.
Subjects
Subjects were required to meet the following inclusion/exclusion criteria: age 18–75 years, inclusive; definite MS by Modified Poser criteria,35 with course classified as relapsing-remitting (RRMS), primary progressive (PPMS), or secondary progressive (SPMS);36 Expanded Disability Status Score (EDSS) of ≤ 7·0;37 no corticosteroids or interferon-beta within 30 days of enrolment; no glatiramer acetate, azathioprine, methotrexate or cyclophosphamide within 90 days of enrolment; no serious medical or psychiatric disorder. Women of childbearing potential could not be pregnant and had to be willing to use an acceptable form of birth control. Subjects with any of the following laboratory values were excluded: creatinine > 1·5 × high normal; haemoglobin < 9·5 mg/dl; platelets < 75 000 cells/μl; serum glutamic oxaloacetic transaminase/serum glutamic pyruvic transaminase (SGOT/SGPT) ≥ 2·5 × high normal. Subjects enrolled in the study included 23 women and four men with an average age of 49 +mn; 10·4 years (mean +mn; SD) and an average duration of MS of 10·8 +mn; 8·3 years. Fourteen patients were diagnosed as RRMS, 10 were SPMS, and three were PPMS.
Toxicity monitoring
At entry and in weeks 24 and 52, samples were taken from subjects for a complete blood count and a 24-channel chemistry panel. Adverse events were recorded at each study visit.
Clinical measures
Patients were assessed using the EDSS, a 25-foot timed walk, and a nine-hole peg test at the week −;4 visit and these assessments were repeated at the week 8, 16, 24, 40 and 52 visits. Subjects were considered ‘better’ or ‘worse’ if they had two consecutive evaluations 6 months apart that were different by EDSS (one EDSS unit for subjects with a starting EDSS ≤ 5·0, or 0·5 EDSS units for patients with a starting EDSS > 5·0).
TCR peptides
The TCR CDR2 peptides (BV5S2, BV6S5 and BV13S1) used in this study were prepared by The Immune Response Corporation (IRC) and consisted of 21, 20 and 19 amino acids, respectively. The amino acid sequences were as follows: BV5S2: 38-Ala-Leu-Gly-Gln-Gly-Pro-Gln-Phe-Ile-Phe-Gln-Thr-Tyr-Glu-Glu-Glu-Glu-Arg-Gln-Arg-Gly-58 [Note: this peptide has a threonine substituted for tyrosine at position 49 (underlined) and has been previously shown to be more immunogenic than the native sequence29] BV6S5: 39-Leu-Gly-Gln-Gly-Pro-Glu-Phe-Leu- Thr-Tyr-Phe-Gln-Asn-Glu-Ala-Gln-Leu-Glu-Lys-Ser-58; BV13S1: 42-Gly-Leu-Arg-Leu-Ile-His-Tyr-Ser-Val-Gly-Ala-Gly-Ile-Thr-Asp-Gln-Gly-Glu-Val-60.
Vaccine preparation and administration
Vaccine (NeuroVax™) was prepared as a 1 : 1 mixture of the three peptides in aqueous solution and IFA (10% surfactant Montanide and 80–90% Drakeol 6 VR light mineral oil supplied by Seppic, Inc., Paris, France). Each single-dose syringe contained 100 µg/ml of each peptide in a nominal volume of 1·1 +mn; 0·2 ml. Pre-filled syringes were for single use only and were stored at 2–8° until use. The vaccine was injected into the deltoid muscle (alternating between the arms) every 4 weeks for the duration of the study.
Limiting dilution assay to quantify TCR-reactive T-cell frequencies
The LDA was used to determine the circulating frequencies of T cells specific for the mixture of the three TCR peptides, as previously described.11 Briefly, PBMC were cultured in 96-well microtitre plates at dilutions of 5, 2·5, 1·25, 0·62, 0·3 and 0·1 × 105 cells/well. At cell concentrations ≤ 1·25 × 105, 105 additional irradiated autologous PBMC were added to each well to serve as antigen- presenting cells. Twelve replicate wells were cultured at each cell concentration. Cultures received 75 µg/ml of the combined tripeptide mixture, or culture medium (control), and tritiated thymidine uptake was measured after 5 days. Individual wells were scored positive if the counts per minute (c.p.m.) exceeded 1·96 standard deviations [95% confidence interval (CI)] of the mean of wells cultured at the same cell concentration without antigen. Using the percentage of non-responding wells at each cell concentration, TCR peptide-specific T-cell frequencies and their 95% CIs were estimated by the χ2 minimization method,38 employing a program adapted for use with a personal computer. This method of analysis provided an estimated T-cell frequency with a 95% CI, permitting statistical comparison of frequencies obtained at different times from the same subject.
Enzyme-linked immunosorbent spot-forming cell assay (ELISPOT) to detect the frequency of TCR peptide-specific cells
ELISPOTs were used as described previously16 to determine the number of TCR peptide-specific cells that produced IFN-γ and IL-10. Ninety-six-well ELISPOT plates (Whatman Unifilter plates) were coated with primary antibodies, IFN-γ (Mabtech, Cincinnati, OH) diluted to 10 µg/ml and IL-10 (BD PharMingen, San Jose, CA) diluted to 4 µg/ml in sterile phosphate-buffered saline (PBS) (100 µl/well) and kept at 4° overnight in a humidifying chamber. After blocking of the plates with 10% fetal calf serum/RPMI, fresh PBMC, isolated from whole blood by density gradient, were seeded at 2 × 105 cells/well (200 µl/well). TCR BV5S2, BV6S5 and BV13S1 CDR2 peptides (NeoMPS, San Diego, CA) were added in quadruplicate at 25 µg/ml, ConA (20 µg/ml) served as a positive control, and medium containing no antigen was used as a negative control in eight wells. The cultures were then incubated at 37° in 7% CO2, for 24 hr for the IFN-γ plate and for 48 hr for the IL-10 plate. After incubation, plates were washed thoroughly using PBS and 0·05% Tween-20 (Sigma, St Louis, MO). Secondary biotinylated anticytokine IFN-γ and IL-10 were diluted in PBS and 0·05% Tween-20 containing 1% bovine serum albumin (BSA) at 1 and 2 µg/ml, respectively. The secondary antibodies were added at 100 µl/well and placed in the dark at ambient temperature for 90 min. Each plate was washed thoroughly with PBS/Tween-20 and incubated for 45 min at ambient temperature after adding 100 µl/well of 1 : 1000 diluted Strepavidin-AP (DAKO Corporation, Carpenteria, CA). After washing, spots showing peptide-specific cells were developed using 100 µl of 5-bromo-4-chloro-3-indoyl-phosphate (BCIP)/nitroblue tetrazolium dye (KPL, Gaithersburg, MD) per well. The number of spots per well was determined using an ImmunoSpot analyser and software (Cellular Technology Ltd, Cleveland, OH).
