Abstract
B7-H1 (also known as CD274 and PD-L1) is a cosignalling molecule regulating T-cell immunity positively or negatively in vivo. However, little is known about the role of endogenous B7-H1 in bacterial infection. We found that B7-H1 expression was up-regulated in various cell populations including CD4+ and CD8+ T cells, natural killer (NK) cells and macrophages following Listeria monocytogenes infection. Administration of the antagonistic B7-H1 monoclonal antibody resulted in a significant increase in mortality in mice infected with a lethal dose of L. monocytogenes compared with mice given the control immunoglobulin. In vivo blockade of B7-H1 greatly inhibited the production of tumour necrosis factor (TNF)-α and nitric oxide, key effector molecules responsible for intracellular killing by macrophages. B7-H1 blockade also suppressed the expression of granzyme B and interferon (IFN)-γ by NK cells. Interestingly, blocking of endogenous B7-H1 selectively inhibited CD8+ T cells rather than CD4+ T cells in response to L. monocytogenes infection, as evidenced by the reduction of IFN-γ production and the expression of effector surface markers including CD62Lint/low and CD44high in CD8+ T cells from mice given anti-B7-H1 monoclonal antibody. In addition, we found that the proliferation of listeriolysin-O (LLO)-specific and IFN-γ-producing L. monocytogenes-reactive CD8+ T cells was significantly decreased not only in the effector phase but also in the memory phase in the presence of anti-B7-H1 antibody. Our findings thus suggest that endogenous B7-H1 can provide positive costimulatory signals for innate and adaptive immunity leading to protection against intracellular bacterial infection.
Keywords: B7-H1, Listeria monocytogenes, innate immunity, adaptive immunity
Introduction
Listeria monocytogenes, a Gram-positive facultative intracellular bacterium, survives and replicates in the cytoplasm of phagocytes and hepatocytes following escape from the phagosome.1 Because of its capacity to induce intensive cell-mediated immunity, the murine L. infection model has been widely used for the investigation of the kinetics and the mechanisms of both innate and adaptive immunity against intracellular bacteria.2–5 The innate immune response to Listeria infection is a complicated process involving not only many cell types, including macrophages, natural killer (NK) cells and neutrophils, but also nitrogen intermediates and cytokines such as tumour necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-12, interferon (INF)-γ, and the more recently identified early T lymphocyte activation (Eta)-1.6–9 It is widely believed that TNF-α and nitric oxide (NO), an end product of inducible nitric oxide synthase (iNOS) produced by macrophages and a newly identified TNF/iNOS-producing dendritic cell (Tip-DC), are key effector molecules responsible for the protection of the host from early Listeria infection in conjunction with INF-γ, which is mainly secreted by NK cells.10–12 Numerous studies also indicate that the CD8+ T-cell immune response plays a prominent role in the complete clearance of Listeria monocytogenes in infected mice through IFN-γ-mediated mechanisms whereby escape of L. monocytogenes from the phagosome is inhibited and macrophages are activated.13 Furthermore, CD4+ T cells are also engaged in antilisterial resistance by providing CD8+ T cells with B7·1/B7·2-mediated costimulation by DCs through the CD40–CD40L interaction,14,15 and by polarizing the immune response towards a T helper type 1 (Th1) pathway.16,17 Specifically, B7·1 and B7·2 costimulatory molecules are reported to be necessary for the production of IFN-γ and IL-2 from Th1 CD4+ T cells during Listeria infection.
B7-H1 (also known as CD274 and PD-L1) is a member of the B7 family that positively or negatively controls T-cell receptor (TCR)-mediated signalling (reviewed by Chen18). The findings of a series of in vivo studies using either the antagonistic anti-B7-H1 antibody or gene knockout mice support the coinhibitory role of endogenous B7-H1; for example, in vivo blockade of B7-H1 with antagonistic monoclonal antibody (mAb) was found to activate effector T cells, leading to an increase in the incidence of autoimmune diabetes in non-obese diabetic (NOD) mice, hapten-induced contact hypersensitivity in normal mice, and susceptibility to experimental autoimmune encephalomyelitis in B7-H1 knockout mice.19–22 However, the findings that transgenic expression of B7-H1 by β-islet cells induces spontaneous diabetes and accelerates the rejection of β-islet cells in allogeneic hosts, and that the antagonistic antibody to B7-H1 inhibits the pathogenesis of inflammatory bowel disease, suggest that B7-H1 plays a costimulatory role in T-cell immunity in vivo.23,24 While B7-H1 has been implicated in T-cell immunity in cancer progression, autoimmunity and graft rejection in many studies, there are few reports elucidating the role of endogenous B7-H1 in infection models. Clerici et al.25 suggested that up-regulation of B7-H1 expression and IL-10 production in antigen-presenting cells from AIDS patients could be responsible for T-cell hyporesponsiveness and loss of protective immunity to human immunodeficiency virus (HIV). More recently, B7-H1 was suggested to be a coinhibitor of T-cell immunity against Schistosoma mansoni infection, with schistosome worms inducing T-cell anergy through the engagement of B7-H1 up-regulated on macrophages.26 To our knowledge, however, there is no report demonstrating the role of B7-H1 in intracellular bacterial infection and, particularly, in the Listeria infection model.
In this study, we present evidence that B7-H1 engagement enhances protective immunity against L. monocytogenes through an in vivo blockade of endogenous B7-H1 with an antagonistic mAb. These findings provide new insight into the effect of endogenous B7-H1 on innate and adaptive immunity against intracellular bacterial infection in vivo.
