Abstract
Cytokine-dependent T helper 1 (Th1) differentiation versus T helper 2 (Th2) differentiation is controlled by distinct transcription factors. Previously, we have demonstrated that immature human dendritic cells (DC) from blood donors with allergies show rapid phosphorylation of the Th2-associated signal transducer and activator of transcription 6 (STAT6) upon contact with protein allergens. In the present study we investigated whether this process is regulated by the downstream molecules suppressor of cytokine signalling (SOCS) and/or by the factors T-bet and GATA3. Therefore, immature DC of grass or birch pollen-allergic donors were treated with the respective Th2-promoting protein allergens, and, for comparison, with the Th1-promoting contact allergen 5-chloro-2-methylisothiazolinone plus 2-methylisothiazolinone (MCI/MI) or with the antigen tetanus toxoid. Changes in the mRNA levels of SOCS1, SOCS3, T-bet and GATA3 were analysed by quantitative real-time polymerase chain reaction. Exposure of DC to protein allergens led to the up-regulation of the Th2-associated genes SOCS3 and GATA3, whereas the contact allergen MCI/MI preferentially enhanced the expression of the Th1-associated gene T-bet. Treatment of immature DC with the antigen tetanus toxoid increased both Th1- and Th2-associated genes. Our data indicate that polarization of type 1 versus type 2 immune responses takes place already at the level of antigen-presenting cells, involving molecules similar to those used in T-cell polarization.
Keywords: allergy, dendritic cells, signal transduction
Introduction
T-cell differentiation towards T helper 1 (Th1) versus T helper 2 (Th2) cells plays a key role in inflammatory diseases, including allergic immune responses. Atopic allergic diseases like allergic asthma, allergic rhinitis or allergic conjunctivitis are characterized by a dominance of Th2 cells, whereas contact allergic reactions are dependent on type 1 immune responses.1–5
The cytokine-dependent Th1 or Th2 differentiation involves the activation of Janus kinases. In an active state, Janus kinases are able to phosphorylate signal transducer and activator of transcription (STAT) family molecules, which then dimerize and translocate into the nucleus, serving among others as transcription factors for cytokine gene expression.6,7 Whereas STAT4 is activated by interleukin (IL)-12 and interferon-α (IFN-α) at sites of Th1-mediated inflammation,8 STAT6 is important for Th2 development.9,10 Two further opposing transcription factors are very important for Th1/Th2 differentiation: T-bet is essential for the development of Th1 cells, and GATA3 performs an equivalent role in Th2 development.11–14
Furthermore, the Janus Kinases (JAK)/STAT pathway is controlled by the family of suppressor of cytokine signalling (SOCS) molecules. This group of intracellular proteins consists of eight members, named SOCS1–7, and CIS. Each of these proteins contain an N-terminal domain of variable length and sequence as well as a C-terminal, 40-amino-acid module called the SOCS box. The SOCS molecules have been shown to regulate cytokine signalling in a classic negative-feedback loop mechanism.15,16 Concerning the differentiation of T helper cells, SOCS1 as well as SOCS3 are important factors for the regulation of Th1 and Th2 development.17,18 For example, SOCS1 is able to inhibit the activation of STAT6 and thereby negatively regulates the IL-4-induced proliferation of Th2 cells.19 On the other hand, SOCS3 is able to prevent IL-12-dependent STAT4 activation and therefore promotes Th2 differentiation.20 In allergic diseases, SOCS3 and SOCS5 are predominantly expressed in Th2 cells, and SOCS3−/− mice show diminished Th2 differentiation.21,22
Previous findings of our group revealed that immature human dendritic cells (DC) show rapid phosphorylation and activation of STAT6 after exposure of these cells to Th2-promoting protein allergens.23 Contact allergens inducing type 1 immune responses have been shown not to cause any activation of STAT6 or other members of the STAT protein family in human DC.24 The present study was carried out to evaluate the role of cytokine signalling factors, such as the downstream SOCS molecules, as well as the transcription factors T-bet and GATA3, in human DC (i.e. at the level of antigen-presenting cells), after exposure to different types of allergens (full antigens and haptens) known to direct the immune response towards Th1 versus Th2 differentiation.
