Abstract
Epigenetic events play an important role in tumour progression and also contribute to escape of the tumour from immune surveillance. In this study, we investigated the up-regulation of major histocompatibility complex (MHC) class I surface expression on tumour cells by epigenetic mechanisms using a murine tumour cell line expressing human E6 and E7 human papilloma virus 16 (HPV16) oncogenes and deficient in MHC class I expression, as a result of impaired antigen-presenting machinery (APM). Treatment of the cells with the histone deacetylase inhibitor Trichostatin A, either alone or in combination with the DNA demethylating agent 5-azacytidine, induced surface re-expression of MHC class I molecules. Consequently, the treated cells became susceptible to lysis by specific cytotoxic T lymphocytes. Further analysis revealed that epigenetic induction of MHC class I surface expression was associated with the up-regulation of APM genes [transporter associated with antigen processing 1 (TAP-1), TAP-2, low-molecular-mass protein 2 (LMP-2) and LMP-7]. The results demonstrate that expression of the genes involved in APM are modulated by epigenetic mechanisms and suggest that agents modifying DNA methylation and/or histone acetylation have the potential to change the effectiveness of antitumour immune responses and therapeutically may have an impact on immunological output.
Keywords: antigen processing, cancer, human papilloma virus, major histocompatibility complex class I, epigenetics
Introduction
Tumour cells are able to escape specific immune responses mediated by cytotoxic T cells (CTLs) by down-modulation of the surface expression of major histocompatibility complex (MHC) class I molecules.1 Reversible MHC class I down-modulation is frequently mediated by inhibition of the transcription of genes involved in the antigen-processing machinery (APM),2,3 namely genes encoding proteasome subunits, low-molecular-mass proteins LMP-2 and LMP-7, and transporters associated with antigen processing TAP-1 and TAP-2. Transcription of these genes can be induced by cytokines [interferon (IFN)-γ and tumour necrosis factor (TNF)-α], leading to restoration of surface expression of MHC class I molecules, which makes tumour cells susceptible to lysis by CTLs.4,5 In general, agents that increase the expression of MHC class I molecules can be of therapeutic significance.6
Epigenetic modifications of the human genome, namely altered histone acetylation or aberrant DNA methylation, represent tumorigenic events that are functionally equivalent to genetic changes.7 Re-expression of genes down-modulated by DNA methylation can be achieved by exposure to inhibitors of DNA methyltransferase, 5-aza-2′-deoxycytidine (DAC) or 5-azacytidine (5-azaC). The effect of these inhibitors can be increased by inhibitors of histone deacetylases, for example Trichostatin A (TSA)8 or sodium butyrate,9 which can also be used for treatment without DNA demethylation agents. The antitumour therapeutic potential of these compounds has been tested in clinical studies, and the targets of choice in the experimental setting are usually epigenetically silenced tumour suppressor genes.10 The expression of immunoactive (e.g. MHC class I or II), costimulatory and adhesive molecules on tumour cells, cytokine production and the expression of tumour-rejecting antigens can also be directly or indirectly modulated by epigenetic mechanisms.11–15 Hence, the reversal of the immune escape phenotype of tumour cells represents one of the possible modalities by which ‘epigenetic agents’ can inhibit growth of the tumour.16
Down-modulation of MHC class I expression by hypermethylation of MHC class I genes has been documented in melanoma cells, and the combination of 5-azaC and TSA promoted the re-expression of HLA class I antigens and subsequent restoration of the antigen-specific immune response after demethylation.17 Histone deacetylase inhibitors have been shown to up-regulate MHC class I expression on several tumour cell lines, including melanoma B16/BL6,18 human neuroblastoma SK-N-MC and mouse adenocarcinoma Colon 26-L5.11 Notably, little is known about the role of the epigenetic mechanisms involved in APM regulation and the associated regulation of MHC class I cell surface expression. An interesting question is whether up-regulation of MHC class I cell surface expression can be mediated by epigenetic induction of the expression of APM genes and how the particular APM components (TAP-1, TAP-2, LMP-2 and LMP-7) are regulated by histone deacetylase inhibitors and DNA demethylation agents.
Cervical neoplasms are mostly attributed to infection with human papilloma virus (HPV), mainly its ‘high risk’ oncogenic types.19 Early viral antigens of the high-risk HPV types, E6 and E7, are necessary for the maintenance of the malignant phenotype and are expressed in all tumour cells. A number of experimental E6/E7 targeting immunotherapeutic protocols designed to elicit a specific CTL-mediated response are currently under development.20 However, their efficiency against E6/E7-expressing tumours with down-modulated MHC class I expression is questionable. Therefore, the problem of the mechanisms of MHC class I up-regulation on HPV-associated tumours is of particular interest. As promotor hypermethylation of multiple genes in carcinoma of the uterine cervix has been described,21,22 the possible modulation of MHC class I gene expression by demethylation is of relevance and has therapeutic potential in cervical carcinoma.23 Considering that the deficiency of surface MHC class I on tumours associated with HPV is frequently caused by APM defects,24 we asked the question of whether epigenetic modifications can modulate surface MHC class I expression indirectly through the silencing or activation of APM genes. To address this problem, we employed an animal model for human HPV16-associated tumours. Using a model of tumour cells expressing E6/E7 antigens with MHC class I down-modulation caused by defects in APM, we examined the impact of histone deacetylase inhibitors and demethylation agents on the surface expression of MHC class I molecules and on the activation of APM genes.
