Abstract
The immune evasion mechanisms of pathogenic trypanosomatids involve a multitude of phenomena such as the polyclonal activation of lymphocytes, cytokine modulation and the enhanced detoxification of oxygen reactive species. A trypanothione cascade seems to be involved in the detoxification process. It was recently described and characterized a tryparedoxin (LiTXN1) involved in Leishmania infantum cytoplasmatic hydroperoxide metabolism. LiTXN1 is a secreted protein that is up-regulated in the infectious form of the parasite, suggesting that it may play an important role during infection. In the present study, we investigated whether recombinant LiTXN1 (rLiTXN1) affects T- and B-cell functions in a murine model. We observed a significant increase in the CD69 surface marker on the B-cell population in total spleen cells and on isolated B cells from BALB/c mice after in vitro rLiTXN1 stimulus. Activated B-cells underwent further proliferation, as indicated by increased [3H]thymidine incorporation. Cytokine quantification showed a dose-dependent up-regulation of interleukin (IL)-10 secretion. B cells were identified as a source of this secretion. Furthermore, intraperitoneal injection of rLiTXN1 into BALB/c mice triggered the production of elevated levels of rLiTXN1-specific antibodies, predominantly of the immunoglobulin M (IgM), IgG1 and IgG3 isotypes, with a minimum reactivity against other heterologous antigens. Taken together, our data suggest that rLiTXN1 may participate in immunopathological processes by targeting B-cell effector functions, leading to IL-10 secretion and production of specific antibodies.
Keywords: Leishmania infantum, LiTXN1, B cells, interleukin-10, specific antibodies
Introduction
Leishmaniasis, a disease caused by protozoan parasites of the genus Leishmania, remains a serious public health problem in areas of the tropics, the subtropics and southern Europe, with approximately 12 million people being affected in these regions.1 Depending on the interaction between the infecting Leishmania species and the immune status of the host, the clinical manifestations can vary from self-healing cutaneous ulceration to progressive visceral dissemination, which is fatal if untreated.2
Leishmania are intracellular parasites that reside primarily within host tissue macrophages and dendritic cells. Interestingly, these cells participate in the first line of defence against pathogens through their antimicrobial and/or antigen-presenting functions.3 In murine models of experimental leishmaniasis, the development of potent type-1 CD4+ cell-mediated immunity was determined to be the general protective mechanism against all Leishmania species. This will ultimately lead to parasite destruction by interferon (IFN)-γ stimulated macrophages through tumour necrosis factor (TNF)-α and nitric oxide (NO)-dependent mechanisms.3Leishmania has developed several strategies to escape the host immune system in order to successfully establish an infection. For instance, Leishmania parasites are capable of modelling the T-cell response and cytokine production towards a non-protective T helper type 2 (Th2) response, characterized by the secretion of anti-inflammatory cytokines such as interleukin (IL)-4, IL-10, IL-13 and transforming growth factor (TGF)-β.4–6 In addition to the suppression of parasite specific Th1 cell-mediated responses during active disease, a marked increase in the humoral response is induced, which is characterized by the secretion of large amounts of parasite non-specific antibodies with self autoreactivity, particularly of the immunoglobulin M (IgM) and IgG isotypes.7 To date, several Leishmania antigens involved in these pathological pathways have been identified. It was shown that a single T-cell epitope derived from the Leishmania homologue of receptor for activator C kinase (LACK) antigen was responsible for early IL-4 production, which is critical for Th2 differentiation, by the Vβ4 Vα8 CD4+ T-cell population in BALB/c mice, contributing to the development of progressive disease in these mice.8 Moreover, we have previously reported the identification of a Leishmania major protein, homologous to the mammalian ribosomal protein S3a, which participates in the immunoregulatory process by inducing polyclonal expansion of non-specific, non-parasite directed B-cell clones and suppression of Th1-type cytokine production.9 Also, other antigens have the capacity to polarize the immune response towards a Th2 phenotype, thus exacerbating the disease; for example, lipophosphoglycan in L. major10 and papLE22 in Leishmania infantum11 do so by reducing the levels of inflammatory cytokines IL-1 and TNF-α on stimulated macrophages and by increasing the level of the immunosuppressive cytokine IL-10, respectively. Thus, it has been argued that it is of critical importance to characterize parasite antigens that potentiate the disease, as an effective vaccine should be capable of preventing the action of these ‘immunopathological’ antigens.11