Materials and methods
Blood samples
Venous heparinized blood or leucocyte-enriched buffy coats (Transfusion Centre, Mainz, Germany) were obtained from allergic patients suffering from allergic rhinoconjunctivitis or asthma to grass or birch pollen. Specific sensitization was documented as a positive skin-prick test and the presence of allergen-specific IgE in the sera (ImmunoCap® class ≥ 2, measured using the ImmunoCap® specific IgE blood test; Phadia AB, Uppsala, Sweden). This study was approved by the local ethical committee. Informed consent was obtained from all subjects before participation in the study.
Generation of monocyte-derived immature DC
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll–Paque 1.077 (PAA, Pasching, Austria) density-gradient centrifugation. Cells (1 × 107) were incubated for 45 min in a six-well plate (Greiner, Frickenhausen, Germany) in Iscove’s modified Dulbecco’s medium containing l-glutamine and 25 mm Hepes (IMDM; PAA) supplemented with an antibiotic/antimycotic solution containing 100 μg/ml of streptomycin, 100 U/ml of penicillin and 250 ng/ml of amphotericin B (PAA), and 3% heat-inactivated autologous plasma. Non-adherent cells were removed by washing gently three times with prewarmed phosphate-buffered saline (PBS). The remaining CD14+ monocytes (> 90% purity) were incubated in 3 ml per well of IMDM containing 1% heat-inactivated autologous plasma, 1000 U/ml of IL-4 (Strathmann Biotec GmbH, Hannover, Germany) and 200 U/ml of granulocyte–macrophage colony-stimulating factor (GM-CSF) (Leukine®; Immunex Corp., Seattle, WA). Every other day, the cells were fed with fresh medium. On day 7, the resulting immature DC were pulsed with 10 μg/ml of birch or grass pollen extract (ALK-Scherax, Wedel, Germany), 1 μg/ml of 5-chloro-2-methylisothiazolinone plus 2-methylisothiazolinone (MCI/MI) (Hermal, Reinbek, Germany) or 1 μg/ml of tetanus toxoid (Chiron Behring GmbH, Marburg, Germany); as a baseline, untreated DC were used. After incubation of up to 1 hr, the DC were harvested, washed twice and used for direct isolation of total RNA.
Isolation of total RNA
Total RNA was isolated from the above-treated immature DC and from PBMC from each single donor by using the RNeasy® Mini Kit (Qiagen, Hilden, Germany), according to the protocol of the manufacturer. The amount of RNA in each sample was determined by photometric analysis.
Generation of cDNA
One-hundred nanograms of total RNA within each sample from every single donor was used to perform a reverse transcription reaction using the Sensiscript® Kit (Qiagen) together with Oligo-d(T) primers (Hoffmann-La Roche, Grenzach-Wyhlen, Germany), according to the protocol of the manufacturer.
Real-time and conventional polymerase chain reaction
Quantitative real-time polymerase chain reaction (PCR) analysis was performed using QuantiTect® Primer Assays (Qiagen). The following primer assays were used in this study:
β-actin: Hs_ACTB_SG_1 (catalogue number QT00095431); SOCS1: Hs_SOCS1_1_SG (catalogue number QT00202475); SOCS3: Hs_SOCS3_SG_1 (catalogue number QT00244580); GATA3: HS_GATA3_SG_1 (catalogue number QT00095501); and T-bet: HS_TBX21_1_SG (catalogue number QT00042217).
Conventional PCR analysis was performed by using the following primers obtained from Roth (Karlsruhe, Germany): CD3 forward: 5′-TGAGGGCAAGAGTGTGTAAG-3′; and CD3 reverse: 5′-TAGTCTGGGTTGGGAACAGG-3′.