Materials and methods
Mice and bacteria
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from Charles River (Tokyo, Japan). All mice were used for the experiments at the age of 8–10 weeks. For infection of the mice, L. monocytogenes (ATCC 19111) was grown in a brain heart infusion (BHI) medium (Difco, Detroit, MI) at 37° and stored in 20% glycerol at −80° until use. For restimulation of immune cells, heat-killed L. monocytogenes (HKLM) was prepared by incubating the L. monocytogenes culture at 70° for 3 hr, followed by washing in phosphate-buffered saline (PBS) three times, and kept at −20° until use.
Reagents and antibodies
LLO91-99/H-2Kd pentamer was obtained from ProImmune (Oxford, UK). Synthetic peptide for listeriolysin O91-99 (GYKDGNEYI) was produced at Peptron (Daejeon, Korea). The neutralizing anti-mouse B7-H1 mAb-producing hybridoma (10B5) was generated as described previously.5 The hybridoma cell line (UC9-1B8) producing hamster anti-DNP immunoglobulin G (IgG) used for isotype control was obtained from the American Type Culture Collection (ATCC, Rockville, MD). The anti-mouse B7-H1 mAb and hamster IgG were purified from ascites using a protein G-column (Sigma, St Louis, MO). The binding activities of the mAbs were tested using mitogen-stimulated spleen cells or mB7-H1-transfected HEK 293 cells as described previously.27 The following mAbs were used for this experiment: fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 (GK1·5), FITC-conjugated anti-mouse CD8α (53-6·7), FITC-conjugated anti-mouse CD11b (M1/70), and FITC-conjugated anti-mouse NK1·1 (PK136), which was obtained from DiNonA (Seoul, Korea). Phycoerythrin (PE)-conjugated anti-mouse programmed death (PD)-1 (J43), PE-conjugated anti-mouse CD44 (IM7), PE-conjugated anti-mouse CD62L (MEL-14), PE-conjugated anti-mouse CD11c (HL3), PE-conjugated anti-mouse IFN-γ (XMG1·2), purified anti-mouse CD16/CD32 (2·4G2), biotin-conjugated hamster IgG1 (A19-3), and FITC-Annexin V were obtained from BD Bioscience (San Diego, CA). FITC-conjugated anti-mouse TNF-α (MP6-XT22) was obtained from e-Bioscience (San Diego, CA). PE-conjugated streptavidin was purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Listeria monocytogenes infection
C57BL/6 mice were infected intravenously (i.v.) with 3000 colony-forming units (CFU) of L. monocytogenes and given intraperitoneally (i.p.) 200 µg of anti-mouse B7-H1 mAb (10B5) or hamster isotype control IgG 1 day before infection for the study of the functions of macrophages and NK cells, or 1 day before and 2 days post-infection (p.i.) for the study of T-cell immunity. For the survival assay, mice treated as above were injected i.v. with a high dose of L. monocytogenes (30 000 CFU). Survival was monitored for 14 days after infection. In some experiments, BALB/c mice previously infected with 3000 CFU of live L. monocytogenes were reinfected with 5000 CFU of live bacteria at day 25 p.i., and then 5 days later proliferation of LLO91-99-specific CD8+ T cells was determined using LLO91-99 pentamer.
Determination of colony-forming units
The number of viable L. monocytogenes in spleens and livers from L. monocytogenes-infected mice was determined as previously described.28 Briefly, the tissues were weighed and homogenized with a tissue grinder (Pyrex, New York, NY) in 0·05% Triton X-100/PBS. The homogenate was serially diluted with BHI medium and plated on BHI agar plates to quantify colony-forming units. Colonies were counted after 24–36 hr of incubation at 37°. The number of colony-forming units per gram of tissue was converted to a log10 value.
Flow cytometry
A single-cell suspension of spleen (H-2b) was used to purify monocytes and NK cells using anti-CD11b or anti-NK1·1 magnetic beads (Miltenyl Biotech, Auburn, CA), according to the manufacturer's instructions. To detect B7-H1 expression, cells were first incubated with FcR blocker (2·4G2) and subsequently stained with biotin-conjugated anti-mouse B7-H1 (clone 10B5). B7-H1 was detected with PE-conjugated streptavidin. Cell populations were determined with FITC-conjugated mAbs for CD4, CD8, CD11b or NK1·1. For analysis of T-cell phenotypes, cells were stained with FITC-conjugated anti-mouse CD4 or CD8 and PE-conjugated anti-mouse CD11c, CD44 or CD62L. For LLO91-99 pentamer staining, spleen cells (H-2d) were stained with FITC-conjugated anti-mouse CD8 and PE-conjugated LLO91-99/H-2Kd pentamer. Analysis was performed using FACSort and CellQuestPro software (BD Bioscience, San Jose, CA). For intracellular TNF-α staining, cells were restimulated with HKLM (1 × 107 CFU) for 6 hr in the presence of brefeldin A (5 µg/ml; BD Biosciences, Mountain View, CA). Cells were first stained with cell surface markers, and subsequently fixed, permeabilized using the Cytofix/Cytoperm Kit (BD Biosciences) according to the manufacturer's instructions, and incubated with FITC-conjugated anti-TNF-α mAb. For intracellular IFN-γ staining, cells were restimulated with LLO91-99 peptide (1 µm) for 48 hr and intracellularly stained with PE-conjugated IFN-γ mAb. For Annexin V staining, cells were suspended in an Annexin V binding buffer (BD Biosciences) and stained with PE-conjugated anti-mouse CD8 and FITC-conjugated Annexin V.