Materials and methods
Cell lines
TC-1 is a malignant, immunogenic cell line of C57BL/6 mouse origin expressing HPV16 E6/E7 antigens.25 The TC-1/A9 cell line is a TC-1 MHC class I-deficient derivative.26 All cells were grown in RPMI 1640 medium supplemented with 10% fetal calf serum, l-glutamine and antibiotics.
In vitro experiments
Cells were cultured in fresh medium for 24 hr, after which the medium was removed and the cells were grown in one of several RPMI/drug groups: 50 µm 5-azaC, 1 µm DAC, 5 µm DAC, 10 ng/ml TSA or 35 µm sodium butyrate (all chemicals were purchased from Sigma, St Louis, MO). The combination of 50 µm 5-azaC and 10 ng/ml TSA was also analysed. The medium was replaced with fresh medium after 48 hr and the cells were cultured for an additional 24 hr in drug-free medium, giving a total of 72 hr, and harvested.
Flow cytometry
Cell surface MHC class I expression of the cell lines was determined by two-step cytoflourometric analysis using anti-mouse H-2Kb/H-2Db monoclonal antibody (clone 28-8-6) and fluorescein isothiocyanate (FITC)-labelled goat anti-mouse immunoglobulin G (IgG) secondary antibody or by one-step labelling using phycoerythrin (PE) anti-H-2Db (KH95) and PE anti-H-2Kb (AF-88·5) antibodies. The following antibodies were used for detection of CD86 and MHC class II molecules: PE anti-CD86 (GL1), FITC anti-I-Ab (AF6-120·1). The cells were initially preincubated with anti-CD16/CD32 to prevent non-specific binding. Analysis of 10 000 cells was carried out with a FACSCAN ELITE cytometer (Coulter, Miami, FL). All antibodies used, including the mouse IgG2a isotope-matching control, were obtained from Pharmingen (San Diego, CA). The proportions of living, dead and apoptotic cells were determined with propidium iodide and the Annexin V-FITC apoptosis detection kit (Sigma, St Louis, MO).
Reverse transcription–polymerase chain reaction (RT-PCR) and real-time quantitative RT-PCR
Total RNA was extracted using the High Pure RNA isolation kit (Roche, Basel, Switzerland). RNA (200 ng) was reverse-transcribed to cDNA using random hexamer primers from the GeneAmp RNA PCR Core Kit (Applied Biosystems, Foster City, CA) in a 20-µl reaction volume at 42° for 30 min. PCR analysis was performed using Amplitaq polymerase (Applied Biosystems). cDNA was amplified under the following conditions: 95° for 2 min, followed by 25 cycles of denaturation at 95° for 30 seconds, annealing at 60° for 1 min, elongation at 72° for 1 min and incubation at 72° for 5 min. Quantification of PCR products was performed in 25 µl of SYBR Green Super Mix (Bio-Rad, Hercules, CA) using an iCycler thermocycler (Bio-Rad). DNA was denatured at 95° for 2 min, followed by 35 cycles of denaturation at 95° for 30 seconds, annealing at 60° for 1 min, elongation at 72° for 1 min and incubation at 72° for 5 min. cDNAs were amplified with specific primers for β-actin, H-2Db, H-2Kb, TAP-1, LMP-2,26,27 TAP-228 and LMP-7.29 A list of the genes and their primer sequences is given in Table 1. Fold changes in transcript levels were calculated using threshold cycle (Ct) values standardized to β-actin, which was used as the endogenous control (reference gene), in order to normalize the quantification of mRNA. All samples were run in triplicate. For statistical analysis the Student t-test was used. Differences between experimental and control samples at P < 0·05 were considered to be statistically significant.
Table 1.
Primers used for polymerase chain reaction (PCR)/quantitative PCR
| Gene | Forward primer (5′−3′) | Reverse primer (5′−3′) |
|---|---|---|
| TAP-1 | GAC AAG AGC CGC TGC TAT TTG G | TGA TAA GAA GAA CCG TCC GAG A |
| TAP-2 | GCC TGT GCT GTT CTC GGG TTC TGC | TGT ACC AGG TGG GCG TAG |
| LMP-2 | CTC TGC ACC AGC ACA TCT T | AGA GTG ATG GCA TCT GTG GT |
| LMP-7 | ATG GCG TTA CTG GAT CTG TGC GGT GC | TCA CAG AGC GGC CTC TCC GTA CTT GTA |
| β-actin | CCA GAG CAA GAG AGG TAT CC | GAG TCC ATC ACA ATG CCT GT |
LMP, low-molecular-mass protein; TAP, transporter associated with antigen processing.
Animals
Two-month-old C57BL/6 mice, purchased from Anlab (Prague, Czech Republic), were used for these experiments. They were maintained in the animal care facility at the Institute of Molecular Genetics according to approved protocols of the Institutional Animal Care Committee at the Institute of Molecular Genetics, Prague.