The conventional PCR was performed in a Primus 96 advanced® Gradient Cycler (Peqlab, Erlangen, Germany), using the HotStarTaq® Master Mix (Qiagen), with an initial heat activation step of 96° for 15 min, followed by 30 cycles of 1 min at 94°, 1 min at 55° and 0·5 min at 72°, according to the protocol of the manufacturer. The size of the expected CD3 amplicon was 234 bp. As negative controls, each PCR was performed with water as template. As a positive control for CD3 we used PBMC from each single donor. A 10-μl sample of the PCR reaction was analysed by means of standard agarose-gel electrophoresis. The amplicon size was verified by applying 6 μl of Gene-Ruler 100 bp Ladder Plus (MBI Fermentas GmbH, St Leon-Rot, Germany).
Quantitative real-time PCR was performed in a LightCycler apparatus (Roche Diagnostics, Mannheim, Germany) using the SYBR Green PCR Mastermix Kit (Qiagen) according to the manufacturer’s protocol along with the above-mentioned primer assays. Each PCR reaction was performed in duplicate. As a negative control, water was used instead of template. Data were collected with the help of lightcycler software 3.5.3 (Roche Diagnostics). The genes analysed in this study were examined for their relative expression by means of the ΔΔCT-method, as described previously by Livak et al.25
Statistics
The Student’s t-test was employed to test the statistical significance of the results. A P-value of ≤ 0·05 was considered significant.
Results
Human DC show a Th2-associated gene expression profile upon contact with protein allergens
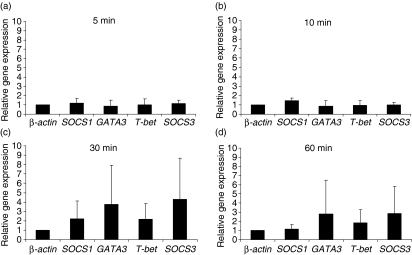
In order to analyse the role of DC in Th2 differentiation, immature human monocyte-derived DC from atopic individuals were pulsed with 10 μg/ml of protein allergen (birch or grass pollen extract) and incubated for 5, 10, 30 or 60 min. Quantitative real-time PCR analysis was performed for the genes SOCS1, SOCS3, GATA3 and T-bet, using β-actin as a housekeeping gene.
After 5 and 10 min of incubation with the protein allergen extracts, no regulation of the genes of interest was observed (Fig. 1a, b). After 30 min of exposure to protein allergen extract, DC showed an up-regulation of the genes SOCS3 and GATA3 (Fig. 1c), which are known to be associated with Th2 differentiation in T cells. T-bet was also up-regulated to a minor degree. This pattern of gene expression was found to persist up to 60 min of incubation (Fig. 1d). To exclude the possibility of contamination with T cells in our DC preparations, conventional reverse transcription PCR for CD3 was performed, which was negative for this T-cell-specific marker, whereas a positive signal was obtained using PBMC from each single donor (data not shown).
Figure 1.
Immature human dendritic cells (DC) were incubated with 10 μg/ml of grass- or birch pollen extract for 5 min (a), 10 min (b), 30 min (c) and 60 min (d). After isolation of RNA and reverse transcription into cDNA, the genes β-actin, SOCS1, SOCS3, GATA3 and T-bet were analysed using a semiquantitative real-time polymerase chain reaction. The results show relative gene expressions, as determined by the ΔΔCT method, at different time-points of incubation. The mean values (± standard deviation) of eight independent experiments are shown.
