Abstract
An enormous body of work supports a role for CD4+ CD25+ regulatory cells (Tregs) in shaping the immune response to tumours. Indeed, there is evidence that the cells impede effective tumour immunosurveillance, inhibit vaccine-induced antitumour immune responses and promote tumour progression. Studies exploring the impact of Tregs on tumour development are discussed in the context of manipulating this T-cell population for the purpose of cancer immunotherapy.
Keywords: cancer, regulation, immune surveillance
Mouse models
Tumour-induced suppressor T cells
A series of studies performed by Robert North and co-workers in the 1980s provided evidence for suppressor T cell involvement in antitumour immune responses. Using a methylcholanthrene-induced fibrosarcoma cell line (called Meth A), the investigators showed that although the tumour cells could induce a T-cell response capable of tumour rejection, these T cells were unable to completely inhibit tumour growth due to the gradual development of tumour-induced suppressor T-cell activity.1 Effective anti-tumour responses, detected by their ability to mediate concomitant immunity, i.e. rejection of a second inoculum of the same tumour cells by a tumour-bearing host, disappeared with time correlating with the acquisition of suppressor T-cell activity. Subsequent studies by the same group indicated that the suppressor T cell population was preferentially destroyed by cyclophosphamide thus treatment of mice with the drug resulted in tumour rejection through ablating the effect of suppressor T cells.2 Characterization of the suppressor T-cell subpopulation revealed them to be CD4+ CD8− and subsequent experiments performed using CD4-specific depleting antibodies indicated that removal of the CD4+ T-cell population resulted in tumour regression.3
CD4+ FoxP3+ regulatory T cells (Tregs) and tumour immunity
The importance of the findings of North and colleagues has been highlighted by the recent resurgence of interest in regulatory T cells, and in particular the naturally occurring regulatory CD4+ T cells (Tregs) characterized by expression of CD25, GITR (glucocorticoid-induced tumour necrosis factor receptor family-related gene), cytotoxic T lymphocyte antigen-4 (CTLA-4), and the most reliable marker, the transcription factor, forkhead box P3 (Foxp3) (reviewed in 4). Originally, Onizuka et al. reported that depletion of CD25+ cells (the majority of these cells in naïve mice are CD4+ Foxp3+) using monoclonal antibodies resulted in T-cell dependent control of a variety of tumour cell lines in mice, including the Meth A tumour described above.5 Subsequently, Shimizu et al. showed that tumour-specific CD8+ T-cell responses as well as natural killer (NK)-like responses were generated in mice inoculated with tumour cells after depletion of CD25+ cells.6 A number of groups confirmed these findings and showed that long-term CD8+ and/or CD4+ T-cell mediated immunity developed in mice that rejected tumour cells after depletion of CD25+ cells.7,8 T cells stimulated in the absence of CD25+ T cells have been shown to contribute to tumour rejection through direct lysis and/or through production of interferon-γ (IFN-γ).9,10 Suppression of concomitant tumour immunity in mice has also been revisited in the context of CD4+ CD25+ Tregs.11 Following inoculation with melanoma cells (B16) engineered to express granulocyte–macrophage colony-stimulating factor (GM-CSF), mice developed concomitant immunity against unmodified B16 cells after depletion of CD4+ cells – treatment with cyclophosphamide was also shown to promote concomitant immunity through inactivation of suppressor T cells.
Tregs and immune surveillance
Burnet hypothesized in the 1950s that the immune system could control and eliminate spontaneous developing tumours, a process later termed immune surveillance.12 Although this theory fell from popularity after the 1970s, a large body of recent work from different groups has demonstrated an increase in both spontaneous and carcinogen-induced tumours in immunocompromised mice (e.g. IFN-γ, IFN-γ-R, perforin, and Rag-2 gene knockouts), lending strong support to the immune surveillance concept.13
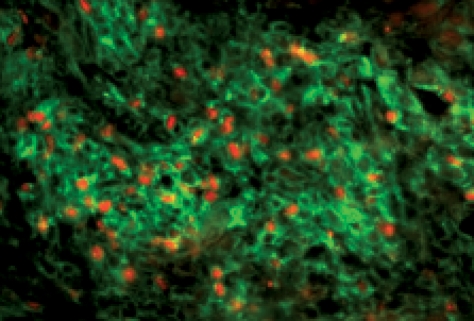
Almost all of the studies investigating the effect of CD25+ Tregs on tumour rejection have been carried out using tumour cell lines and have not examined whether these cells also impinge upon the development of tumours in vivo and consequently impede immune surveillance. A study by ourselves and others14,15 indicate that tumours induced by the chemical carcinogen methylcholanthrene (MCA), develop more slowly and less frequently in mice depleted of CD25+ cells; remarkably this effect is observed even if the Treg depletion is only for a short transient period when the mice are first injected with MCA. Strikingly MCA-induced tumours are infiltrated with an abundance of FoxP3+ CD4+ T cells (Fig. 1); this enrichment in Tregs in the tumour infiltrating lymphocytes is not reflected by a relative increase in Tregs in other compartments such as spleen, blood or lymph node.15 Whether control of tumour growth is an immune mediated process in these mice requires further investigation but these findings suggest that Tregs are playing a central role in tumour immune surveillance.