Cytokine enzyme-linked immunosorbent assay (ELISA)
Soluble TNF-α, IFN-γ and IL-12 were measured by ELISA using a cell culture supernatant harvested from the spleen cells restimulated in vitro with HKLM (1 × 107 CFU) for 24 hr according to the manufacturer's specifications (e-Bioscience). In brief, microtitre plates (Costar, New York, NY) were coated with capture antibodies at 4° overnight. After blocking with 5% fetal bovine serum (FBS) in PBS for 1 hr at room temperature, undiluted supernatant was incubated for 2 hr at room temperature. Subsequently the cytokines were detected using biotin-conjugated detecting antibodies and the colour was developed using horseradish peroxidase (HRP)-conjugated streptavidin and tetramethyl benzidine as a substrate (Endogen, Rockford, IL). Optical densities were measured at 450 nm with an ELISA plate reader (Molecular Devices, Union City, CA).
Reverse transcription–polymerase chain reaction (RT-PCR)
NK cells were purified (> 97%) from splenocytes using biotin-conjugated NK1·1 plus streptavidin-conjugated magnetic beads at day 3 p.i. Total RNA was isolated from purified NK cells using the TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was reverse-transcribed into cDNA using a PCR cDNA synthesis kit (Clontech Laboratories, Palo Alto, CA). PCR was performed using sense/antisense primers. The PCR primer sequences were as follows: mouse perforin: forward, 5′-TGC TAC ACT GCC ACT CGG TCA-3′; reverse, 5′-TTG GCT ACC TTG GAG TGG GAG-3′; mouse granzym B: forward, 5′-CTC CAC GTG CTT TCA CCA AA-3′; reverse, 5′-GGA AAA TAG TAC AGA GAG GCA-3′; and mouse GAPDH: forward, 5′-TCG TGG AGT CTA CTG GCG TCT T-3′; reverse, 5′-TGG CATTGA TGG CAT GGA CTG T-3′. PCR products were analysed by electrophoresis on 1% agarose gels.
Nitrite assay
A quantity of 2 × 105 spleen cells per well in 96-well plates were restimulated with 1 × 107 CFU of HKLM for 48 hr. The supernatants were harvested, subsequently mixed with Griess Reagent (Sigma) and incubated for 10 min at room temperature. Optical densities were measured at 450 nm with an ELISA plate reader (Molecular Devices). NaNO2 was used as the standard.
Statistical analysis
CFU data were analysed using a two-tailed Mann–Whitney U-test. Differences were considered significant when P < 0·05.
Results
B7-H1 expression was up-regulated on immune cells following Listeria monocytogenes infection
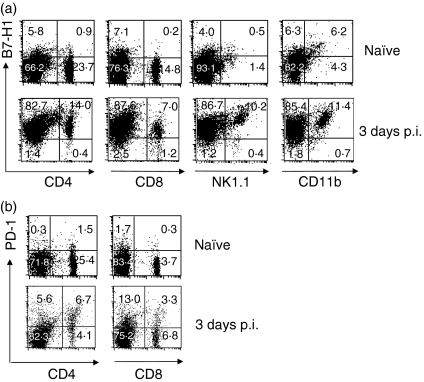
We first examined B7-H1 expression in splenic immune cells following the L. monocytogenes infection. Although naïve cell populations expressed B7-H1 at a low level even before L. monocytogenes infection, B7-H1 expression was up-regulated in more than 90% of each cell population, including CD4+ and CD8+ T cells, NK cells and macrophages (Fig. 1a). B7-H1 was up-regulated from day 1 p.i. and subsequently reached a peak at days 3–5 p.i. (data not shown). Expression of PD-1, the cognate receptor for B7-H1, was induced at a higher level in CD4+ T cells than in CD8+ T cells at day 3 p.i. (Fig. 1b). Consistent with other reports, these results indicate that B7-H1 is induced immediately after bacterial infection in various immune cells.
Figure 1.
Expression of B7-H1 and programmed death (PD)-1 in response to Listeria monocytogenes infection. Mice (C57BL/6) were infected intravenously (i.v.) with 3000 colony-forming units (CFU) of L. monocytogenes, and spleens were harvested at the indicated times. (a) Expression of B7-H1 on splenic immune cells. Splenocytes were isolated and stained with biotin-conjugated anti-mouse B7-H1 (10B5) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse monoclonal antibodies (mAbs) for CD4, CD8, NK1·1 or CD11b. B7-H1 was detected using phycoerythrin (PE)-conjugated streptavidin and analysed by flow cytometry. (b) Expression of PD-1 on splenic lymphocytes. Splenocytes were isolated on day 3 post-infection (p.i.) and stained with PE-conjugated anti-mouse PD-1 and FITC-conjugated anti-mouse CD4 and CD8 and analysed by flow cytometry. The dot plot result is representative of three independent experiments, each showing similar results.