Gene expression in human DC treated with the contact allergen MCI/MI resembles a Th1 pattern
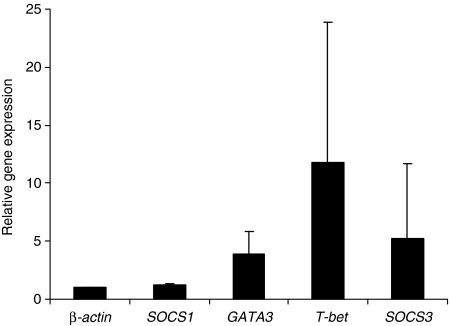
To analyse whether human DC show an alteration in the gene expression profile upon exposure to the contact allergen (hapten) MCI/MI and whether it differs from the expression profile observed upon exposure to protein allergens, human DC were incubated with 1 μg/ml of MCI/MI for 60 min and quantitative real-time PCR was performed. The gene expression profile showed an up-regulation of GATA3 and SOCS3 and a predominant relative gene expression of T-bet, known to be associated with Th1 differentiation in T cells. The expression of the gene SOCS1 remained at baseline level (Fig. 2).
Figure 2.
Immature human dendritic cells (DC) were incubated with 1 μg/ml of 5-chlor-2methyl-2,3-dihydroisothiazol-3-on/-methyl-2,3-dihydroisothiazol-3-on (MCI/MI) for 60 min. After isolation of RNA and reverse transcription into cDNA, the genes β-actin, SOCS1, SOCS3, GATA3 and T-bet were analysed using a semiquantitative real-time polymerase chain reaction. The results show relative gene expressions, as determined by the ΔΔCT method, after 60 min of incubation. The mean values (± standard deviation) of four independent experiments are shown.
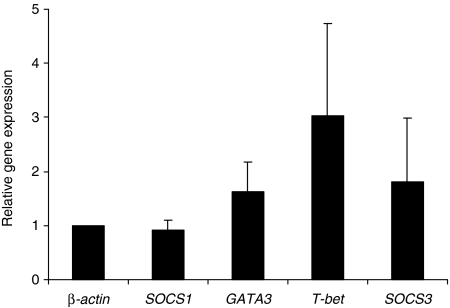
Tetanus toxoid induces a ‘Th1/Th2-neutral’ gene expression profile in human DC
In order to verify the findings of gene expression in human DC pulsed with either contact allergen or protein allergen extracts, we incubated DC for 60 min with 1 μg/ml of tetanus toxoid. Tetanus toxoid is known to induce Th1 as well as Th2 immune responses in T cells and is therefore considered as a ‘Th1/Th2-neutral’ antigen. The relative expression of the analysed genes showed an up-regulation of the Th2-associated genes GATA3 and SOCS3 as well as an up-regulation of the Th1-associated gene T-bet (Fig. 3).
Figure 3.
Immature human dendritic cells (DC) were incubated with 1 μg/ml of tetanus toxoid for 60 min. After isolation of RNA and reverse transcription into cDNA, the genes β-actin, SOCS1, SOCS3, GATA3 and T-bet were analyzed using a semiquantitative real-time polymerase chain reaction. The results show relative gene expressions, as determined by the ΔΔCT method, after 60 min of incubation. The mean values (± standard deviation) of three independent experiments are shown.
Discussion
In an earlier study we demonstrated that immature human DC showed a rapid activation of STAT6 upon contact with protein allergens.23 In the present study we analysed whether the regulation of this process also involves the downstream molecules of the SOCS family and extended our observations to the expression of the Th1- or Th2-associated transcription factors T-bet and GATA3 and the effects of contact allergens/haptens. Our results demonstrated that human DC from grass or birch pollen allergic donors show an alteration of their gene expression profile that is dependent on the type of antigen they come into contact with. After exposure to protein allergens, DC show a gene expression profile that is associated with Th2 differentiation so far only described for T cells (up-regulation of SOCS3 and GATA3), whereas upon contact with the contact allergen MCI/MI, human DC show an enhanced expression of the gene T-bet, which is essential for Th1 immune responses. After exposure to tetanus toxoid, human DC show a gene expression profile that is associated with a Th1 response as well as a Th2 response: the genes GATA3, T-bet and SOCS3 are up-regulated.