Figure 1.
CD4+ Foxp3+ cells in methylcholanthrene-induced tumours in mice. Frozen sections of MCA-induced tumours were stained with CD4− (green) and Foxp3− (red) specific antibodies.
Human Tregs
The observation that Tregs exhibiting phenotypic and functional characteristics similar to those described in mice are also found in humans led to the assumption that this T-cell population also play a role in controlling antitumour immune responses in humans. However, where animal models allow experimental studies to be performed, human studies are more restricted and largely observational in nature, making definitive conclusions more difficult to obtain. We shall consider the following two questions: (i) what is the evidence that immune responses impede the growth of human adult epithelial adenocarcinomas that are not known to be associated with chronic infections? and (ii) is there a role for Tregs in controlling antitumour immune responses?
Does the immune response control the growth of tumours in humans?
Probably yes, though this is a difficult question to answer absolutely for the following reasons: firstly, if an effective antitumour immune response developed that could control the growth of a tumour, or even destroy it, then this event is unlikely to be recorded as the individual would not present clinically to physicians. Conversely, if a patient is diagnosed with a cancer, by definition, mechanisms for controlling the dysregulated cell growth of the tumour, including possible antitumour immune responses, have failed. Second, we do not currently have the knowledge, tools or means to measure accurately the immune response to a tumour that has been eliminated. Immunity to previous infections may be recorded by specific serological and cellular responses, but not enough is known about the antigens and antitumour responses to enable similar measurements to be made for most tumours. So after the event, without a calling card to read, there is no way of knowing how many subjects have fought and cleared tumours through effective specific immune responses.
Is there any indirect evidence for immune surveillance? Despite the extensive use of mild to moderate immunosuppressive agents in humans for a wide variety of diseases, the main overall increase in tumours is seen in those associated with chronic viral infections like Epstein–Barr virus and lymphoma; human papillomavirus and cervical cancer; and human herpes virus 8 and Kaposi’s sarcoma in patients who are markedly immunosuppressed after organ transplantation.16 Some epidemiological studies suggest there is an increase in relative risk compared to the general population for tumours of non-viral origin as well (including breast, lung, pancreas, colon, renal tract and skin tumours) in recipients of solid organ transplants, although conflicting results exist between centres.16
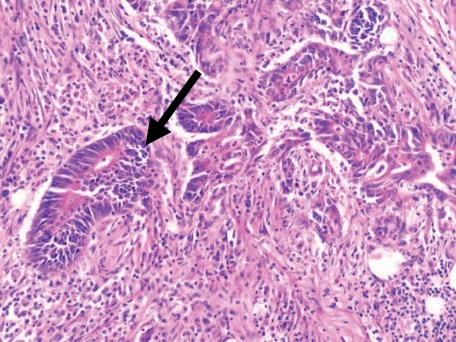
It has been recognized for many years that for certain tumours, there is a correlation between the degree of infiltration of the tumour with lymphocytes and the likelihood of ‘cure’ after surgery. In addition to the epidemiological data above, studies dating from the 1970s indicate a correlation between better patient prognosis and an increase in tumour infiltrating lymphocytes (TILs).17,18Figure 2 shows a histological section of a resected colorectal cancer with an infiltrate of lymphocytes seemingly attacking and destroying malignant cells, perhaps offering the most visual of arguments that anti-tumour immune responses do develop. Presumably an immune response that attacks these malignant cells and inhibits their growth, or kills the cells, is of benefit to the individual. The survival advantage of lymphocytic infiltration in colorectal cancer (CRC) was commented on by McCarty in the 1930s19 and since this time there have been several studies suggesting a positive effect of colorectal TILs on survival.17,18,20,21 Ropponen et al. demonstrate quite clearly that an increase in TILs correlates with earlier stage disease and hence better prognosis. In this study of 276 patients, an association was found between the clinical Dukes’ staging of the cancer and TILs. Clinically, colorectal cancer staging is one of the most accurate in predicting patient survival (e.g. Dukes’ A tumour confined to the bowel wall has a 85–90% 5 year survival, Dukes’ B tumour penetrating the wall of bowel 55–85%; Dukes’ C tumour involving lymph nodes 20–55%; Dukes’ D tumour with distant spread <20%). The frequency of TILs decreases with more aggressive disease staging. A more recent study by Naito