B7-H1 blockade impaired the clearance of Listeria monocytogenes in vivo
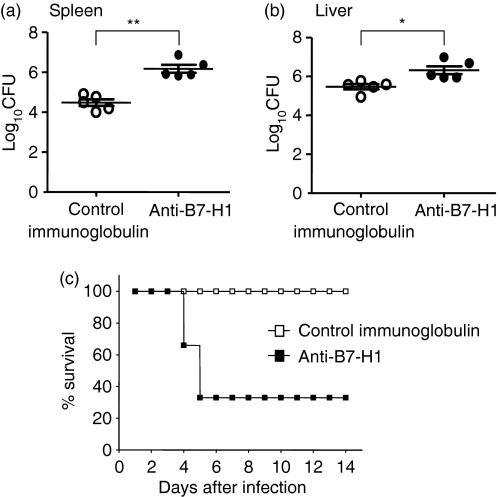
Next, we examined the bacterial load in livers and spleens from the mice infected with L. monocytogenes to determine the physiological role of endogenous B7-H1 in bacterial clearance in vivo. When challenged with L. monocytogenes, the bacterial load in spleens from mice infused with antagonistic anti-B7-H1 mAb was higher than that in control mice given hamster isotype immunoglobulin (6·1 ± 0·4 log10 CFU in the anti-B7-H1-treated group versus 4·4 ± 0·3 log10 CFU in the control group; P < 0·001) (Fig. 2a). We observed a similar pattern of bacterial load in liver tissues from mice administered anti-B7-H1 mAb (6·3 ± 0·4 log10 CFU in the anti-B7-H1-treated group versus 5·4 ± 0·3 log10 CFU in the control group; P < 0·05). Consistent with this result, B7-H1 blockade resulted in a significant increase in mortality compared with the mice given control immunoglobulin [survival 10 of 10 (control immunoglobulin-treated mice) versus three of 10 (anti-B7-H1-treated mice); P < 0·001] (Fig. 2b). These results indicate that B7-H1 is involved in resistance to L. monocytogenes infection in vivo.
Figure 2.
Failure of mice administered anti-B7-H1 monoclonal antibody (mAb) to reduce their Listeria monocytogenes burden. (a) Mice were infected intravenously (i.v.) with 3000 colony-forming units (CFU) of L. monocytogenes. Mice were given 200 µg of anti-B7-H1 mAb or control immunoglobulin by intraperitoneal (i.p.) injection 24 hr before infection. (a) Livers and spleens were harvested at day 3 post-infection (p.i.). Colony-forming units were determined by plating the serially diluted liver or spleen homogenate on brain-heart infusion agar plates. Data represent the means for five mice per group. Similar results were obtained from three independent experiments. **P < 0·001, *P < 0·05, compared with control immunoglobulin. The plotted data are mean ± standard deviation. (b) A high dose (30 000 CFU) of L. monocytogenes was injected i.v. into mice given 200 µg of anti-B7-H1 mAb or control immunoglobulin 1 day before infection. Survival was monitored for 14 days after infection for four mice per group. Similar results were obtained in two independent experiments.
In vivo blockade of B7-H1 suppressed innate immunity against Listeria monocytogenes infection
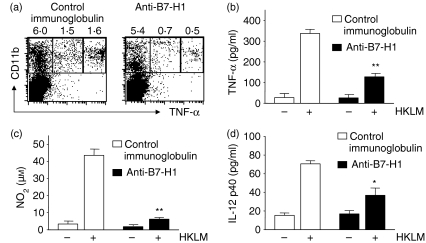
We next tested whether neutralization of endogenous B7-H1 affected the intracellular bacterial killing mechanisms of innate immune cells, including macrophages and NK cells. In the in vitro study using macrophages restimulated with HKLM, we found that expression of intracellular TNF-α was greatly diminished in macrophages from the mice given anti-B7-H1 mAb compared with the mice given control immunoglobulin at day 3 p.i. (Fig. 3a). A similar result for the production of soluble TNF-α was obtained for mice given anti-B7-H1 mAb (127 ± 16·6 in anti-B7-H1-treated mice versus 336·7 ± 20·0 pg/ml in the control group; P < 0·001) (Fig. 3b). Consistent with these findings, when spleen cells were restimulated with HKLM for 2 days in vitro, macrophages from the mice given anti-B7-H1 mAb produced NO at a significantly lower level in response to HKLM in vitro compared with the control group (7·4 ± 1·9 in the anti-B7-H1-treated group versus 43·8 ± 4·8 in the control group; P < 0·01) (Fig. 3c). We also observed that macrophages from anti-B7-H1-treated mice produced much less IL-12p40 than those from control mice (36·7 ± 7·8 in the anti-B7-H1-treated group versus 70·5 ± 3·3 in the control group; P < 0·05) (Fig. 3d).
Figure 3.
Reduction of tumour necrosis factor (TNF)-α, nitric oxide (NO) and interleukin (IL)-12 production by macrophages following infusion of anti-B7-H1 monoclonal antibody (mAb). Mice were infected and given mAbs as in Fig. 2. Splenocytes were harvested from infected mice at day 3 post-infection (p.i.). (a) Expression of intracellular TNF-α. Spleen cells (2 × 105) were restimulated with heat-killed Listeria monocytogenes[HKLM; 1 × 107 colony-forming units (CFU)] for 6 hr in the presence of brefeldin A. The stimulated cells were stained for CD11b and intracellular TNF-α and then analysed by flow cytometry. Each fluorescence-activated cell sorting (FACS) plot is a representative of three independent experiments. CD11b+ macrophages (2 × 105) were restimulated with HKLM for 24 hr in 96-well plates. Soluble TNF-α (b) and IL-12 p40 (d) in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA). (c) CD11b+ macrophages (2 × 105) were restimulated with HKLM for 48 hr. Nitrite (NO2) in culture supernatants was measured using the Griess Reagent Kit. Results are representative of three independent experiments. *P < 0·05, compared with control immunoglobulin. Plotted data are mean ± standard deviation.