In this study, the relative expression of the genes SOCS3 and GATA3 peaked at 30 min of incubation with protein allergens and showed a decrease at the 60-min time-point. These changes in gene expression may be associated with a differentiation of DC towards Th2-inducing antigen-presenting cells, as DC pulsed with such protein allergens have been described to induce Th2 responses.26,27 The fact that the up-regulation of SOCS3 may be involved also in DC is further supported by Li et al., who demonstrated that the expression of SOCS3 in murine DC induces a DC2 phenotype that is able to induce Th2 differentiation in vitro and in vivo.28 Additionally, increased SOCS3 protein levels in DC, and an increased number of SOCS3-positive cells in skin from patients with atopic dermatitis, were shown in a recently performed cDNA microarray study.29 We were not able to detect SOCS1, SOCS3, GATA3, or T-bet protein by Western blot, flow cytometry or immunofluorescence staining after stimulation of immature DC with allergen, contact allergen or tetanus toxoid at different time-points (1–24 hr) in our in vitro study. This suggests that the protein levels of these transcription factors are rather low after exposure to these stimuli.
It is well known that DC are able to induce Th1 as well as Th2 differentiation. Signals that are involved in Th1 induction by antigen-presenting cells have been investigated in greater details (e.g. the cytokines IL-12, IL-18 and IL-23).30,31 IL-12 and IL-18 are required for antimicrobial responses to intracellular pathogens and differentiation of naïve T cells into IFN-γ-producing Th1 cells, whereas the new IL-12 family member, IL-23, drives a novel T-cell subset (distinct from Th1) characterized by the production of IL-17, which plays a central role in mediating chronic inflammatory responses.32,33 However, signals involved in the induction of Th2 responses are understood in much less detail. IL-13 secreted by T cells and DC may play a role in this process.23,34,35 Further studies have divided DC into two subtypes (DC1 and DC2), based on their Th-polarizing capabilities. DC1 are Th1-polarizing DC that produce high amounts of IL-12; DC2 are DC that produce less IL-12 and induce Th2 differentiation, with prostaglandin E2 (PGE2) being an important factor for the differentiation of DC2.30,36,37 As described previously, when employing the same culture system as used in this investigation, the application of PGE2– although essential for the induction of Th2 responses – did not prevent DC from inducing Th1 responses, which were critically dependent on the type of allergen involved.38
The gene expression profile of human DC upon contact with the contact allergen MCI/MI shows a pattern that resembles that of Th1 cells, with up-regulation of the gene T-bet. Our findings are in line with studies of Lugo-Villarino et al. and Wang et al., who have shown that DC are able to produce T-bet in amounts similar to those in Th1 cells and that T-bet is required for the optimal production of IFN-γ in T cells and the optimal activation of antigen-specific Th1 cells.39,40 More recently, Li et al. have also demonstrated that T-bet and IFN-γ mRNA and protein can be induced in human immature and mature DC by bryostatin-1, a protein kinase C modulator with antitumour activity.41
Taken together, the results of the present study support the hypothesis that – besides other modulating factors – the type of allergen (protein allergen versus hapten) is decisive for the polarization of the specific Th immune response initiated and that this polarization already takes place at the level of antigen-presenting cells, employing molecular factors similar to those in T-cell polarization.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft grant SA 483/6-2.