et al. examined 131 cases of colorectal cancer and suggested an increase in CD8+ cells infiltrating the tumour correlates with better survival.21 The resected colorectal cancers were histologically analysed, the numbers of CD8+ T cells semi-quantitated in three areas: the cancer cell nests; the stroma of the cancer, and the interface with normal tissue. An increase in CD8+ cells infiltrating the cancer cell nests was the area that positively correlated with patient survival. However, this finding was also shown to correlate with earlier stages of colorectal cancer with a favourable Dukes’ staging. It is apparent that early tumours tend to be infiltrated with lymphocytes, and hence a lymphocytic infiltrate is associated with better survival. Both these studies pose the difficult question as to which came first: does an earlier tumour allow a lymphocyte reaction to develop which naturally diminishes with time and tumour progression, or does the lymphocyte reaction control the tumour and prevent or delay its progress? A degree of caution must therefore be applied to the interpretation of these types of observational studies.
Figure 2.
Histological section prepared from a resected colonic carcinoma. The neoplastic glands can be seen advancing into the wall of the colon. As indicated by the arrow, tumour tissue appears to be invaded and destroyed by a lymphocytic infiltrate.
However, a recent study by Galon et al. has added further insight into the role of the immune system in colorectal cancer (CRC) prognosis.22 This study, involving the use of several hundred samples, demonstrated a correlation between increased T helper 1 (Th1) gene expression in tumours (e.g. IFN-γ, CD3ζ, CD8, granzyme B, etc.) and decreased tumour recurrence after surgery. Intriguingly, when they analysed semi-automatedly the presence of CD3+, CD8+ and CD45RO+ T cells in the histological samples by immunofluoresence, they observed an independent correlation between the presence of these cells and the prognosis of the patients after surgery. These interesting observations need repeating in a prospective study, but they strongly confirm what McCarty commented on earlier, that patients with tumours more infiltrated with immunocytes had a better prognosis.19 These data suggest that the degree of T-cell infiltration (CD8+ CD45RO+ cells) may be as important as clinicopathological staging for prognosis, and support an essential role for the immune system in controlling tumour recurrence.
Is there a role for Tregs in controlling antitumour immune responses?
There are many immunology studies demonstrating specific cellular and serological immune responses to antigens expressed by tumours. So why does effective antitumour immunity not develop, and do Tregs play a part? It seems likely that tumour antigens, regardless of whether they are self-antigens or tumour-specific antigens, are not particularly effective in generating immune responses. This is probably due to: (i) mechanisms which preserve tolerance to peripherally expressed antigens in normal tissue including Tregs; (ii) the poor immunogenicity of antigens expressed in solid tumours; (iii) the immunosuppressive environment created by the tumour; and (iv) the fact that most solid epithelial tumours are very slow growing, accruing the necessary mutations to become malignant over many years; in this context even ‘neoantigens’ may appear like ‘self’ antigens and invoke mechanisms of peripheral tolerance, including Tregs.
It seems likely that Tregs do play a role in tumour development in humans. Before the recent expansion of interest and publications in naturally occurring Tregs, there was already published evidence that antitumour T cells are subjected to regulatory influences. It was observed in the 1980s that although TILs derived from a variety of human solid tumours including breast, colon, lung, skin and oesophageal carcinomas could be cultured, expanded, and shown to exhibit anti-tumour effector functions in vitro, the cells were far more difficult to grow compared to lymphocytes derived from peripheral blood mononuclear cells (PBMCs).23 Studies at a similar time identified CD4+ lymphocytes, which appeared to down-regulate antitumour CD8+ T cell responses in patients with melanoma.24 With these and the murine studies in mind, it seems likely that certain populations of cells reside in tumours and nodes which control or prevent effective antitumour responses. This may explain the curious observation that TILs appear to exist which recognize tumour antigens in vitro but are unable to clear the tumour in vivo.