51Cr microcytotoxicity assay
Mice (three per goup) were immunized with irradiated (150 Gy) TC-1 or TC-1/A9 and either untreated or treated with 10 ng/ml TSA. In all immunization protocols, the dosage was 107 cells per mouse, and the mice were immunized twice at a 3-week interval as previously described.30 Eight days after the second immunization, spleen cells from immunized and control mice were extracted and used as effector cells. After lysis of erythrocytes with Tris-NH4Cl buffer, the effector cells were mixed with the 51Cr-labelled tumour target cells and incubated at four different target-to-effector cell ratios (1 : 25, 1 : 50, 1 : 100 and 1 : 200) for 18 hr in triplicate in 96-well round-bottom microtitre plates (Nunc, Roskilde, Denmark). The medium was also enriched with 10−5 m mercaptoethanol. To assess the role of CTLs, CD8-positive cells were depleted with specific monoclonal antibody 2·4331 and complement (Baby Rabbit Complement; Cederlane, Hornby, Ontario, Canada) prior to the mixing of effector cells with targets. The percentage of specific 51Cr release was expressed according to the formula[(c.p.m. experimental release – c.p.m. control release)/(c.p.m. maximum release – c.p.m. control release)] × 100, where c.p.m. is counts per minute.30,32 For statistical analyses, Student's t-test was used.
Chromatin immunoprecipitation assay
The assay was performed using a chromatin immunoprecipitation assay kit (ChIP; Upstate Biotechnologies Inc., Billerica, MA) as per the manufacturer's instructions, with some modifications. Briefly, proteins were cross-linked to DNA by adding 1% formaldehyde directly to the medium for 10 min at 37°. Cross-linking was stopped by the addition of 0·125 m glycine at 37° for 15 min. The medium was removed and the cells were washed with phosphate-buffered saline (PBS) containing protease inhibitors [1 mm phenylmethylsulphonyl fluoride (PMSF), 1 µg/ml aprotinin and 1 µg/ml pepstatin]. The cells were then resuspended in sodium dodecyl sulphate (SDS) and sonicated. The DNA/protein mixture was precleared with salmon sperm DNA/protein A agarose/50% slurry and then incubated with the antibody for acetylated histone H3 (Upstate Biotechnologies Inc.). After several washes, DNA bound to the immunocomplexes was obtained and then incubated with proteinase K. DNA was recovered via phenol/chloroform extraction. For promoter analysis, we designed the following PCR primers which span the TAP-1, LMP-2 bidirectional promoter: TAPsh-F GGC AAA TCT GCC CAG AGA and TAPbd R CCT AGC CTG GGA CTC TCG AC.
Bisulphite modification and methylation-specific PCR (MSP)
DNA from TC-1/A9 cells was treated with sodium bisulphite using a previously established protocol.33 In order to identify CpG islands within the promoter region of the antigen-processing genes, MSP analysis was performed with primers designed with the program MethPrimer,34 which spanned the 570-bp region of the bidirectional promoter TAP-1/LMP-2,35 1000 bp of the promoter region of TAP-236 and the 1622-bp upstream region of LMP-7(Table 2).
Table 2.
Methylation-specific polymerase chain reaction (MSP) primers
| Gene | Forward primer (5′−3′) | Reverse primer (5′−3′) |
|---|---|---|
| TAP-1 (unmeth) | GTA AGT TAG TTT TAG AAG GAG GTG T | ATC CTA ACC TAA AAC TCT CAA CAT C |
| TAP-1 (meth) | GTA AGT TAG TTT TAG AAG GAG GCG T | ATC CTA ACC TAA AAC TCT CGA CGT |
| TAP-2 (unmeth) | TTT TTT AAA ATA TGT TTG GAG GTT G | CCA ACA AAT AAC ACC TAT CAA TTT ACA |
| TAP-2 (meth) | GTT TTT TAA AAT ATG TTC GGA GGTC | AAC AAA TAA CGC CTA TCA ATT TAC G |
| LMP-7 (unmeth) | TTT TGA TTT GTT TTT TAT TAG ATG G | CCT TTC TCT CTA TAC ACT TTA AAC ATT |
| LMP-7 (meth) | TTT TTC GAT TCG TTT TTT ATT AGA | CCT TTC TCT CTA TAC ACT TTA AAC GTT |
LMP, low-molecular-mass protein; meth, methylated; TAP, transporter associated with antigen processing; unmeth, unmethylated.
Western blot analysis
Whole-cell protein extracts were prepared from cell lines TC-1 and TC-1/A9 using lysis buffer containing 20 mm HEPES (pH 7·9), 150 mm NaCl, 1 mm ethylenediaminetetraacetic acid (EDTA), 1% Nonidet P-40, 10% glycerol, 1 mm dithiotreitol, 1 mm phenylmethylsulphonyl fluoride and 0·2 mm protease inhibitor cocktail (Sigma). Proteins (30 µg) were separated on a 7·5% SDS-PAGE gel and transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was blocked in 0·1%/tris buffered saline tween (TBST)/5% skimmed milk for 1 hr at room temperature and incubated overnight at 4° with goat polyclonal antibody against TAP-1 (Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1 : 1000). After incubation with anti-goat-horseradish peroxidase (HRP) (Santa Cruz Biotechnology), followed by extensive washing, the bound antibodies were visualized using enhanced chemiluminescence (West Femto; Pierce, Rockford, IL). Levels of actin were analysed using goat polyclonal antibody (Santa Cruz Biotechnology; dilution 1 : 1000) and were used as a control for equal loading.