References
- 1.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 2.Wohlfahrt JG, Kunzmann S, Menz G, Kneist W, Akdis CA, Blaser K, Schmidt-Weber CB. T cell phenotype in allergic asthma and atopic dermatitis. Int Arch Allergy Immunol. 2003;131:272–82. doi: 10.1159/000072139. [DOI] [PubMed] [Google Scholar]
- 3.Yssel H, Groux H. Characterization of T cell subpopulations involved in the pathogenesis of asthma and allergic diseases. Int Arch Allergy Immunol. 2000;121:10–8. doi: 10.1159/000024292. [DOI] [PubMed] [Google Scholar]
- 4.Ricci M, Rossi O, Bertoni M, Matucci A. The importance of Th2-like cells in the pathogenesis of airway allergic inflammation. Clin Exp Allergy. 1993;23:360–9. doi: 10.1111/j.1365-2222.1993.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 5.Girolomoni G, Gisondi P, Ottaviani C, Cavani A. Immunoregulation of allergic contact dermatitis. J Dermatol. 2004;31:264–70. doi: 10.1111/j.1346-8138.2004.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 6.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117(Pt 8):1281–3. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 7.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Clin Invest. 2002;109:1279–83. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frucht DM, Aringer M, Galon J, et al. Stat4 is expressed in activated peripheral blood monocytes, dendritic cells, and macrophages at sites of Th1-mediated inflammation. J Immunol. 2000;164:4659–64. doi: 10.4049/jimmunol.164.9.4659. [DOI] [PubMed] [Google Scholar]
- 9.Christodoulopoulos P, Cameron L, Nakamura Y, et al. Th2 cytokine-associated transcription factors in atopic and nonatopic asthma: evidence for differential signal transducer and activator of transcription 6 expression. J Allergy Clin Immunol. 2001;107:586–91. doi: 10.1067/mai.2001.114883. [DOI] [PubMed] [Google Scholar]
- 10.Kurata H, Lee HJ, O’Garra A, Arai N. Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity. 1999;11:677–88. doi: 10.1016/s1074-7613(00)80142-9. [DOI] [PubMed] [Google Scholar]
- 11.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4 + T cells. Nat Immunol. 2002;3:549–57. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 12.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–96. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 13.Kiwamoto T, Ishii Y, Morishima Y, et al. Transcription factors T-bet and GATA-3 regulate development of airway remodeling. Am J Respir Crit Care Med. 2006;174:142–51. doi: 10.1164/rccm.200601-079OC. [DOI] [PubMed] [Google Scholar]
- 14.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–3. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 15.Elliott J, Johnston JA. SOCS: role in inflammation, allergy and homeostasis. Trends Immunol. 2004;25:434–40. doi: 10.1016/j.it.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Agnello D, Lankford CS, Bream J, Morinobu A, Gadina M, O’Shea JJ, Frucht DM. Cytokines and transcription factors that regulate T helper cell differentiation: new players and new insights. J Clin Immunol. 2003;23:147–61. doi: 10.1023/a:1023381027062. [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto M, Tsutsui H, Yumikura-Futatsugi S, Ueda H, Xingshou O, Abe T, et al. A regulatory role for suppressor of cytokine signaling-1 in T(h) polarization in vivo. Int Immunol. 2002;14:1343–50. doi: 10.1093/intimm/dxf094. [DOI] [PubMed] [Google Scholar]
- 18.Knisz J, Rothman PB. Suppressor of cytokine signaling in allergic inflammation. J Allergy Clin Immunol. 2007;119:739–45. doi: 10.1016/j.jaci.2006.12.620. [DOI] [PubMed] [Google Scholar]
- 19.Yu CR, Mahdi RM, Ebong S, Vistica BP, Chen J, Guo Y, Gery I, Egwuagu CE. Cell proliferation and STAT6 pathways are negatively regulated in T cells by STAT1 and suppressors of cytokine signaling. J Immunol. 2004;173:737–46. doi: 10.4049/jimmunol.173.2.737. [DOI] [PubMed] [Google Scholar]
- 20.Egwuagu CE, Yu CR, Zhang M, Mahdi RM, Kim SJ, Gery I. Suppressors of cytokine signaling proteins are differentially expressed in Th1 and Th2 cells: implications for Th cell lineage commitment and maintenance. J Immunol. 2002;168:3181–7. doi: 10.4049/jimmunol.168.7.3181. [DOI] [PubMed] [Google Scholar]