Proliferation assay to detect the reactivity of recall antigen and neuroantigen-specific cells
After collection of heparinized blood, 10 ng/ml of IL-7 and 5 ng/ml of IL-15 (R & D Systems, Minneapolis, MN) were injected through the rubber septum of each green-topped vacutainer using a 1-ml syringe. The blood samples were then placed on an orbital rocker for 30 min. Afterwards, mononuclear cells were separated on a density gradient and resuspended in 1% human type AB serum (Genotech, St. Louis, MO). Three 96-well U-bottomed plates were then seeded with 2 × 105 cells/well (200 µl/well) in 60 wells of the plates (in some subjects, more plates were set up depending on the cell yield). Each plate was divided into six sets of 10 wells; two sets for medium controls, one set for recall antigen [either tetanus toxoid (CalBiochem, San Diego, CA) or herpes simplex virus type 1 (BioWhitaker, Walkersville, MD)] and three sets for neuroantigen peptides that had been identified in a prescreening assay as possibly reactive. The plates were then incubated at 37° and 7% CO2 for 7 days. On day 6 the cells were pulsed with 3H-Tdy (0·5 μCi) for the last 18 hr prior to harvesting. Wells were identified as positive when the c.p.m. of wells stimulated with antigen was greater than the mean c.p.m. + 2 standard deviations of wells incubated without antigen. Fourteen peptides (from MBP, PLP and MOG) were used in a screening assay carried out prior to the first TCR peptide injection in six MS subjects, and the two most reactive peptides for each subject were used as marker antigens to follow over the course of the trial. MOG 145–160 peptide (VFLCLQYRLRGKLRAE) used to follow neuroantigen responses in two responder patients was synthesized by NeoMPS (> 95% purity).
Proliferation response to unique CDR2 determinants
PBMC from MS subjects and HC donors were cultured at 200 000 cells/well in triplicate and stimulated for 60 hr in the presence of 25 μg of each of the 113 unique TCR CDR2 peptides, 5 μg/ml ConA, or medium alone. 3H-Tdy (0·5 μCi) was added for the last 18 hr of culture, and the cells were harvested and counted by liquid scintillation. For a complete listing of CDR2 peptide sequences, see Vandenbark et al.39
Determination of response to vaccination
Subjects underwent LDA in response to the tripeptide mixture at week 0 immediately prior to the first injection and at weeks 8/9, 12, 24 and 48. Subjects were considered to have responded immunologically to the tripeptide mixture if two or more LDA frequency determinations were significantly elevated above the reference baseline frequency (week 0) and at least one of these postimmunization frequencies was > 2 × 10−;6.
Isolation of T-cell subpopulations using magnetic beads
Blood was collected into heparinized tubes and mononuclear cells separated by Ficoll density centrifugation. CD4+ CD25+ and CD4+ CD25– cells were isolated from 70 million PBMC using the Miltenyi (Auburn, CA) magnetic bead separation protocol. These cells were first incubated with the Miltenyi CD4+ No Touch T Cell kit containing antibodies that remove non-CD4+ cells, including CD8+ and γδ+ T cells, B cells, NK cells, monocytes, dendritic cells, granulocytes, platelets and erythroid cells. The CD4+ cells were then separated using anti-CD25 monoclonal antibody (mAb)-conjugated magnetic beads into the CD25+ T-cell fraction (> 90% pure) and the remaining CD25– fraction.
FoxP3 expression: real-time polymerase chain reaction
T-cell subpopulations were analysed for FoxP3 expression using real-time polymerase chain reaction (PCR) as described previously.6 Briefly, total RNA was isolated from frozen cell pellets using the Qiagen RNAeasy kit (Qiagen, Valencia, CA). RNA was DNase-treated using Turbo-DNA free (Ambion, Austin, TX) and cDNA was synthesized in a 20-µl volume using Superscript II reverse transcriptase (Life Technologies, Gaithersburg, MD) and random primers (Invitrogen, Carlsbad, CA) following the manufacturer's recommendations. FoxP3 message expression was determined by the TaqMan (Applied Biosystems, Foster City, CA) method of real-time PCR, using hypoxanthine guanine phosphoribosyl transferase (HPRT)1 as an endogenous control. TaqMan Universal PCR Master Mix, and both the FoxP3 primer/probe (part #Hs00203958_m1) and the HPRT1 primer/probe sets (part #4333768F) were purchased directly from Applied Biosystems. HPRT1 was chosen as an endogenous control after comparing several different housekeeping genes (18sRNA, phosphoglycerate kinase (PGK) 1, GAPDH and HPRT1) with the goal of finding one that did not vary with the type of sorted cell population, or the culture conditions used in this study (data not shown).
FoxP3 expression: western blot analysis
Sorted cells were lysed in lysis buffer [25 mm Tris-Cl, pH 8·8, 1 mm ethylenediaminetetraacetic acid (EDTA) and 2% sodium dodecyl sulphate (SDS)] and analysed by western blotting with 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gels as described previously.6 Rabbit anti-human FoxP3 antibody (1 : 1000) (a gift from Dr S. Ziegler, Benaroya Research Institute, Seattle, WA) and goat anti-rabbit immunoglobulin G (IgG) horseradish peroxidase (HRP)-conjugated antibody (1 : 20 000) (Pierce, Rockford, IL) and the enhanced ECL™ Western Blotting System (Amersham, Arlington Heights, IL) were used for the detection of FoxP3 protein. Actin was subsequently detected with mouse anti-actin antibody (1 : 1000) (Chemicon, Temecula, CA) and goat anti-mouse IgG HRP-conjugated antibody (1 : 20 000) (Pierce) as an internal control.