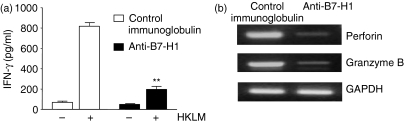
As IFN-γ is believed to be critically important in macrophage activation, which leads to early resistance to Listeria infection through the production of free radicals and various intermediates,29,30 we decided to examine IFN-γ production in NK cells, an important cellular source of IFN-γ. As shown in Fig. 4(a), IFN-γ production was greatly reduced in NK cells from the mice given anti-B7-H1 mAb compared with the control mice (196 ± 29·1 in the anti-B7-H1-treated mice versus 818·1 ± 32·5 in the control group; P < 0·001). In addition, cytotoxic granules such as granzyme B and perforin were greatly decreased in NK cells from anti-B7-H1-treated mice (Fig. 4b). These results indicate that endogenous B7-H1 is involved in early activation of innate immune cells including macrophages and NK cells, which leads to early protection against L. monocytogenes infection.
Figure 4.
Reduction of natural killer (NK) cell activities following administration of anti-B7-H1 monoclonal antibody (mAb). Mice were infected and given mAbs as in Fig. 2. NK cells were negatively (a) or positively (b) purified from spleen cells at day 3 post-infection (p.i.). (a) Interferon (IFN)-γ production. Cells (2 × 105) were restimulated with heat-killed Listeria monocytogenes (HKLM) for 24 hr. The level of IFN-γ in culture supernatants was determined by enzyme-linked immunosorbent assay (ELISA). **P < 0·001, compared with control immunoglobulin. Plotted data are mean ± standard deviation. (b) Expression of effector molecules. Total RNA was extracted from NK cells treated as in (a), and reverse transcription–polymerase chain reaction (RT-PCR) for perforin and granzyme B was performed as described in the ‘Materials and methods’ section.
Blocking of endogenous B7-H1 selectively inhibited the proliferation and activation of effector CD8+ T cells
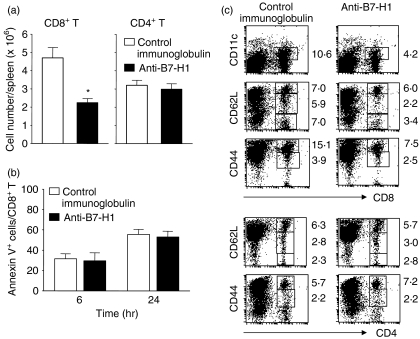
We then decided to further investigate the mechanisms underlying the failure of mice given anti-B7-H1 mAb to reduce their L. monocytogenes burden by examining the T-cell immune responses to L. monocytogenes infection. T-cell immunity reaches its peak 7–8 days following L. monocytogenes infection and plays a critical role in complete clearance of L. monocytogenes in infected mice.31 We found that the number of CD8+ T cells in spleens from infected mice given anti-B7-H1 mAb was significantly decreased at day 7 p.i. (2·2 × 106 ± 0·2 × 106 in the anti-B7-H1 mAb-treated group versus 4·7 × 106 ± 0·5 × 106 in the control group; P < 0·05), whereas the numbers of CD4+ T cells were comparable in the two groups (Fig. 5a). To rule out the possibility of depletion of CD8+ T cells by apoptosis, the amount of T-cell apoptosis was determined by Annexin V staining of spleen cells restimulated with HKLM for 6 or 24 hr in vitro. No difference in Annexin V+ CD8+ T-cell populations was observed between the two groups (Fig. 5b). This result suggests that the reduced number of CD8+ T cells in the anti-B7-H1-treated mice was unlikely to have been caused by apoptosis of B7-H1-expressing CD8+ T cells.
Figure 5.
Impairment of generation of effector CD8+ T cells following administration of anti-B7-H1 monoclonal antibody (mAb). Mice were infected as in Fig. 2 and were given anti-B7-H1 or control immunoglobulin (200 µg per injection) 1 day before and 2 days after infection. Splenocytes were isolated from mice at day 7 post-infection (p.i.). (a) Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD8 or anti-CD4, and analysed by flow cytometry. *P < 0·05, compared with control immunoglobulin. Plotted data are mean ± standard deviation. (b) Percentage of CD8+ T cells positive for Annexin V. Cells were restimulated with heat-killed Listeria monocytogenes (HKLM) [1 × 107 colony-forming units (CFU)] for 6 or 24 hr. Cells were stained with phycoerythrin (PE)-conjugated anti-CD8 and FITC-conjugated Annexin V. Plots were gated on CD8+ T cells. (c) Expression of effector surface markers on splenic T cells. Cells were stained with FITC-conjugated anti-CD8 or -CD4 and PE-conjugated anti-CD11c, -CD44 or -CD62L, and analysed by flow cytometry. The numbers indicate the percentages of gated cells. Each fluorescence-activated cell sorting (FACS) plot is representative of three independent experiments.