Results
Epigenetic agents up-regulate MHC class I expression on the surface of MHC class I-deficient TC-1/A9 cells
The effect of 5-azaC and TSA on MHC class I expression in MHC class I-deficient TC-1/A9 was studied. Treatment of this cell line with 5-azaC and TSA induced expression of MHC class I molecules on the cells (Fig. 1). The effect was weaker than that observed in the IFN-γ-treated control, in which expression reached the level found in the parental TC-1 cells. In the next series of experiments, we studied the effects of TSA and 5-azaC separately. The results revealed that even treatment with TSA alone resulted in MHC class I up-regulation. The data were supported by results of experiments with sodium butyrate and DAC. Importantly, both agents were able to up-regulate MHC class I expression. Control experiments were performed on the parental MHC class I-positive cell line TC-1, in which no changes in MHC class I surface expression were observed. All cell lines remained MHC class II and CD86 negative (data not shown).
Figure 1.
Epigenetic agents induce major histocompatibility complex (MHC) class I cell-surface expression on TC-1/A9 cells. MHC class I expression on TC-1/A9 and TC-1 after 5-azacytidine (5-azaC) and Trichostatin A (TSA) treatments was determined by flow cytometry. The efficacy of the 5-azaC and TSA treatment was compared to the effects of interferon (IFN)-γ. In the control experiments the effects of sodium butyrate and 5-aza-2′-deoxycytidine (DAC) on TC-1/A9 cells were demonstrated. All experiments were performed at least three times with similar results.
Proapoptotic effects of TSA and 5-azaC treatments
In order to assess the proapoptotic and cytotoxic effects of the epigenetic agents, a percentage of living, apoptotic and dead cells after treatment was analysed using annexin and propidium iodide labelling (Fig. 2). The percentages of dead and living TSA-treated cells were similar to those of the untreated controls, demonstrating that TSA at the concentrations used in the experiments had no effect on cell viability. After 5-azaC treatment, either alone or in combination, an increase in the portion of apoptotic cells was observed.
Figure 2.
Determination of the proapoptotic effects of the 5-azacytidine (5-azaC) and Trichostatin A (TSA) treatments. The proportions of viable, apoptotic and dead cells were determined by dual labelling with annexin V and propidium iodide; viable cells were double-negative, apoptotic cells were labelled only with annexin V and dead cells were double-positive. The percentages of the apoptotic cells (lower right quadrant) and dead cells (upper right quadrant) are depicted. The experiment was performed twice with similar results.
TSA-treated TC-1/A9 cells are effectively lysed with spleen cells from immunized animals
To determine whether MHC class I up-regulation by TSA was sufficient for the recognition of tumour cells by CTLs, we used TSA-treated TC-1/A9 cells as targets in a chromium-release microcytotoxic test with the spleen cells from animals immunized with TC-1 cells(Fig. 3). 5-azaC- and TSA-treated TC-1/A9 cells were effectively lysed in this experiment. In vitro depletion of CD8+ cells in the spleen cell mixture revealed that lysis was mediated by cytotoxic T cells. However, spleen cells from animals immunized with TSA-treated TC-1/A9 cells lysed MHC class I-positive TC-1 cells more efficiently than spleen cells from mice immunized with untreated TC-1/A9 cells.
Figure 3.
Trichostatin A (TSA)-treated TC-1/A9 cells are susceptible to cytotoxic T lymphocyte (CTL)-mediated lysis and TSA treatment augments the CTL-mediated immune response. (a) Spleen cells from mice immunized with TC-1 cells or control mice (pooled from three mice) were used as effector cells. Significant differences were observed between the following groups: target cells TC-1: P < 0·01 (200 : 1), P < 0·05 (100 : 1 and 50 : 1); anti-TC-1 immune effectors × control effectors, and anti-TC-1 immune effectors × anti-TC-1 immune effectors, CD8+ cells depleted; target cells TC-1/A9-TSA: P < 0·05 (200 : 1, 100 : 1 and 50 : 1); anti-TC-1 immune effectors × control effectors, and anti-TC-1 immune effectors × anti-TC-1 immune effectors, CD8+ cells depleted. (b) Spleen cells from mice immunized with TC-1/A9 or TSA-treated TC-1/A9 cells (pooled from three mice) or control mice were used, and TC-1 cells, TSA-treated TC-1/A9 cells and untreated TC-1/A9 cells were used as targets. Significant differences were observed between the following groups: P < 0·05 (200 : 1 and 100 : 1); anti-TC-1/A9 TSA immune effectors × control effectors. All experiments were performed twice with similar results.