Statistical analyses
The Student–Newman–Keuls test and Dunnett's test for comparing multiple groups were used to test the significance of the difference between the mean FoxP3 message and FoxP3 protein levels in HC and MS subjects. χ2 and Fisher's exact test were used to compare proportions of responses to TCR CDR2 peptides, neuroantigens and recall antigens.
Results
Subjects
A total of 27 MS subjects who had not been treated previously with TCR peptides (14 RRMS, 10 SPMS and three PPMS) were enrolled in the study. Blood samples were also obtained from 18 age- and gender-matched HC subjects. Demographic data and clinical outcome at exit from the trial are shown for all subjects in Table 1. Of the 27 subjects, 23 completed the 1-year trial, with the remaining four subjects (three RRMS and one SPMS) exiting the trial after 8 weeks (one subject) or 24 weeks (three subjects). Of the 23 subjects who completed the trial, 19 remained stable (no sustained change in EDSS, nine-hole peg test, or timed walk), including 10 of 11 subjects with RRMS, three of three subjects with PPMS and six of nine subjects with SPMS, after 1 year of treatment. Four of the 23 subjects were worse. Of the four subjects who exited the study early, all were stable.
Table 1.
Subject demographics and clinical outcome
| Completed trial? | ID | M/F | Age at entry (years) | Type of MS | Duration of MS at entry (years) | EDSS at entry | Clinical outcome |
|---|---|---|---|---|---|---|---|
| N | 5101 | F | 51 | RRMS | 6 | 4 | Stable at early exit |
| Y | 5102 | F | 43 | SPMS | 19 | 4 | Stable |
| Y | 5103 | F | 59 | PPMS | 14 | 4 | Stable |
| Y | 5104 | F | 62 | RRMS | 32 | 2·5 | Stable |
| Y | 5105 | F | 38 | RRMS | 6 | 3 | Stable |
| Y | 5106 | F | 51 | SPMS | 13 | 6 | Worse |
| Y | 5107 | F | 41 | RRMS | 3 | 1·5 | Stable |
| N | 5108 | F | 51 | SPMS | 18 | 6 | Stable at early exit |
| Y | 5109 | F | 46 | SPMS | 17 | 4 | Worse |
| Y | 5110 | F | 61 | RRMS | 8 | 3 | Stable |
| Y | 5111 | F | 59 | RRMS | 5 | 2 | Stable |
| N | 5112 | F | 37 | RRMS | 1 | 1·5 | Stable at early exit |
| Y | 5113 | M | 52 | RRMS | 4 | 6 | Stable |
| Y | 5114 | F | 55 | PPMS | 12 | 6 | Stable |
| Y | 5115 | M | 55 | SPMS | 23 | 6 | Stable |
| Y | 5116 | F | 42 | SPMS | 18 | 6 | Stable |
| Y | 5117 | M | 39 | SPMS | 14 | 6 | Worse |
| Y | 5118 | F | 53 | SPMS | 6 | 7·5 | Stable |
| Y | 5119 | F | 48 | SPMS | 20 | 6 | Stable |
| Y | 5120 | M | 70 | PPMS | 3 | 4 | Stable |
| Y | 5121 | F | 52 | RRMS | 3 | 2 | Stable |
| Y | 5122 | F | 50 | SPMS | 23 | 6·5 | Stable |
| N | 5123 | F | 34 | RRMS | 9 | 6 | Stable at early exit |
| Y | 5124 | F | 24 | RRMS | 2 | 0 | Stable |
| Y | 5125 | F | 35 | RRMS | 0·6 | 2·5 | Stable |
| Y | 5126 | F | 44 | RRMS | 1 | 2 | Stable |
| Y | 5127 | F | 61 | RRMS | 11 | 3 | Worse |
| Mean +mn; SD | 23F/4M | 49 +mn; 10·4 | 10·8 +mn; 8·3 | 4·4 +mn; 2 |
EDSS, Expanded Disability Status Score; F, female; ID, subject identification number; M, male; MS, multiple sclerosis; N, no; PPMS, primary progressive MS; RRMS, relapsing-remitting MS; SD, standard deviation; SPMS, secondary progressive MS; Y, yes.
Frequency of TCR-reactive T cells in vaccinated subjects
In our previous blinded study, we established that all subjects receiving tripeptides (BV5S2, BV6S5 and BV13S1) in IFA (injected i.m. every 4 weeks) had a significant increase in the frequency of PBMC T cells reactive to the TCR vaccine, as measured by proliferation in the limiting dilution assay. In the current open-label study, we tested a subgroup of seven subjects by LDA and ELISPOT for changes in response to the vaccinating peptides. Similar to our previous studies, there was a low frequency of proliferating TCR-reactive T cells (0·5 cells/million PBMC) prior to vaccination which increased strongly to a maximum frequency of 52 cells/million PBMC after 12 weeks of therapy, and then gradually decreased over the course of the trial to 11 cells/million PBMC at exit (Fig. 1). Additionally, there was a detectable starting frequency of IL-10-secreting TCR-reactive cells in PBMC prior to vaccination (∼ 12 cells/million PBMC as measured by ELISPOT) which increased after vaccination to a maximum frequency of 87 cells/ million PBMC after 15 weeks of therapy, and then decreased to starting levels (13 cells/million PBMC) at exit from the trial (Fig. 1). This pattern was mirrored by smaller changes in the frequency IFN-γ-secreting cells which reached a maximum (54 cells/million PBMC) after 18 weeks (Fig. 1). This result validates our previous studies showing that TCR vaccination predominately boosted IL-10-secreting cells.
Figure 1.