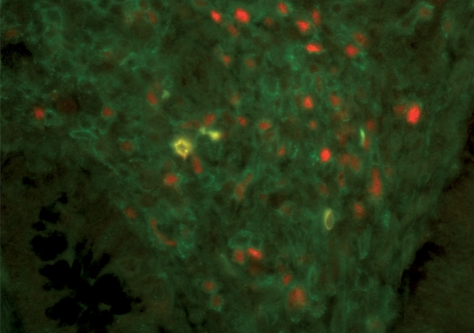
Are CD4+ Foxp3+ Tregs involved in regulating immune responses to tumours? A detailed account of Treg frequencies in blood and sometimes TIL and draining lymph nodes, in a variety of human tumours has been extensively reviewed elsewhere.25 The overall trend that has been observed is an increase in Treg frequencies in patients affected with a wide variety of tumours compared to healthy controls. The implication of this observation, in the context of previous work performed using animal models, is that these cells are suppressing antitumour immune responses. Furthermore, when we compared the frequency of Tregs in the blood of CRC patients with a control group with colonic inflammation, this increased frequency was still present suggesting it was not simply caused by chronic inflammation within the colon (26 and unpublished data). In addition, staining of sections of colorectal tumours demonstrates that CD4+ Foxp3+ cells are readily detectable (Fig. 3).
Figure 3.
CD4+ Foxp3+ cells in a paraffin-embedded section of a human colon carcinoma. Tumour sections were stained with CD4− (green) and Foxp3− (red) specific antibodies.
Studies using lymphocytes from human patients also suggest that TILs bearing the phenotypic characteristics of Treg cells can suppress the activity of conventional effector cells. Wang et al. generated a panel of CD4+ T-cell clones isolated from a melanoma. One of the clones, which expressed CD25, GITR and Foxp3, recognized the cancer-testis antigen LAGE-1 and was shown to inhibit the proliferation of conventional CD4+ T cells following stimulation with CD3-specific antibodies.27 Later, a study by Curiel et al. demonstrated that Tregs, isolated from malignant ascites obtained from patients suffering from ovarian cancer, were able to suppress the cytotoxic activity of tumour-specific T cells.28 The same study demonstrated that Tregs appear to co-localize with CD8+ T cells within solid ovarian tumours. The mechanisms through which the cells exert their suppressive effects remains elusive although studies performed in vitro using tumour-isolated Treg cells indicate that suppression is cell-contact dependent. However, it is possible that the suppressive effects of the Tregs are more far-reaching in vivo perhaps involving many different mechanisms.
As mentioned above, several studies have attempted to correlate the presence of TILs with patient outcome. The types of lymphocytes present within the TILs will of course influence the conclusions drawn from such studies. This has been strikingly illuminated by two papers on ovarian carcinoma.28,29 Zhang et al. published a report in 2002 measuring CD3+ TILs in 186 ovarian tumour biopsies taken from patients with advanced stage III/IV disease. The CD3+ cells had a steady ratio of CD4+ and CD8+ T cells and patients with TIL containing CD3+ cells had an overall 5-year survival of 38%, absence of CD3+ cells from the TILs diminished survival to 4·5%. This seemed a clear message: in advanced ovarian carcinoma, TILs containing CD3+ T cells are good for you.29 Move forward to 2004 to the study described by Curiel et al.28 which looked at subtypes of T cells invading tumours including Tregs (CD4+ CD25+ Foxp3+). In a total of 104 tumours, biopsies from patients with stage I disease (n = 7) contained less Tregs (about 12% of CD4+ T cells infiltrating the tumour were CD25+) compared to more advanced disease in stages II–IV (n = 97; 25–30% of CD4+ T cells were CD25+). When absolute numbers of Treg cells per high power field were measured, an increase in Treg numbers corresponded to poorer survival. So putting these two studies together, perhaps one may say that CD3+ TILs improve patient outcome with ovarian cancer, but not if a significant proportion of them are CD4+ CD25 CD4+ Foxp3+, and by inference, regulatory in function.
Although the parameters governing the trafficking of Treg versus effector T cells into tumours are not well defined, the study described above suggested that Tregs migrate into ovarian tumours in response to production of the chemokine CCL22 by tumour-infiltrating macrophages.28 Earlier, we considered whether immune responses do develop during the early stages of tumour development, which naturally diminish as the tumour progresses, or does the immune response control the tumour and prevent or delay its progress? A similar line of reasoning may apply to the presence of Tregs in these ovarian tumours: does late stage disease hijack natural regulatory processes or does the presence of Tregs at an early stage allow the tumour to progress?