We next examined the effector surface markers, including CD62L, CD44 and CD11c, of CD4+ and CD8+ T cells in both groups. Specifically, CD11c, a member of the β2-integrin family, has been reported to be a useful effector marker for CD8+ T cells in an acute virus infection.32 We found that CD11c+ CD8+ T cells were generated in 5–7 days following infection (data not shown). The CD11c+ CD8+ T-cell population from the mice given anti-B7-H1 mAb was reduced by two- to threefold (4·1 ± 1·6% in the anti-B7-H1-treated group versus 11·0 ± 1·5% in the control group) (Fig. 5c, upper panel). Similarly, CD8+ T cells with activation phenotypes including CD62Llow/int or CD44high were diminished in the mice given anti-B7-H1 mAb (CD62Llow/int, 5·5 ± 0·7% in the anti-B7-H1-treated group versus 13·1 ± 1·2% in the control group; CD44high, 10·3 ± 0·8% in the anti-B7-H1-treated group versus 19·4 ± 1·5% in the control group) (Fig. 5c, upper panel). In contrast to CD8+ T cells, there was no significant difference in the number of CD4+ T cells showing the CD44high or CD62Llow/int phenotype between the two groups (Fig. 5c, lower panel). These findings indicate that endogenous B7-H1 selectively activates effector CD8+ T cells rather than CD4+ T cells during the early phase of L. monocytogenes infection.
Blockade of endogenous B7-H1 repressed L. monocytogenes-specific T-cell generation
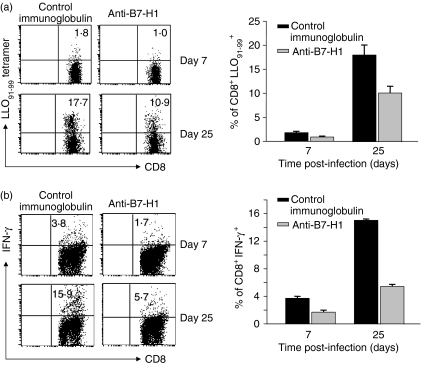
Having determined that blockade of endogenous B7-H1 impeded the proliferation and activation of CD8+ T cells from L. monocytogenes-infected mice, we next examined whether B7-H1 blockade resulted in a decrease in L. monocytogenes-specific CD8+ T-cell generation. We immunized control immunoglobulin- or anti-B7-H1-treated mice with live L. monocytogenes and then determined the percentage of LLO91-99-specific CD8+ T cells using LLO91-99 pentamer (Fig. 6a) and intracellular IFN-γ staining (Fig. 6b). The percentage of LLO91-99-specific effector CD8+ T cells in the mice given anti-B7-H1 mAb was significantly reduced compared with the control mice at day 7 p.i. (LLO91-99 pentamer, 0·9 ± 0·2% in the anti-B7-H1-treated group versus 1·8 ± 0·3% in the control group; IFN-γ; 1·6 ± 0·3% in the anti-B7-H1-treated group versus 3·7 ± 0·3% in the control group) (Figs 6a,b, upper panel). To determine whether B7-H1 blockade also suppresses L. monocytogenes-specific memory T cells, we immunized control immunoglobulin- or anti-B7-H1-treated mice with live L. monocytogenes and then rechallenged the mice 25 days later. As expected, L. monocytogenes-specific CD8+ memory T cells were generated at a much lower level in the mice given anti-B7-H1 mAb than in the control mice (LLO91-99 pentamer, 10·2 ± 1·4% in the anti-B7-H1-treated group versus 18·1 ± 2·1% in the control group; IFN-γ, 5·5 ± 0·3% in the anti-B7-H1-treated group versus 15·1 ± 0·2% in the control group) (Figs 6a,b, lower panel). Taken together, our results thus indicate that B7-H1 costimulation is required for the generation of L. monocytogenes-specific effector and memory T cells.
Figure 6.
Reduction of expansion of Listeria monocytogenes-specific T cells following administration of anti-B7-H1 monoclonal antibody (mAb). Mice (C57BL/6 or BALB/c) were infected and given mAbs as in Fig. 2. Splenocytes were isolated from Ab-treated mice on day 7 post-infection (p.i.). Other mice were reinfected at day 25 p.i. with 5000 colony-forming units (CFU) of L. monocytogens. Splenoctyes were isolated 5 days after reinfection. (a) Cells were stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD8 and phycoerythrin (PE)-conjugated listeriolysin-O (LLO91-99) pentamer, and analysed by flow cytometry. Plots were gated on CD8+ T cells. (b) Splenocytes were restimulated in vitro with LLO91-99 peptide for 48 hr. Interferon (IFN)-γ production was measured by intracellular staining. Plots were gated on CD8+ T cells. Each fluorescence-activated cell sorting (FACS) plot is representative of two independent experiments.