Frequencies of T-cell receptor (TCR) peptide-reactive peripheral blood mononuclear cells (PBMC) from TCR tripeptide-vaccinated subjects with multiple sclerosis (MS). Reactivity to the tripeptide mixture of complementarity determining region (CDR) 2 peptides from BV5S2, BV6S5 and BV13S1 was assessed in seven MS subjects in terms of proliferation responses using the limiting dilution assay and in terms of secretion of interleukin (IL)-10 or interferon (IFN)-γ using the enzyme-linked immunosorbent spot-forming cell assay (ELISPOT). Blood samples were collected from the subjects prior to vaccination and at weeks 8/9, 12, 24 and 48 of the trial and cultured as described in the Materials and methods. Data are presented as mean frequency +mn; standard deviation for MS subjects at entry, at maximum postvaccination response, and at exit from the trial. ELISPOT frequencies represent the sum of responses to each of the three vaccinating TCR CDR2 peptides.
TCR vaccination restores deficient expression of FoxP3 in CD4+ CD25+ Treg
We and others previously demonstrated that MS subjects had decreased expression of the master regulator gene, FoxP3, with a concomitant reduction in the ability of CD4+ CD25+ Treg to inhibit CD4+ CD25– indicator cells.5,6 In the light of the observation that selection of Treg occurs in the thymus to host self antigens,40 we further suggested that CD4+ TCR-reactive T cells may represent a subset of Treg.16 If this is true, expansion of TCR-reactive T cells after vaccination might restore missing Treg activity. We thus evaluated FoxP3 mRNA and protein expression from CD4+ C25+ cells from MS subjects before and at various times after treatment with TCR tripeptides. In accordance with our previous study,6 expression of FoxP3 mRNA was significantly decreased in CD4+ CD25+ cells from MS subjects prior to vaccination compared with age- and gender-matched HC donors (Fig. 2a). However, by 12 weeks of therapy with the trivalent TCR peptides, FoxP3 expression was significantly increased compared with entry levels and was similar to that of the matched HC donors. It is noteworthy that TCR vaccination also significantly increased the FoxP3 message that occurs at low levels in CD4+ CD25– T cells which reportedly can be induced to become Treg,29 although there was no reduction in FoxP3 expression in this cell fraction in MS subjects versus HC donors prior to vaccination (Fig. 2b). The restored expression of FoxP3 message after TCR vaccination was further validated by a similar reduction in FoxP3 protein in CD4+ CD25+ T cells from MS subjects at baseline, followed by a subsequent significant increase to normal levels after TCR vaccination (Fig. 2c). The increase in FoxP3 message occurred in a significant proportion of vaccinated MS subjects (13/16 = 81%P < 0·001; Fig. 3) who largely represented those who had reduced expression levels prior to vaccination compared with HC donors.
Figure 2.
T-cell receptor (TCR) vaccination restored forkhead box (Fox) P3 message and protein expression. Peripheral blood mononuclear cells (PBMC) were collected from subjects with multiple sclerosis (MS) prior to vaccination and at the indicated time-points during treatment with TCR tripeptides, as well as from age- and gender-matched healthy control (HC) donors. The cells were sorted into CD4+ CD25+ and CD4+ CD25– populations, and mRNA was extracted and evaluated for expression of FoxP3 and hypoxanthine guanine phosphoribosyl transferase (HPRT)1 genes. Sorted cells from the same subjects were also evaluated for FoxP3 and HPRT-1 protein by western blots. (a) FoxP3 expression in CD4+ CD25+ T cells showed a significant increase after vaccination. Note reduced FoxP3 expression in the CD4+ CD25+ cells from MS subjects versus HC donors prior to vaccination, and significantly enhanced expression to levels even higher than those in HC donors at week 12 and in all subsequent weeks tested during the vaccination procedure. (b) FoxP3 expression in CD4+ CD25– T cells increased significantly after vaccination. FoxP3 expression was much lower in the CD4+ CD25– population and was not different between MS subjects and HC donors prior to vaccination, but was significantly enhanced to levels higher than those in the HC donors in the MS subjects during vaccination with TCR tripeptides. (c) FoxP3 protein showed a significant increase after vaccination. FoxP3 protein levels were reduced in CD4+ CD25+ T cells from MS subjects relative to those in HC donors prior to vaccination, but were restored to levels higher than those in HC donors in MS subjects during vaccination. Error bars represent the standard error of the mean.
Figure 3.
Forkhead box (Fox) P3 message expression was enhanced in a significant proportion (13/16; 81%P < 0·001) of vaccinated subjects with multiple sclerosis (MS). FoxP3 message levels (normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT)1) are shown for 13 MS subjects who had increased expression (a) and three MS subjects (19%) who had decreased expression (b) over the course of treatment with T-cell receptor (TCR) tripeptides.
TCR vaccination reduced the frequency of neuroantigen-specific cells in a subset of MS subjects, but did not affect T-cell responses to recall antigens
Of importance to the mechanism of action of the TCR peptide therapy approach is the effect of vaccination on responses to potentially encephalitogenic neuroantigens, as well as possible effects on responses to recall antigens. We thus evaluated entry and exit proliferation responses of PBMC to candidate neuroantigen peptides (identified for each subject using a prescreening assay with 14 known neuroantigenic peptides) and to either type 1 herpes simplex virus (HSV) or tetanus toxoid antigen in a subset of six MS subjects. As shown in Table 2, only two of the six subjects had a detectable response to neuroantigen (MOG 145–160 but not other tested peptides) at entry, and in both subjects the response at exit from the trial was significantly reduced (Table 2 and Fig. 4; P ≤ 0·001). In contrast, responses to herpes simplex virus or tetanus toxoid antigen were stable or increased (one subject) over the course of the study. It is noteworthy that subjects without a significant response to the marker neuroantigen peptides at baseline did not develop increased responses during the trial. These findings demonstrate that TCR peptide vaccination can significantly inhibit neuroantigen-specific T cells without affecting recall responses, consistent with our previous report.12
Table 2.