Expression of APM genes is up-regulated by TSA and 5-azaC
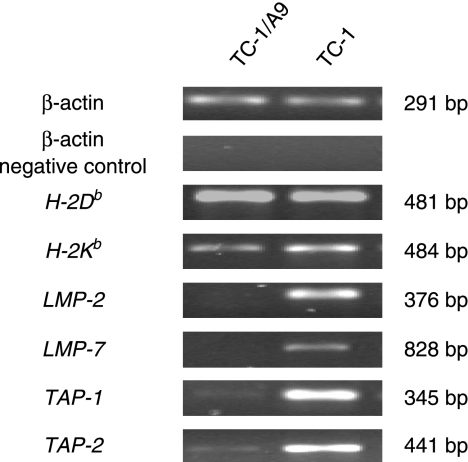
The expression of MHC I molecules does not occur on the surface of TC-1/A9 tumour cells. We showed, via RT-PCR, that the two loci that produce MHC class I antigens, H-2Db and H-2Kb, were fully functional in TC-1 and TC-1/A9 cell lines (Fig. 4). However, there was a decrease in mRNA expression of TAP-1, TAP-2, LMP-2 and LMP-7 in TC-1/A9 cells compared with TC-1 cells.
Figure 4.
Reverse transcription–polymerase chain reaction (RT-PCR) analysis of the expression of major histocompatibility (MHC) class I H-2Db and H-2Kb genes and antigen-processing machinery (APM) components transporter associated with antigen processing 1 (TAP-1), TAP-2, low-molecular-mass protein 2 (LMP-2) and LMP-7. Twenty-five PCR cycles were performed to distinguish the different patterns of gene expression between particular cell lines.
The effects of epigenetic agents on selected APM genes were studied in detail by quantitative real-time RT-PCR (Fig. 5a). After administering the combination of 5-azaC and TSA to the TC-1/A9 cell line, we observed an increase in the expression of APM genes, namely TAP-2 and LMP-7. Furthermore, we analysed the expression of each gene after the administration of 5-azaC and TSA alone. Statistically significant up-regulation of TAP-1, TAP-2, LMP-2 and LMP-7 was observed after the addition of TSA, and statistically significant up-regulation of TAP-1, TAP-2 and LMP-7 after the 5-azaC treatment. In control experiments, epigenetic agents also up-regulated the expression of APM genes in MHC class I-positive TC-1 cells; TSA treatment resulted in a significant increase in expression of TAP-1, LMP-2 and LMP-7. TAP-2 and LMP-7 expression was significantly up-regulated by 5-azaC. Notably, the effects of TSA and 5-azaC on particular genes differed and were not synergistic. The effects of the particular treatments on TAP-1 and LMP-2 were very similar.
Figure 5.
Quantitative polymerase chain reaction (PCR) and western blot analysis of expression levels of antigen-processing machinery (APM) components in TC-1/A9 and TC-1 cells. (a)Quantitative PCR: transporter associated with antigen processing 1 (TAP-1), TAP-2, low-molecular-mass protein 2 (LMP-2) and LMP-7 mRNA expression after 5-azacytidine (5-azaC)/Trichostatin A (TSA), 5-azaC and TSA treatments and in untreated cells was related to expression of β-actin. Cells were given 5-azaC in combination with TSA, 5-azaC alone, or TSA alone. In both major histocompatibility complex (MHC) class I+ and MHC class I– cell lines, TAP-1 and LMP-2 were up-regulated after the administration of TSA. TAP-2 and LMP-7 were up-regulated after treatment with 5-azaC and TSA and 5-azaC alone. The samples whose relative expression exhibited a P-value less than 0·05, compared with controls, were determined to be significant and are denoted by an asterisk. (b) Western blot: TAP-1 and TAP-2 expression at the protein level after treatment with epigenetic agents was determined in TC-1/A9 cells. β-actin expression served as a control.
Western blot analysis determined that the protein levels of TAP-1 and TAP-2 in cells treated with 5-AzaC, TSA or a combination of the two drugs were increased when compared with the untreated TC-1/A9 control (Fig. 5b). Detectable protein expression was detected in untreated cells; however, the intensity of the band was greater in cells treated with 5-azaC alone and in combination with TSA for both proteins. TAP-1 protein expression was increased in cells exposed to TSA; however, the TAP-2 expression level appeared to be unchanged.
Antigen-processing genes are demethylated after 5-azaC and TSA treatment
Bisulphite-treated DNA was analysed with primers designed to distinguish between modified and unmodified DNA. MSP analysis of the bidirectional promoter of TAP-1/LMP-2 demonstrated that the promoter was partially methylated in TC-1/A9. Upon treatment with 5-azaC the promoter was demethylated (Fig. 6a). Interestingly, TSA alone appeared to induce demethylation of TAP-1. The promoter region of TAP-2, as well as the upstream sequence of LMP-7, was also found to be partially methylated before treatment. However, after 5-azaC treatment, TAP-2 was demethylated while there appeared to be no change in the methylation status of LMP-7. There were no detectable changes after treatment with the combination of drugs.
Figure 6.