- 21.Seki Y, Inoue H, Nagata N, et al. SOCS-3 regulates onset and maintenance of T(H)2-mediated allergic responses. Nat Med. 2003;9:1047–54. doi: 10.1038/nm896. [DOI] [PubMed] [Google Scholar]
- 22.Kubo M, Inoue H. Suppressor of cytokine signaling 3 (SOCS3) in Th2 cells evokes Th2 cytokines, IgE, and eosinophilia. Curr Allergy Asthma Rep. 2006;6:32–9. doi: 10.1007/s11882-006-0007-6. [DOI] [PubMed] [Google Scholar]
- 23.Bellinghausen I, Brand P, Bottcher I, Klostermann B, Knop J, Saloga J. Production of interleukin-13 by human dendritic cells after stimulation with protein allergens is a key factor for induction of T helper 2 cytokines and is associated with activation of signal transducer and activator of transcription-6. Immunology. 2003;108:167–76. doi: 10.1046/j.1365-2567.2003.01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valk E, Zahn S, Knop J, Becker D. JAK/STAT pathways are not involved in the direct activation of antigen-presenting cells by contact sensitizers. Arch Dermatol Res. 2002;294:163–7. doi: 10.1007/s00403-002-0309-z. [DOI] [PubMed] [Google Scholar]
- 25.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 26.Kleinjan A, Willart M, Van Rijt LS, Braunstahl GJ, Leman K, Jung S, Hoogsteden HL, Lambrecht BN. An essential role for dendritic cells in human and experimental allergic rhinitis. J Allergy Clin Immunol. 2006;118:1117–25. doi: 10.1016/j.jaci.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Traidl-Hoffmann C, Mariani V, Hochrein H, Karg K, Wagner H, Ring J, Mueller MJ, Jakob T, Behrendt H. Pollen-associated phytoprostanes inhibit dendritic cell interleukin-12 production and augment T helper type 2 cell polarization. J Exp Med. 2005;201:627–36. doi: 10.1084/jem.20041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol. 2006;177:1679–88. doi: 10.4049/jimmunol.177.3.1679. [DOI] [PubMed] [Google Scholar]
- 29.Ekelund E, Saaf A, Tengvall-Linder M, et al. Elevated expression and genetic association links the SOCS3 gene to atopic dermatitis. Am J Hum Genet. 2006;78:1060–5. doi: 10.1086/504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation [see comments] Science. 1999;283:1183–6. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 31.Kapsenberg ML, Hilkens CM, Wierenga EA, Kalinski P. The paradigm of type 1 and type 2 antigen-presenting cells. Implications for atopic allergy. Clin Exp Allergy. 1999;29(Suppl 2):33–6. [PubMed] [Google Scholar]
- 32.Langrish CL, McKenzie BS, Wilson NJ, de Waal MR, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 33.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Saint Vis B, Fugier Vivier I, Massacrier C, et al. The cytokine profile expressed by human dendritic cells is dependent on cell subtype and mode of activation. J Immunol. 1998;160:1666–76. [PubMed] [Google Scholar]
- 35.Webb DC, Cai Y, Matthaei KI, Foster PS. Comparative roles of IL-4, IL-13, and IL-4Ralpha in dendritic cell maturation and CD4 + Th2 cell function. J Immunol. 2007;178:219–27. doi: 10.4049/jimmunol.178.1.219. [DOI] [PubMed] [Google Scholar]
- 36.Kalinski P, Moser M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–60. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 37.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–41. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 39.Lugo-Villarino G, Maldonado-Lopez R, Possemato R, Penaranda C, Glimcher LH. T-bet is required for optimal production of IFN-gamma and antigen-specific T cell activation by dendritic cells. Proc Natl Acad Sci USA. 2003;100:7749–54. doi: 10.1073/pnas.1332767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Fathman JW, Lugo-Villarino G, Scimone L, von Andrian U, Dorfman DM, Glimcher LH. Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells. J Clin Invest. 2006;116:414–21. doi: 10.1172/JCI26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Wojciechowski W, Dell’Agnola C, Lopez NE, Espinoza-Delgado I. IFN-gamma and T-bet expression in human dendritic cells from normal donors and cancer patients is controlled through mechanisms involving ERK-1/2-dependent and IL-12-independent pathways. J Immunol. 2006;177:3554–63. doi: 10.4049/jimmunol.177.6.3554. [DOI] [PubMed] [Google Scholar]