Tregs and vaccination
To date studies aimed at generating effective antitumour immune responses in patients have been disappointing.30 Perhaps a strategy in which Tregs are depleted or inactivated as part of a vaccination regimen will prove useful, not only for increasing the immunogenicity of the vaccine but also by ensuring that Tregs which favour tumour growth are not promoted. Denileukin diftitox (Ontak) is a fusion protein comprising interleukin-2 (IL-2) and diphtheria toxin that has recently been tested for its ability to deplete Treg cells in cancer patients. Dannull et al. vaccinated a group of patients with dendritic cells transduced with tumour RNA and reported enhanced T-cell responses to tumour antigens in patients who had received Ontak prior to vaccination compared to those receiving vaccine alone.31 The effectiveness of Ontak for Treg depletion is still uncertain because most, but not all investigators have demonstrated a reduction in Treg numbers in recipients of the drug.32,33 An inadvertent effect of targeting CD25 for the depletion of Tregs is the attendant elimination of conventional CD25+ T helper cells, which may be essential for promoting tumour rejection. Other approaches may involve conventional cytotoxic agents such as cyclophosphamide which, at low doses, can be used to selectively deplete Tregs in humans.34 In a rat model, a combination of cyclophosphamide with tumour cell vaccination led to regression of established tumours.35 This strategy may offer an effective method for boosting antitumour immunity in cancer patients.
Treatment with CTLA-4-specific antibodies has also shown promise for the eradication of tumours in patients with a variety of cancers.36,37 Blockade of CTLA-4 using non-depleting antibodies may promote rejection of tumours through inhibiting Tregs and directly boosting conventional effector T cells.7 Similarly, antibodies, which stimulate signaling through GITR, may inhibit Treg activity whilst directly promoting effector T-cell activity.38,39 It may, however, prove difficult, using these types of approaches, to elicit tumour immunity without invoking autoimmunity although this may be considered an acceptable consequence compared to solid organ adenocarcinomas with a poor prognosis.
Adoptive transfer of tumour-specific T cells represents another strategy for treatment of established tumours. The results of a recent study indicate that adoptive transfer of CD4+ CD25− T cells and melanocyte antigen (gp100)-specific CD8+ T cells followed by vaccination with recombinant viruses expressing gp100 resulted in regression of established melanoma only in irradiated mice and was abrogated by the co-transfer of CD4+ CD25+ T cells.40 Adoptive immunotherapy using in vitro expanded antitumour CD4+ and CD8+ T cells can lead to tumour regression in patients41 and the ability of adoptively transferred T cells to control or destroy tumours is improved by lymphodepleting the host prior to transfer. It is plausible that lymphodepletion allows the removal of Tregs, as well as making room for the rapid expansion of effectors in vivo. Furthermore, it may be important to transfer over certain populations of T cells, e.g. central memory rather than effector memory cells.42 These strategies are at a preliminary stage and await a greater understanding of which T cells are critical for tumour clearance and the generation of protective memory responses.
Conclusion
Collectively, the results of both the murine and human studies described in this review support the idea that finding ways of overcoming the suppressive activity of Tregs, or of selectively depleting these cells, represents an important therapeutic strategy aimed at inducing effective tumour-specific immune responses capable of controlling or eradicating tumours. Currently the method by which this is best achieved still has to be identified.
Direct targeting of the cells based on their phenotypic characteristics may not work and other more indirect approaches may be needed. Fundamental gaps in our knowledge exist which may hold the key to understanding how the immune response can be manipulated and key questions need to be addressed such as why Tregs are present in such large numbers in tumours; what is the role of antigen in this process; and what type of immune cells are crucial for recognizing and destroying tumour cells?
Acknowledgments
We wish to thank Professor Geraint Williams, Dr Emma Jones and Gareth Betts for kindly providing the histological sections shown. We are grateful to Dr Matthew Wise for reading this manuscript. Andrew Godkin is a Consultant Physician at the University Hospital of Wales and a Reader in Hepatology and Gastroenterology in Cardiff University; Awen Gallimore is an MRC funded Senior Research Fellow in Cardiff University.