Epigenetic agents induce DNA demethylation and histone deacetylation.(a) DNA from the TC-1/A9 cell line treated with epigenetic agents was treated with bisulphite. Methylation-specific polymerase chain reaction (MSP) analysis revealed partial methylation of transporter associated with antigen processing 1 (TAP-1), low-molecular-mass protein 2 (LMP-2) and LMP-7 in control cells not treated with epigenetic agents. Administration of 5-azacytidine (5-azaC)/Trichostatin A (TSA) led to demethylation. 5-azaC alone resulted in the demethylation of TAP-1 and LMP-2. TSA had an effect on the TAP-1 promoter but no effect on TAP-2. LMP-7 remained methylated. U, unmethylated primer; M, methylated primer. Histone H3 is associated with the TAP-1/LMP-2 bidirectional promoter. (b) ChiP analysis of chromatin isolated from TC-1A9 treated cells demonstrated an increase in acetylated histone H3 after 5-azaC and TSA treatment. Experiments were repeated at least twice with similar results.
Histone H3 is re-acetylated at the bidirectional promoter after 5-azaC/TSA treatment
A ChIP assay was performed to determine whether the combined dose of 5-azaC/TSA, which is sufficient to reverse the methylation of the TAP-1/LMP-2 promoter, was able to modify the histones associated with this promoter (Fig. 6b). The assay demonstrated that histone H3 was re-acetylated after treatment with both epigenetic agents alone as well as in combination. Acetylated histone H3 was detected at this region in untreated TC-1/A9 cells at a low level.
Discussion
The aim of this study was to elucidate whether epigenetic modifications influence MHC class I cell-surface expression in tumour cells that contain reversible MHC class I expression deficiencies caused by defects in APM components. Histone deacetylase inhibitors and DNA demethylation agents partially restored the expression of APM components and induced the expression of MHC class I molecules on the surface of the tumour cells. The level of expression did not reach that of the parental MHC class I-positive cells, but was sufficient for lysis by effector spleen cells from mice immunized with parental TC-1 tumour cells.
A plausible mechanism of the MHC class I up-regulation is activation of the APM components. Induction of the expression of these genes was observed in the TC-1/A9 cell line, although not all investigated APM genes were up-regulated in the same manner. We also cannot exclude the possibility of a role for other factors in MHC class I up-regulation, as a number of other genes are affected by epigenetic agents. MHC class I up-regulation was also induced by TSA, suggesting a role in the modification of histone H3 associated with TAP-1 and LMP-2, resulting in the transformation from heterochromatin to euchromatin. However, 5-azaC appears to have a greater influence with regard to histone re-acetylation, probably as a result of its action on the methylated promoter inhibiting DNA methyltransferases and promoting an environment in which histone acetyltransferases can act on chromatin. In tandem, changes in DNA demethylation and histone acetylation lead to the re-expression of the epigenetically silent gene. TAP-1 and LMP-2 genes were influenced by the two epigenetic agents similarly. This is not surprising as these genes are regulated by the same promoter.34
Interestingly, the APM-related genes analysed were also significantly epigenetically up-regulated in MHC class I-positive TC-1 cells, with no effects on the levels of MHC class I cell-surface expression. The results demonstrated that, in this case, the APM components were epigenetically up-regulated in the cells with functional antigen processing and presentation, but their up-regulation was not the limiting factor for a further increase in MHC class I expression.
We also investigated the effect of 5-azaC/TSA on other immunoactive molecules. Interestingly, in neither of our cell lines was MHC class II cell surface expression up-regulated, contrary to what has been found in some other cell lines.12,15,37 MHC class II and also MHC class I induction can be associated with up-regulation of MHC class II transactivator (CIITA) coactivators,38,39 which has been reported to be modulated by histone acetylation.15
We must also take into consideration the possibility that the MHC class I induction could be related to the proapoptotic effects of the agents or a result of their effects on the cell cycle. This possibility cannot be excluded, as TAP-1 and TAP-2 expression has been found to be cell-cycle dependent.40 However, unlike 5-azaC, TSA was used in our experiments in concentrations that did not induce apoptosis of the treated cells compared with untreated controls. These mechanisms, as well as the problem of possible involvement of the IFN-γ pathway in epigenetic induction of APM components, should be studied in more detail.
It is now generally accepted that epigenetic non-mutational changes represent important mechanisms in the multistep process of tumour development. There is mounting evidence that epigenetic modifications are important factors driving the tumour cell to escape from immune surveillance. However, understanding of the epigenetic regulation of APM modulation, which is a frequent mechanism of reversible inhibition of MHC class I cell surface molecules in cervical carcinoma, is limited.41 We have demonstrated that MHC class I re-expression in APM-defective tumour cells can be induced by treatment with TSA either alone or in combination with 5-azaC. This up-regulation was associated with the activation of down-modulated genes of the APM. The mechanisms of this activation remain to be elucidated in future studies. It remains to be determined whether these genes are regulated directly, independently or, as in the case of IFN-γ treatment, by upstream regulatory elements. It is also noteworthy that MHC class I reconstitution on tumour cells has not been as effective as, for instance, with IFN-γ treatment. These results indicate that epigenetic mechanisms can substantially contribute to MHC class I expression but are not the primary mechanism.
The increased sensitivity of the treated tumour cells to a specific immune response is an important mechanism of antitumour effects in addition to tumour-suppressor gene activation and proapoptotic effects, and the usage of epigenetic agents can improve the efficacy of immunotherapeutic protocols.