Discussion
We have demonstrated in this study that blockade of B7-H1 impaired protective immunity mediated primarily by macrophages, NK cells and CD8+ T cells against L. monocytogenes infection. It has long been known that early resistance to L. monocytogenes is attributable to effector molecules such as INF-γ, TNF-α, lymphotoxin-β and NO produced by the innate immune cells, including macrophages, NK cells, γδ T cells, neutrophils29,30,33,34 and the recently identified Tip-DCs.10 Numerous studies have demonstrated that, following L. monocytogenes infection, macrophages produce IL-12 which, in turn, activates NK cells to release IFN-γ, a major cytokine for macrophage activation.35,36 The activated macrophages subsequently produce TNF-α and NO, two key mediators responsible for intracellular bacterial killing (reviewed by Lara-Tejero and Pamer37). We have shown that the production of NK cell IFN-γ and macrophage IL-12 was down-regulated following administration of antagonistic B7-H1 mAb, which was directly correlated with a reduction in TNF-α and NO production by macrophages, leading to a failure of anti-B7-H1-treated mice to decrease the L. monocytogenes burden in spleens and livers compared with the control mice. The results suggest that endogenous B7-H1 positively regulates the effector functions of innate immune cells, leading to early protection against intracellular bacterial infection. However, we cannot exclude the possibility that engagement of B7-H1 expressed on macrophages and NK cells by mAb may directly deliver an inhibitory signal back into these cells. This idea comes from recent observations suggesting that costimulatory molecules expressed on DCs can deliver signals back into the cells, thus influencing the functions of the cells.38,39
Accumulating data indicate that αβ T cells play a significant role in complete bacterial clearance and long-term protective immunity,40,41 and, of these, CD8+ T cells contribute much more to protective immunity than CD4+ T cells.42 Interestingly, blockade of endogenous B7-H1 selectively inhibited the proliferation and activation of effector CD8+ T cells rather than CD4+ T cells in response to L. monocytogenes infection, as indicated by the finding that the expression of surface activation markers such as CD62Llow/int and CD44high was greatly reduced in CD8+ T cells from mice given anti-B7-H1 mAb. In contrast, there was no difference in the number of CD4+ T cells. In particular, endogenous B7-H1 blockade reduced the CD11c+ CD8+ T-cell population, a newly identified T-cell population that is expanded following acute infection with the leukotriene cytomegalovirus (LCMV).32 However, the reduction of CD8+ T cells in vivo was not mediated by apoptosis or in vivo depletion, as demonstrated by the observation that there was no difference in the Annexin V+ cell population between the mice given control immunoglobulin and those given anti-B7-H1 mAb, and also by our previous reports.43,44 In the setting of in vivo blockade of B7-H1, the question of whether there is differential regulation of the proliferation of CD4+ and CD8+ T cells will need to be answered in a future study.
The observation that B7-H1 blockade down-regulated the expansion of LLO-specific or IFN-γ-producing L. monocytogenes-reactive effector and memory CD8+ T cells indicates that endogenous B7-H1 is required for costimulation of L. monocytogenes-specific CD8+ T cells in response to L. monocytogenes infection. However, there was no apparent difference in bacterial burden in the livers and spleens between control immunoglobulin- and anti-B7-H1-treated mice which were rechallenged with L. monocytogenes at day 25 p.i. (data not shown). This finding could not be simply interpreted as a failure of B7-H1 costimulation to generate antibacterial memory CD8+ T cells, which is believed to be required to complete resolution of listerial infection.15 The result indicating a positive role for endogenous B7-H1 in T-cell immunity is consistent with results obtained in the murine model of chronic inflammation,23 wherein, following infusion of antagonistic B7-H1 mAb, experimental colitis was prevented and the production of cytokines, including IFN-γ, TNF-α and IL-2, was significantly reduced. Our findings are also in agreement with those of our previous studies on the costimulatory role of B7-H1 in vitro and in vivo, in which we found that immobilized B7-H1Ig or cell-associated B7-H1 enhanced T-cell proliferation and cytokine production,27,45 and administration of B7-H1Ig fusion protein increased keyhole limpet haemocyanin-specific T-cell proliferation.45 On the basis our observations, we can speculate that endogenous B7-H1 acts as a costimulator by delivering a positive signal through an as yet unidentified costimulatory receptor other than PD-1 on T cells. The idea that an unidentified costimulatory receptor is present comes from several lines of evidence that a B7-H1 mutant in which a PD-1-binding site is abolished remains costimulatory for T cells, and that PD-1-deficient T cells are still activated by B7-H1. However, there is a report indicating that B7-H1 expressed on T cells can deliver a costimulatory signal back into T cells through a reverse signalling pathway.46 Therefore, we cannot rule out the possibility that anti-B7-H1 mAb may hinder the ligation of B7-H1 expressed on T cells to PD-1, resulting in inhibition of transmission of costimulatory reverse signal transmission into T cells through B7-H1.
Collectively, the results indicate that endogenous B7-H1 may act as a costimulator not only enhancing the activation and expansion of antigen-specific CD8+ T cells but also positively regulating the innate immune response against L. monocytogenes infection. Our findings thus shed new light on the physiological role of endogenous B7-H1 in bacterial infection.
Acknowledgments
This work was supported by a Korea Research Foundation grant from the Korean Government (KRF-2005-041-E00120 to SKS), and by a Korea Science and Engineering Foundation (KOSEF) grant, also from the Korean Government (MOST) (R13-2007-023-00000-0 to IC).