References
- 1.North RJ, Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2- suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J Exp Med. 1984;159:1295–311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med. 1982;155:1063–74. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awwad M, North RJ. Immunologically mediated regression of a murine lymphoma after treatment with anti-L3T4 antibody. A consequence of removing L3T4+ suppressor T cells from a host generating predominantly Lyt-2+ T cell-mediated immunity. J Exp Med. 1988;168:2193–206. doi: 10.1084/jem.168.6.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6:345–52. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 5.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–33. [PubMed] [Google Scholar]
- 6.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 7.Sutmuller RP, van Duivenvoorde LM, van Elsas A, et al. Synergism of cytotoxic T lymphocyte-associated antigen 4 blockade and depletion of CD25(+) regulatory T cells in antitumor therapy reveals alternative pathways for suppression of autoreactive cytotoxic T lymphocyte responses. J Exp Med. 2001;194:823–32. doi: 10.1084/jem.194.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones E, Dahm-Vicker M, Simon AK, Green A, Powrie F, Cerundolo V, Gallimore A. Depletion of CD25+ regulatory cells results in suppression of melanoma growth and induction of autoreactivity in mice. Cancer Immun. 2002;2:1. [PubMed] [Google Scholar]
- 9.Casares N, Arribillaga L, Sarobe P, et al. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931–9. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 10.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–24. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnet M. Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. Br Med J. 1957;5023:841–7. doi: 10.1136/bmj.1.5023.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–8. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 14.Tawara I, Take Y, Uenaka A, Noguchi Y, Nakayama E. Sequential involvement of two distinct CD4+ regulatory T cells during the course of transplantable tumor growth and protection from 3-methylcholanthrene-induced tumorigenesis by CD25-depletion. Jpn J Cancer Res. 2002;93:911–6. doi: 10.1111/j.1349-7006.2002.tb01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betts G, Twohig J, Van den Broek M, Sierro S, Godkin A, Gallimore A. The impact of regulatory T cells on carcinogen-induced sarcogenesis. Br J Cancer. 2007;96:1849–54. doi: 10.1038/sj.bjc.6603824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penn I. Posttransplant malignancies. Transplant Proc. 1999;31:1260–2. doi: 10.1016/s0041-1345(98)01987-3. [DOI] [PubMed] [Google Scholar]
- 17.House AK, Watt AG. Survival and the immune response in patients with carcinoma of the colorectum. Gut. 1979;20:868–74. doi: 10.1136/gut.20.10.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jass JR. Lymphocytic infiltration and survival in rectal cancer. J Clin Pathol. 1986;39:585–9. doi: 10.1136/jcp.39.6.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty M. Principles of prognosis in cancer. J Am Med Assoc. 1931;96:30–3. [Google Scholar]
- 20.Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Prognostic value of tumour-infiltrating lymphocytes (TILs) in colorectal cancer. J Pathol. 1997;182:318–24. doi: 10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 22.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 23.Miescher S, Whiteside TL, Moretta L, von Fliedner V. Clonal and frequency analyses of tumor-infiltrating T lymphocytes from human solid tumors. J Immunol. 1987;138:4004–11. [PubMed] [Google Scholar]
- 24.Mukherji B, Guha A, Chakraborty NG, Sivanandham M, Nashed AL, Sporn JR, Ergin MT. Clonal analysis of cytotoxic and regulatory T cell responses against human melanoma. J Exp Med. 1989;169:1961–76. doi: 10.1084/jem.169.6.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Betts GJ, Clarke SL, Richards HE, Godkin AJ, Gallimore AM. Regulating the immune response to tumours. Adv Drug Deliv Rev. 2006;58:948–61. doi: 10.1016/j.addr.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Clarke SL, Betts GJ, Plant A, et al. CD4+CD25+FOXP3+ regulatory T cells suppress anti-tumor immune responses in patients with colorectal cancer. PLoS ONE. 2006;1:e129. doi: 10.1371/journal.pone.0000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang HY, Lee DA, Peng G, Guo Z, Li Y, Kiniwa Y, Shevach EM, Wang RF. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20:107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 28.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–3. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–33. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahnke K, Schonfeld K, Fondel S, et al. Depletion of CD4+CD25+ human regulatory T cells in vivo: kinetics of Treg depletion and alterations in immune functions in vivo and in vitro. Int J Cancer. 2007;120:2723–33. doi: 10.1002/ijc.22617. [DOI] [PubMed] [Google Scholar]
- 33.Attia P, Maker AV, Haworth LR, Rogers-Freezer L, Rosenberg SA. Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma. J Immunother (1997) 2005;28:582–92. doi: 10.1097/01.cji.0000175468.19742.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 36.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 37.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2003;100:8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 39.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–14. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 40.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]