TCR vaccination inhibits neuroantigen but not recall antigen response
| Antigen responses (proliferation) | ||||
|---|---|---|---|---|
| Recall antigens (HSV/TT) [no. positive/total wells (%)] | Neuroantigen peptides (MBP/MOG) [no. positive/total wells1 (%)] | |||
| Subject no. | Entry | Exit | Entry | Exit |
| 5120 | 56/60 (93) | 29/30 (97) | 0/60 (0) | 0/30 (0) |
| 5121 | 60/60 (100) | 30/30 (100) | 0/60 (0) | 0/30 (0) |
| 5122 | 30/30 (100) | 50/50 (100) | 0/30 (0) | 0/50 (0) |
| 5124 | 39/40 (98) | 30/30 (100) | 0/40 (0) | 0/30 (0) |
| 5126 | 30/30 (100) | 30/30 (100) | 12/30 (40) | 1/30 (3)*** |
| 5127 | 30/40 (75) | 30/30 (100)** | 16/40 (40) | 1/30 (3)*** |
| Sum | 245/260 (94) | 199/200 (100)** | 28/260 (11) | 2/200 (1)*** |
| Mean +mn; SD (%) | 94 +mn; 4 | 100 +mn; 1 (NS2) | 13 +mn; 8 | 1 +mn; 1 (NS2) |
Background subtracted.
Paired t-test.
Asterisks indicate significant change at exit (Fisher's exact test or χ2 test;
P < 0·01;
P ≤ 0·001).
HSV, herpes simplex virus; MBP, myelin basic protein; MOG, myelin oligodendrocyte glycoprotein; NS, not significant; SD, standard deviation; TT, tetanus toxoid.
Figure 4.
Vaccination with T-cell receptor (TCR) tripeptides reduced reactivity to myelin oligodendrocyte glycoprotein (MOG) 145–160 peptide while maintaining or increasing reactivity to a recall antigen [herpes simplex virus (HSV)]. Peripheral blood mononuclear cells (PBMC) from two subjects with multiple sclerosis (MS) (subjects 5126 and 5127) were cultured in replicate wells of 200 000 cells with or without HSV antigen or MOG 145–160 peptide, and evaluated for proliferation responses in terms of uptake of 3H-Tdy. Wells were determined to be reactive to the antigen if the counts per minute (c.p.m.) exceeded 2 standard deviations of the mean c.p.m. of the unstimulated control wells. Significance was determined by comparing the rates of positive wells at entry and exit using Fisher's exact test (**P < 0·01; ***P < 0·001).
Multiple TCR injections expand the breadth of network recognition of TCR V genes
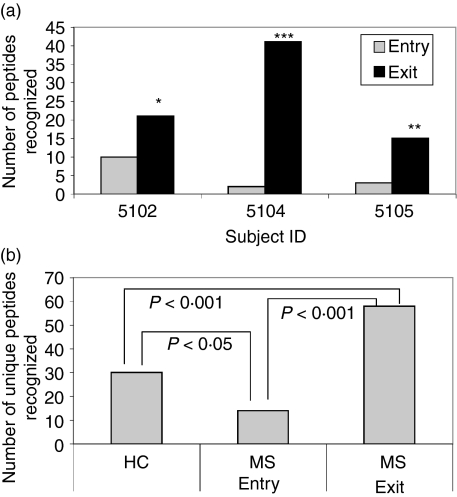
The data presented above clearly demonstrated that injection of TCR tripeptides increased the frequency of T cells responsive to the included TCR CDR2 peptides from BV5S2, BV6S5 and BV13S1. In theory, this expansion of tripeptide-reactive T cells should be polyclonal, and could lead to the activation of additional T cells specific for up-regulated V gene determinants that would be present in association with MHC class II on the surface of newly activated tripeptide-specific T cells.41,42 Moreover, alleles with very similar amino acid sequences to the recognized TCR peptides may be cross-reactive.39 To determine if expansion of TCR-reactive T-cell specificities occurred during multiple treatments with the tripeptide mixture, we evaluated proliferation responses from three MS subjects at baseline and in week 52 (at exit) to a set of 113 CDR2 peptides that represent essentially the entire AV and BV gene repertoire of > 160 alleles (some alleles have identical peptide sequences within CDR2 and thus some peptides represent more than one allele). As shown in Fig. 5(a), the number of TCR alleles recognized by each of the three MS subjects at entry (2–10 alleles) was increased significantly at exit (15–42 alleles; P < 0·05). The total number of alleles recognized by HC donors (30) was significantly greater than the number recognized by MS subjects at entry (14) (P < 0·05; Figs 5b and 6), and this number was significantly increased to 58 recognized alleles in vaccinated MS subjects at exit (P < 0·001; Figs 5b and 6). It is noteworthy that five common CDR2 alleles were recognized in HC donors versus only one common allele in multiple MS subjects at baseline, compared with 14 common alleles in MS subjects after TCR vaccination. This increase in commonly recognized CDR2 sequences suggests a similar sensitization pattern after vaccination with the tripeptide vaccine.
Figure 5.
T-cell receptor (TCR) peptide vaccination increased the number of V gene sequences recognized by peripheral blood mononuclear cells (PBMC). (a) PBMC from three subjects with multiple sclerosis (MS) were evaluated prior to vaccination and at exit (week 52) for proliferation responses (determined by a t-test for triplicate wells stimulated with medium or the given peptide) to a panel of 113 AV and BV CDR2 peptides. The increase in the total number of peptides recognized by each of the three subjects after vaccination was significant (*P < 0·05; **P < 0·01; ***P < 0·001). (b) The number of unique peptides recognized by the MS subjects was reduced at entry compared with healthy control (HC) donors but increased significantly after vaccination.
Figure 6.
Identity of the AV and BV CDR2 peptides recognized by the three healthy control (HC) donors and three subjects with multiple sclerosis (MS) before and after vaccination. The amino acid sequences of the indicated peptides are listed in Vandenbark et al.39 Multiple boxes indicate recognition by more than one HC donor or MS subject.