Abbreviations
- CFSE
carboxyfluorescein succinimidyl ester
- CFU
colony-forming unit
- HKLM
heat-killed Listeria monocytogenes
- LLO
listeriolysin-O
References
- 1.Kocks C, Gouin E, Tabouret M, Berche P, Ohayon H, Cossart P. L. monocytogenes-induced actin assembly requires the actA gene product, a surface protein. Cell. 1992;7:521–31. doi: 10.1016/0092-8674(92)90188-i. [DOI] [PubMed] [Google Scholar]
- 2.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–68. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunn PL, North RJ. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of αβ and γδT cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–6. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 5.Edelson BT, Unanue ER. Immunity to Listeria infection. Curr Opin Immunol. 2000;12:425–31. doi: 10.1016/s0952-7915(00)00112-6. [DOI] [PubMed] [Google Scholar]
- 6.Shiloh MU, MacMicking JD, Nicholson S, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 7.Buchmeier NA, Schreiber RD. Requirement of endogenous interferon-γ production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci USA. 1985;82:7404–8. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–4. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 10.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 11.Meraz MA, White JM, Sheehan KCF, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84:431–42. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 12.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and - dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 13.Portnoy DA, Schreiber RD, Connelly P, Tilney LG. Gamma-interferon limits access of Listeria monocytogenes to the macrophage cytoplasma. J Exp Med. 1989;170:2141–6. doi: 10.1084/jem.170.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding FA, Allison JP. CD28–B7 interactions allow the induction of CD8+ cytotoxic T lymphocytes in the absence of exogenous help. J Exp Med. 1993;177:1791–6. doi: 10.1084/jem.177.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–51. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann SH. Listeria monocytogenes specific T-cell lines and clones. Infection. 1988;16:S128–36. doi: 10.1007/BF01639735. [DOI] [PubMed] [Google Scholar]
- 18.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–47. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 19.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 20.Latchman YE, Liang SC, Wu Y, et al. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci USA. 2004;101:10691–6. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;7:63–83. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsushima F, Iwai H, Otsuki N, et al. Preferential contribution of B7-H1 to programmed death-1-mediated regulation of hapten-specific allergic inflammatory responses. Eur J Immunol. 2003;33:2773–82. doi: 10.1002/eji.200324084. [DOI] [PubMed] [Google Scholar]
- 23.Kanai T, Totsuka T, Uraushihara K, et al. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol. 2003;171:4156–63. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]
- 24.Subudhi SK, Zhou P, Yerian LM, et al. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest. 2004;113:694–700. doi: 10.1172/JCI19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trabattoni D, Saresella M, Biasin M, et al. B7-H1 is up-regulated in HIV infection and is a novel surrogate marker of disease progression. Blood. 2003;101:2514–20. doi: 10.1182/blood-2002-10-3065. [DOI] [PubMed] [Google Scholar]
- 26.Smith P, Walsh CM, Mangan NE, et al. Schistosoma mansoni worms induce anergy of T cells via selective up-regulation of programmed death ligand 1 on macrophages. J Immunol. 2004;173:1240–8. doi: 10.4049/jimmunol.173.2.1240. [DOI] [PubMed] [Google Scholar]
- 27.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 28.Bandroft GJ, Schreiber RD, Unanue ER. Natural immunity: a T-cell independent pathway macrophage activation, defined in the scid mouse. Immunol Rev. 1991;124:5–24. doi: 10.1111/j.1600-065x.1991.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 29.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–9. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Havell EA. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J Immunol. 1989;143:2894–9. [PubMed] [Google Scholar]
- 31.Busch DH, Pamer EG. T lymphocyte dynamics during Listeria monocytogenes infection. Immunol Lett. 1999;65:93–8. doi: 10.1016/s0165-2478(98)00130-8. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Roberts TJ, Sriram V, Cho S, Brutkiewicz RR. Myeloid marker expression on antiviral CD8+ T cells following an acute virus infection. Eur J Immunol. 2003;33:2736–43. doi: 10.1002/eji.200324087. [DOI] [PubMed] [Google Scholar]
- 33.Ehlers S, Hölscher C, Scheu S, et al. The lymphotoxin receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J Immunol. 2003;170:5210–8. doi: 10.4049/jimmunol.170.10.5210. [DOI] [PubMed] [Google Scholar]
- 34.Kaufmann S. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–63. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 35.MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage fuction. Annu Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 36.Trinchieri G. Interleukin-12. A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–76. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 37.Lara-Tejero M, Pamer EG. T cell responses to Listeria monocytogenes. Curr Opin Microbiol. 2004;7:45–50. doi: 10.1016/j.mib.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen LT, Radhakrishnan S, Ciric B, et al. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;18:1393–8. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 40.Belz GT, Wodarz D, Diaz G, Nowak MA, Doherty PC. Compromised influenza virus-specific CD8(+)-T-cell memory in CD4(+)-T-cell-deficient mice. J Virol. 2002;76:12388–93. doi: 10.1128/JVI.76.23.12388-12393.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+)-T-cell responses in CD4-depleted lg(–/–) mice. J Virol. 2000;74:9762–5. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Egan PJ, Carding SR. Downmodulation of the inflammatory response to bacterial infection by γδ T cells cytotoxic for activated macrophages. J Exp Med. 2000;191:2145–58. doi: 10.1084/jem.191.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–5. [PubMed] [Google Scholar]
- 44.Hirano F, Kaneko K, Tamura K, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–96. [PubMed] [Google Scholar]
- 45.Tamura H, Dong H, Zhu G, Sica GL, Flies DB, Tamada K, Chen L. B7-H1 costimulation preferentially enhances CD28-independent T-helper cell function. Blood. 2001;97:1809–16. doi: 10.1182/blood.v97.6.1809. [DOI] [PubMed] [Google Scholar]
- 46.Dong H, Strome SE, Matteson EL, et al. Costimulating aberrant T cell responses by B7-H1 autoantibodies in rheumatoid arthritis. J Clin Invest. 2002;111:363–70. doi: 10.1172/JCI16015. [DOI] [PMC free article] [PubMed] [Google Scholar]