Acknowledgments
We are grateful to Dr T. C. Wu, who kindly provided the TC-1 cells, to Dr M. Šmahel for making available the TC-1/A9 cells. This work was supported by the Grant Agency of the Czech Republic (grant nos 301/04/0492 and 301/07/1410), by the League against Cancer, Prague, by the Academy of Sciences of the Czech Republic (grant no. AVOZ50520514) by EU-FP6-NoE Clinigene Project no. 018933, and by the Ministry of Education of the Czech Republic (project no. MSM0021620806).
Abbreviations
- 5-azaC
5-azacytidine
- APM
antigen-processing machinery
- ChIP
chromatin immunoprecipitation
- c.p.m.
counts per minute
- Ct
threshold cycle
- CTL
cytotoxic T lymphocyte
- DAC
5-aza-2′-deoxycytidine
- HPV
human papilloma virus
- IFN-γ
interferon gamma
- MHC
major histocompatibilty complex
- MSP
methylation-specific polymerase chain reaction
- PBS
phosphate-buffered saline
- PMSF
phenylmethylsulphonyl fluoride
- RT-PCR
reverse transcription–polymerase chain reaction
- TSA
Trichostatin A
References
- 1.Garrido F, Ruiz-Cabello F, Cabrera T, Perez-Villar JJ, Lopez-Botet M, Duggan-Keen M, Stern PL. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol Today. 1997;18:89–95. doi: 10.1016/s0167-5699(96)10075-x. [DOI] [PubMed] [Google Scholar]
- 2.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–64. doi: 10.1016/s0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 3.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–86. doi: 10.1016/s1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 4.Lu Y, Yamauchi N, Koshita Y, et al. Administration of subtumor regression dosage of TNF-alpha to mice with pre-existing parental tumors augments the vaccination effect of TNF gene-modified tumor through the induction of MHC class I molecule. Gene Ther. 2001;8:499–507. doi: 10.1038/sj.gt.3301429. [DOI] [PubMed] [Google Scholar]
- 5.Mikyskova R, Bubenik J, Vonka V, Smahel M, Indrova M, Bieblova J, Simova J, Jandlova T. Immune escape phenotype of HPV16-associated tumours: MHC class I expression changes during progression and therapy. Int J Oncol. 2005;26:521–7. [PubMed] [Google Scholar]
- 6.Bubenik J. MHC class I down-regulation: tumor escape from immune surveillance? Int J Oncol. 2004;25:487–91. [PubMed] [Google Scholar]
- 7.Rountree MR, Bachman KE, Herman JG, Baylin SB. DNA methylation, chromatin inheritance, and cancer. Oncogene. 2001;20:3156–65. doi: 10.1038/sj.onc.1204339. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–9. [PubMed] [Google Scholar]
- 9.Chiurazzi P, Pomponi MG, Pietrobono R, Bakker CE, Neri G, Oostra BA. Synergistic effect of histone hypermethylation and DNA methylation in the reactivation of the FMR1 gene. Human Mol Genet. 1999;8:2317–23. doi: 10.1093/hmg/8.12.2317. [DOI] [PubMed] [Google Scholar]
- 10.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 11.Reitz MS, Jr, Mann DL, Eiden M, Trainor CD, Clarke MF. DNA methylation and expression of HLA-DR alpha. Mol Cell Biol. 1984;4:890–7. doi: 10.1128/mcb.4.5.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magner WJ, Kazim AL, Stewart C, et al. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–24. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 13.Maeda T, Towatari M, Kosugi H, Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96:3847–56. [PubMed] [Google Scholar]
- 14.Nie Y, Liao J, Zhao X, Song Y, Yang GY, Wang LD, Yang CS. Detection of multiple gene hypermethylation in the development of esophageal squamous cell carcinoma. Carcinogenesis. 2002;23:1713–20. doi: 10.1093/carcin/23.10.1713. [DOI] [PubMed] [Google Scholar]
- 15.Chou SD, Khan AN, Magner WJ, Tomasi TB. Histone acetylation regulates the cell type specific CIITA promoters, MHC class II expression and antigen presentation in tumor cells. Int Immunol. 2005;17:1483–94. doi: 10.1093/intimm/dxh326. [DOI] [PubMed] [Google Scholar]
- 16.Tomasi TB, Magner WJ, Khan AN. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–84. doi: 10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serrano A, Tanzarella S, Lionello I, Mendez R, Tranversari C, Ruiz-Cabello F, Garrido F. Re-expression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer. 2001;94:243–51. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 18.Komastu Y, Hayashi H. Histone deacetylase inhibitors up-regulate the expression of cell surface MHC class-I molecules in B16/BL6 cells. J Antibiot. 1998;51:89–91. doi: 10.7164/antibiotics.51.89. [DOI] [PubMed] [Google Scholar]
- 19.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 20.Stanley MA. Human papillomavirus vaccines. Rev Med Virol. 2006;16:139–49. doi: 10.1002/rmv.498. [DOI] [PubMed] [Google Scholar]