Discussion
T-cell immunoregulation directed at TCRs appears to result from self-presentation of naturally processed TCR chains with expressed MHC class I and II molecules on the surface of activated T cells.43 T cells with such presentation could serve as both stimulators and targets of a second set of T cells with receptors that bind to the unique MHC–TCR peptide complexes present on the targeted T cells.44 The repertoire of TCR peptide-reactive T cells is positively selected in the thymus after depletion of negatively selected clonotypes, as was demonstrated in our earlier study showing that T cells from healthy donors generally could recognize CDR2 peptides from essentially all known TCR α and β alleles.16 In contrast, T cells from MS subjects had significantly reduced recognition of the expressed CDR2 repertoire, suggesting a deficiency in this natural autoregulatory network.9 Regulatory deficiencies have long been suspected in MS45 and were recently further suggested by reduced suppressive activity of CD4+ CD25+ FoxP3+ T cells.5,6 Because CD4+ CD25+ FoxP3+ Treg are also positively selected in the thymus to self determinants,40 we hypothesized that TCR-specific T cells might represent a subset of the naturally induced Treg, and that vaccination with TCR peptides would both expand TCR reactivity and enhance expression of FoxP3.9
The current open-label study was designed to evaluate in more detail both the in vivo activation process of TCR-specific T cells and effects on circulating T cells, including those specific for potentially encephalitogenic neuroantigens and memory T cells specific for recall antigens. Additionally, the study explored the possible relationship between TCR vaccination and induction of FoxP3+ Treg. The results presented herein demonstrate conclusively that treatment of MS subjects with TCR tripeptides induced a surge of proliferating, IL-10-secreting TCR peptide-specific T cells concomitant with restoration of deficient FoxP3 expression to higher than normal levels. The induction of these regulatory phenotypes by the TCR tripeptides for 15 weeks preceded lower but still significant levels of TCR-reactive IFN-γ-secreting cells that peaked in week 18. Surprisingly, TCR-reactive ELISPOT frequencies for both cytokines returned to baseline levels by week 48, when proliferation responses still remained. This result suggested that the weakly proliferating TCR-reactive cells detectable in week 48 had a diminished capacity to secrete either cytokine, a finding consistent with maturation of Treg that have limited proliferation and reduced cytokine expression. The stable enhancement of FoxP3 in PBMC from TCR-vaccinated patients coupled with the drop in cytokine secretion levels by TCR-reactive cells during the later stages of the trial suggested that the FoxP3+ Treg may have been derived from both the ‘natural’ thymus-derived FoxP3+ lineage and from antigen-induced, TGF-β-dependent CD4+ CD25– FoxP3– peripheral T cells.33
We demonstrated previously6 that FoxP3 expression in CD4+ CD25+ T cells was significantly reduced in MS subjects, a finding that has since been confirmed.46 In the light of the responsiveness to TCR peptides in T-cell populations that are enriched in Treg, we postulated that TCR-specific T cells may represent an important specificity of naturally occurring Treg. The current study demonstrated for the first time that TCR peptide vaccination significantly restored the reduced FoxP3 message and protein expression levels in the CD4+ CD25+ Treg fraction of MS subjects, enhancing FoxP3 message in 13 of 16 subjects. Although this study utilized bead separation rather than fluorescence-activated cell sorting (FACS) to enrich for Treg, the purified CD4+ CD25+ fraction obtained was highly enriched for FoxP3 expression (> 15×) compared with the CD4+ CD25– fraction. However, based on residual proliferation responses to anti-CD3/CD28 (data not shown), it is likely that our bead sorted fraction contained some FoxP3– non-Treg.
It is noteworthy that TCR vaccination also boosted FoxP3 expression in the ‘inducible’ CD4+ CD25– T-cell fraction. In both cell fractions, the increase in FoxP3 was clearly significant within 12 weeks, and ultimately the levels actually exceeded expression in age- and gender-matched HC donors, indicating that the Treg induction process was ongoing with continued boosting injections of TCR peptides. A previous study demonstrated that in vitro activation of CD4+ CD25– T cells with glatiramer acetate could induce FoxP3+ CD4+ CD25+ Treg.47 Moreover, a later report from this group demonstrated that IL-2 receptor (IL-2R)-reactive T-cell lines derived from MS subjects previously vaccinated with activated and attenuated neuroantigen-reactive T cells had increased expression of FoxP3 and increased secretion of both IL-10 and IFN-γ.48 In both of these reports, the increase in FoxP3 expression occurred as a result of in vitro activation, a finding highly consistent with another recent report showing that TCR engagement acts directly on the FoxP3 promoter.49
The striking increase in the number of TCR sequences recognized after vaccination with only three TCR peptides was predicted by the network theory41 but was still surprising given the consistency of the observation in all three subjects tested. We demonstrated in a previous study that essentially all of the 113 unique CDR2 sequences from the ∼ 165 known AV and BV alleles could be recognized in HC donors (by ELISPOT detection of IL-10- and IFN-γ-secreting cells) and that MS subjects had more limited responses.16 This result was confirmed in the present study using a proliferation assay, in which HC donors recognized a total of 30 TCR alleles compared with only 14 for MS subjects at entry to the trial. After TCR peptide vaccination, the MS subjects recognized a total of 58 TCR alleles, indicating a significant broadening of recognition of TCR targets in vivo. Although it remains unknown why there was a lower number of TCR epitopes recognized by MS subjects at baseline, the boosting effect of vaccination with only three TCR determinants probably resulted from expansion of the tripeptide-reactive T cells that expressed a variety of different V genes. As postulated previously, activation of these TCR-reactive T cells would result in increased expression of MHC classes I and II which could form immunogenic complexes in combination with these additional internally processed TCR chains.42 Recognition of different TCR determinants may also occur through cross-reactivity of similar peptide sequences present in closely related alleles (which may have only a single amino acid difference).39 Broadening of TCR recognition with concomitant enhancement of FoxP3 expression represents an important consequence of polyclonal T-cell activation in response to the injected TCR peptides, and would be expected to enhance both peptide-specific TCR-mediated immunoregulation and non-specific suppression mediated by Treg. The eventual loss of cytokine-secreting TCR-reactive T cells at the end of the study could result from maturation of earlier cytokine-secreting Treg to a non-secreting phenotype, or from non-specific Treg suppression.
A key issue concerning induction of TCR-reactive T cells in vivo is their potential for suppressing target T cells specific for potentially encephalitogenic neuroantigens. Our previous study demonstrated a significant downward trend in response to MBP in successfully vaccinated MS subjects,12 and we sought to readdress this issue in the current study by comparing the frequency of antigen-stimulated wells at exit versus entry in a limited subset of vaccinated MS subjects. To find suitable neuroantigens, we prescreened six of the MS subjects prior to initiating therapy to a battery of 14 previously identified neuroantigen peptides, and then used two of the most reactive peptides as marker antigens for assessing changes in T-cell reactivity during the trial. We found that only two of six subjects screened in this manner had a significant response to just one of the neuroantigen peptides (MOG 145–160) at entry, and, in both cases, treatment with the tripeptide vaccine resulted in a highly significant reduction in reactivity. Moreover, subjects without neuroantigen responses at baseline did not develop new responses during the trial. In contrast, responses to recall antigens in all six subjects remained the same or increased at exit from the study. These results suggest that there may be some selectivity in the regulatory effects of the TCR peptide-reactive T cells induced by vaccination for neuroantigen responses. This would seem plausible if the neuroantigen-reactive cells were in a more activated state than recall antigen-reactive cells in vivo, as activation would enhance display of processed TCR determinants expressed with up-regulated self MHC. This indeed could be the case given the demonstrated presence of increased HPRT– mutant cells specific for neuroantigens in MS.50,51
In summary, TCR peptide vaccination using a tripeptide mixture induced strongly increased frequencies of TCR-reactive T cells with expanded recognition of the TCR repertoire which had potent regulatory effects in a subset of subjects on T-cell responses to neuroantigens but not recall antigens. The regulatory mechanisms probably involved an early induction of IL-10-secreting TCR-reactive T cells concomitant with an increased expression of FoxP3 in ‘natural’ CD4+ CD25+ Treg and in the Treg ‘inducible’ CD4+ CD25– population after vaccination. Such regulation would involve local inhibition of both target cells expressing the cognate TCR recognized by the TCR-specific cells and bystander cells affected by secreted IL-10 or by cell–cell contact with activated FoxP3+ Treg, perhaps through production of excess adenosine.52 These data support the potential of vaccination using only three commonly expressed TCR peptides as an immunoregulatory therapy for MS.
Acknowledgments
The authors wish to thank Eva Niehaus for her assistance in preparation of the manuscript. This work was supported by Orchestra Therapeutics Inc. (formerly known as The Immune Response Corporation), NIH grants NS23221 and NS23444, The National Multiple Sclerosis Society, The Nancy Davis MS Center Without Walls, and the Department of Veterans Affairs Department of Biomedical Research.
References
- 1.Bielekova B, Sung M-H, Kadom N, Simon R, McFarland H, Martin R. Expansion and functional relevance of high-avidity myelin-specific CD4+ T cells in multiple sclerosis. J Immunol. 2004;172:3893–904. doi: 10.4049/jimmunol.172.6.3893. [DOI] [PubMed] [Google Scholar]
- 2.Lunemann JD, Ruckert S, Kern F, Wendling U, vanderZee R, Volk HD, Zipp F. Cross-sectional and longitudinal analysis of myelin-reactive T cells in patients with multiple sclerosis. J Neurol. 2004;251:1111–20. doi: 10.1007/s00415-004-0493-1. [DOI] [PubMed] [Google Scholar]
- 3.Vandenbark AA, Finn T, Barnes D, et al. Diminished frequency of IL-10 secreting, TCR peptide-reactive T-cells in multiple sclerosis patients may allow expansion of activated memory T-cells bearing the cognate BV gene. J Neurosci Res. 2001;66:171–6. doi: 10.1002/jnr.1209. [DOI] [PubMed] [Google Scholar]
- 4.Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–7. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huan J, Culbertson N, Spencer L, et al. Decreased FoxP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 7.Antel J, Bania M, Noronha A, Neely S. Defective suppressor cell function mediated by T8+ cell lines from patients with progressive multiple sclerosis. J Immunol. 1986;137:3436–9. [PubMed] [Google Scholar]
- 8.Feldmann M, Steinman L. Design of effective immunotherapy for human autoimmunity. Nature. 2005;435:612–9. doi: 10.1038/nature03727. [DOI] [PubMed] [Google Scholar]
- 9.Vandenbark AA. TCR peptide vaccination in multiple sclerosis: Boosting a deficient regulatory network that may involve TCR-specific CD4+CD25+ Treg cells. Curr Drug Targets – Inflamm Allergy. 2005;4:85–94. doi: 10.2174/1568010053586327. [DOI] [PubMed] [Google Scholar]
- 10.Vandenbark AA, Culbertson NE. Trivalent T cell receptor peptide vaccine for treatment of multiple sclerosis targets predominant V genes widely implicated in autoimmune diseases and allergy. In: Zhang J, editor. Immune Regulation and Immunotherapy in Autoimmune Disease. New York, NY: Springer Life Sciences; 2007. pp. 369–8. [Google Scholar]
- 11.Bourdette DN, Edmonds E, Smith C, et al. A highly immunogenic trivalent T cell receptor peptide vaccine for multiple sclerosis. Multiple Sclerosis. 2005;11:552–61. doi: 10.1191/1352458505ms1225oa. [DOI] [PubMed] [Google Scholar]
- 12.Vandenbark AA, Chou YK, Whitham R, et al. Treatment of multiple sclerosis with T cell receptor peptides: Results of a double-blind pilot trial. Nature Med. 1996;2:1109–15. doi: 10.1038/nm1096-1109. [DOI] [PubMed] [Google Scholar]
- 13.Vandenbark AA, Morgan E, Bourdette D, et al. TCR peptide therapy in human autoimmune diseases. Neurochem Res. 2001;26:713–30. doi: 10.1023/a:1010951706830. [DOI] [PubMed] [Google Scholar]
- 14.Pender MP, Csurhes PA, Greer JM, et al. Surges of increased T cell reactivity to an encephalitogenic region of myelin proteolipid protein occur more often in patients with multiple sclerosis than in healthy subjects. J Immunol. 2000;165:5322–31. doi: 10.4049/jimmunol.165.9.5322. [DOI] [PubMed] [Google Scholar]
- 15.Hellings N, Baree M, Verhoeven C, D'hooghe MB, Medaer R, Bernard CC, Raus J, Stinissen P. T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls. J Neurosci Res. 2001;63:290–302. doi: 10.1002/1097-4547(20010201)63:3<290::AID-JNR1023>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 16.Buenafe AC, Tsaknaridis LJ, Spencer L, et al. Specificity of regulatory CD4+CD25+ T cells for self-T cell receptor determinants. J Neurosci Res. 2004;76:129–40. doi: 10.1002/jnr.20066. [DOI] [PubMed] [Google Scholar]