- 21.Dong SM, Kim HS, Rha SH, Sidransky D. Promoter hypermethylation of multiple genes in carcinoma of the uterine cervix. Clin Cancer Res. 2001;7:1982–6. [PubMed] [Google Scholar]
- 22.Sova P, Feng Q, Geiss G, Wood T, Strauss R, Rudolf V, Lieber A, Kiviat N. Discovery of novel methylation biomarkers in cervical carcinoma by global demethylation and microarray analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:114–23. doi: 10.1158/1055-9965.EPI-05-0323. [DOI] [PubMed] [Google Scholar]
- 23.Bubenik J. Prospects for immunotherapy of MHC class I-deficient tumors. Folia Biol (Praha) 2003;49:95–9. doi: 10.14712/fb2003049030095. [DOI] [PubMed] [Google Scholar]
- 24.Keating PJ, Cromme FV, Duggan-Keen M, Snijders PJ, Walboomers JM, Hunter RD, Dyer PA, Stern PL. Frequency of down-regulation of individual HLA-A and -B alleles in cervical carcinomas in relation to TAP-1 expression. Br J Cancer. 1995;72:405–11. doi: 10.1038/bjc.1995.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 26.Smahel M, Sima P, Ludvikova V, Marinov I, Pokorna D, Vonka V. Immunisation with modified HPV 16, E7 genes against mouse oncogenic TC-1 cell sublines with downregulated expression of MHC class I molecules. Vaccine. 2003;21:1125–36. doi: 10.1016/s0264-410x(02)00519-4. [DOI] [PubMed] [Google Scholar]
- 27.Smahel M, Sobotkova E, Bubenik J, et al. Metastic MHC class I-negative mouse cells derived by transformation with human papillomavirus type 16. Br J Cancer. 2001;84:374–80. doi: 10.1054/bjoc.2000.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuchtey J, Chefalo PJ, Gray RC, Ramachandra L, Harding CV. Enhancement of dendritic cell antigen cross-presentation by CpG DNA involves type I IFN and stabilization of class I MHC mRNA. J Immunol. 2005;175:2244–51. doi: 10.4049/jimmunol.175.4.2244. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Lora A, Martinez M, Algarra I, Gaforio JJ, Garrido F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int J Cancer. 2003;106:521–7. doi: 10.1002/ijc.11241. [DOI] [PubMed] [Google Scholar]
- 30.Bubenik J, Zeuthen J, Indrova M, Bubenikova D, Simova J. Kinetics and function of peritoneal exudate cells during local IL-2 gene therapy of cancer. Int J Oncol. 1994;4:13–6. doi: 10.3892/ijo.4.1.13. [DOI] [PubMed] [Google Scholar]
- 31.Sarmiento M, Glasebrook AL, Fitch FW. IgG or IgM monoclonal antibodies reactive with different determinants on the molecular complex bearing Lyt 2 antigen block T cell-mediated cytolysis in the absence of complement. J Immunol. 1980;125:2665–72. [PubMed] [Google Scholar]
- 32.Indrova M, Mikyskova R, Jandlova T, Vonka V, Bubenik J, Bieblova J. Adjuvant cytokine treatment of minimal residual disease after surgical therapy in mice carrying HPV16-associated tumours: cytolytic activity of spleen cells from tumour regressors. Folia Biol (Praha) 2003;49:217–22. doi: 10.14712/fb2003049060217. [DOI] [PubMed] [Google Scholar]
- 33.Herman JG, Graff JR, Myohanen S, Nelkin B, Baylin SB. Methylation-specific PCR. A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–6. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18:1427–31. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 35.Wright KL, White LC, Kelly A, Beck S, Trowsdale J, Ting JP. Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J Exp Med. 1995;181:1459–71. doi: 10.1084/jem.181.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arons E, Kunin V, Schechter C, Ehrlich R. Organization and functional analysis of the mouse transporter associated with antigen processing 2 promoter. J Immunol. 2001;166:3942–51. doi: 10.4049/jimmunol.166.6.3942. [DOI] [PubMed] [Google Scholar]
- 37.Wright KL, Ting JP. Epigenetic regulation of MHC-II and CIITA genes. Trends Immunol. 2006;27:405–12. doi: 10.1016/j.it.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Martin BK, Chin KC, Olsen JC, Skinner CA, Dey A, Ozato K, Ting JP. Induction of MHC class I expression by the MHC class II transactivator CIITA. Immunity. 1997;6:591–600. doi: 10.1016/s1074-7613(00)80347-7. [DOI] [PubMed] [Google Scholar]
- 39.van den Elsen PJ, Gobin SJ, van Eggermond MC, Peijnenburg A. Regulation of MHC class I and II gene transcription: differences and similarities. Immunogenetics. 1998;48:208–21. doi: 10.1007/s002510050425. [DOI] [PubMed] [Google Scholar]
- 40.Alpan RS, Zhang M, Pardee AB. Cell cycle-dependent expression of TAP1, TAP2, and HLA-B27 messenger RNAs in a human breast cancer cell line. Cancer Res. 1996;56:4358–61. [PubMed] [Google Scholar]
- 41.Ritz U, Momburg F, Pilch H, Huber C, Maeurer MJ, Seliger B. Deficient expression of components of the MHC class I antigen processing machinery in human cervical carcinoma. Int J Oncol. 2001;19:1211–20. doi: 10.3892/ijo.19.6.1211. [DOI] [PubMed] [Google Scholar]