- 17.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 18.Shevach EM. Regulatory T cells in autoimmunity. Ann Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 19.Curiel TJ. Regulatory T-cell development: Is FoxP3 the decider? Nature Med. 2007;13:250–3. doi: 10.1038/nm0307-250. [DOI] [PubMed] [Google Scholar]
- 20.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated FoxP3 expression. Nature. 2007;445:766–70. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 21.Wu Y, Borde M, Heissmeyer V, et al. FoxP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 22.Cabarrocas J, Cassan C, Magnusson F, et al. FoxP3+CD25+ regulatory T cells specific for a neo-self-antigen develop at the double-positive thymic stage. Proc Natl Acad Sci USA. 2006;103:8453–8. doi: 10.1073/pnas.0603086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S. Naturally arising FoxP3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 24.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting Edge. Direct suppression of B cells by CD4+CD25+ regulatory T cells. J Immunol. 2005;175:4180–3. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Menard C, Terme M, et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505–11. doi: 10.1084/jem.20050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi S. Regulatory T cells: Key controllers of immunological self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 28.Walker LSK, Chodos A, Abbas AK. Antigen-dependent proliferation of CD4+CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–58. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. Do novo generation of antigen specific CD4+CD25+ regulatory T cells from human CD4+CD25– cells. Proc Natl Acad Sci USA. 2005;102:4103–8. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy J, Illes Z, Zhang X, et al. Myelin proteolipid protein- specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2004;101:15434–9. doi: 10.1073/pnas.0404444101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu P, Gregg RK, Bell JJ, et al. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J Immunol. 2005;174:6772–80. doi: 10.4049/jimmunol.174.11.6772. [DOI] [PubMed] [Google Scholar]
- 32.Ochoa-Raparaz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol. 2007;178:1791–9. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gavin MA, Torgerson TR, Houston E, et al. Single-cell analysis of normal and FoxP3-mutant human T cells: FoxP3 expression without regulatory T cell development. Proc Nat Acad Sci USA. 2006;103:6659–64. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolson KS, O'Neill EJ, Sundstedt A, Streeter HB, Minaee S, Wraith DC. Antigen-induced IL-10+ regulatory T cells are independent of CD25+ regulatory cells for their growth, differentiation, and function. J Immunol. 2006;176:5329–37. doi: 10.4049/jimmunol.176.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 36.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: Results of an international survey. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- 37.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 38.Taswell C. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J Immunol. 1981;26:1614. [PubMed] [Google Scholar]
- 39.Vandenbark AA, Culbertson N, Finn T, et al. Human TCR as antigen: Homologies and potentially cross-reactive HLA-DR2-restricted epitopes within the AV and BV CDR2 loops. Crit Rev Immunol. 2000;20:57–83. [PubMed] [Google Scholar]
- 40.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Hoelenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self- peptide. Nature Immunol. 2001;2:301–6. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 41.Cohen IR, Young DB. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991;12:105–10. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- 42.Vandenbark AA, Hashim GA, Offner H. T cell receptor peptides in treatment of autoimmune disease: rationale and potential. J Neurosci Res. 1996;43:391–402. doi: 10.1002/(SICI)1097-4547(19960215)43:4<391::AID-JNR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 43.Jiang H, Sercarz E, Nitecki D, Pernis B. The problem of presentation of T cell receptor peptides by activated T cells. Ann N Y Acad Sci. 1991;636:28–32. doi: 10.1111/j.1749-6632.1991.tb33435.x. [DOI] [PubMed] [Google Scholar]
- 44.Vandenbark AA, Chou YK, Bourdette DN, Whitham R, Offner H. Immunogenicity is critical to the therapeutic application of T cell receptor peptide. Drug News Persp. 1997;10:341–6. [Google Scholar]
- 45.Antel J, Brown M, Nicholas MK, Blain M, Noronha A, Reder A. Activated suppressor cell function in multiple scleoris – clinical correlations. J Neuroimmunol. 1988;17:323–30. doi: 10.1016/0165-5728(88)90123-3. [DOI] [PubMed] [Google Scholar]
- 46.Venken K, Hellings N, Hensen K, et al. Secondary progressive in contrast to relapsing-remitting multiple sclerosis patients show a normal CD4+CD25+ regulatory T-cell function and FoxP3 expression. J Neurosci Res. 2006;83:1432–46. doi: 10.1002/jnr.20852. [DOI] [PubMed] [Google Scholar]
- 47.Hong J, Li N, Zhang X, Zheng B, Zhang JZ. Induction of CD4+CD25+ regulatory T cells by copolymer-1 through activation of transcription factor FoxP3. Proc Natl Acad Sci USA. 2005;102:6449–54. doi: 10.1073/pnas.0502187102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong J, Zang YCQ, Nie H, Zhang JZ. CD4+ regulatory T cell responses induced by T cell vaccination in patients with multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:5024–9. doi: 10.1073/pnas.0508784103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mantel P-Y, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, Schmidt-Weber CB. Molecular mechanisms underlying FoxP3 induction in human T cells. J Immunol. 2006;176:3593–602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 50.Allegretta M, Nicklas JA, Sriram S, Albertini RJ. T cells responsive to myelin basic protein in patients with multiple sclerosis. Science. 1990;247:718–21. doi: 10.1126/science.1689076. [DOI] [PubMed] [Google Scholar]
- 51.Lodge PA, Allegretta M, Steinman L, Sriram S. Myelin basic protein peptide specificity and T-cell receptor gene usage of HPRT mutant T-cell clones in patients with multiple sclerosis. Ann Neurol. 1994;36:734–40. doi: 10.1002/ana.410360508. [DOI] [PubMed] [Google Scholar]
- 52.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediated immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]