As the major Leishmania species complexes diverged some 40–80 million years ago,12 it is not surprising that different correlatives of protection are found for each pathology. For example, in chronic murine visceral leishmaniasis, while TGF-β has been shown to inhibit the Th1-associated resolution of infection,13 IL-4 has been shown not to contribute to the disease outcome.14 Nevertheless, there is considerable evidence supporting a central immunosuppressive role for the endogenous IL-10.15 Antigen-induced production of IL-10 is of major interest because of the antagonistic effects of IL-10 on IFN-γ.16 This Th1 suppressive cytokine is also responsible for compromising antigen specific T-cell stimulation and for impairment of macrophage activation.16 Thus, IL-10 is generally considered to be the major cytokine involved in the progression to visceral disease.17
In a previous study, we identified a cytosolic L. infantum tryparedoxin (LiTXN1) that belongs to a special class of oxidoreductases found in trypanosomatids and that is related to the ubiquitous thioredoxins. LiTXN1 is expressed throughout the parasite life cycle.18 However, it was observed to be up-regulated and excreted/secreted only in the infective stages.19
The purpose of our study was to characterize the effect of this secreted protein on the immune system, focusing on T- and B-cell functions. The results showed that rLiTXN1 is a potent B-cell activator in the presence or absence of T lymphocytes. The activated B cells underwent both proliferation and differentiation, as shown by the production of specific antibodies. However, it also promoted the B-cell production of IL-10, suggesting a potential role in disease progression.
Materials and methods
Mice
Six-week-old male BALB/c mice were obtained from Harlen Iberica (Barcelona, Spain) and 6-week-old male C3H/HeJ mice were purchased from Charles River (L'Arbresle, France).
Purification of rLiTXN1
The L. infantum TXN1 protein was produced from pretransformed Escherichia coli BL21 clones.18 The protein used was obtained as a recombinant protein (rLiTXN1) containing six histidine residues at its N-terminal. rLiTXN1 purity was determined by sodium dodecyl sulphate–polyacrilamide gel electrophoresis (SDS-PAGE) and staining with Coomassie blue. For biological assays, the protein was dialysed against phosphate-buffered saline (PBS) using PD10 desalting columns (Amersham, Arlington, IL). The recombinant protein content was determined using the Folin procedure.20
Injection of mice
Six- to seven-week-old BALB/c mice were injected three times intraperitoneally (i.p.), at 7-day intervals, with 50 µg of rLiTXN1. Control mice were injected with PBS. Two weeks after the final injection, spleens and sera were collected.
Spleen B-cell isolation
After cervical dislocation, spleens were removed and homogenized to obtain a single cell suspension. The cells were washed two times in RPMI-1640 culture medium (BioWittaker, Verviers, Belgium) and adjusted to 107 cells/ml in RPMI-1640 culture medium supplemented with 10% fetal calf serum (FCS), 2 mm glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin and 20 mm HEPES. Spleen B cells were isolated by depletion of non-B cells using the B-cell isolation kit MACS® (Miltenyi Biotec, Auburn, CA) following the manufacturer's instructions. The purity of enriched B cells was evaluated by flow cytometry, using double labelling with specific monoclonal antibodies (mAb; anti-µ+, anti-CD4+ and anti-CD8+). B-cell purity above 97% was obtained after the purification process.
Cell culture and proliferation assays
Single cell suspensions (2 × 105 cells/well) of spleen cells or isolated B cells, from normal or rLiTXN1 injected BALB/c mice or from normal C3H/HeJC mice, were cultured in 96-well flat-bottom plates at a final volume of a 200 µl. Cells were stimulated with concanavalin A (ConA; 6 µg/ml), lipopolysaccharide (LPS; 10 µg/ml) or rLiTXN1 (5, 10 or 50 µg/ml) and incubated at 37° in 5% CO2. On days 1, 2 and 3 of culture, the cells were pulsed with 1 µCi of [3H]thymidine (Amersham) for the last 8 hr. Pulsed splenocytes were harvested on a glass filter using an automated multiple sample harvester and dried. Incorporation of radioactive thymidine was then determined by liquid scintillation as specified in a standard protocol. Assays were performed in triplicate, and the stimulatory index was calculated by dividing the arithmetic mean counts per minute (c.p.m.) obtained from stimulated cultures by the arithmetic mean c.p.m. obtained from control cultures without stimulation.
Flow cytometry
Spleen cells or isolated B cells from normal or rLiTXN1-injected BALB/c mice obtained as single cell suspensions were washed in PBS supplemented with 2% FCS. A total of 106 viable cells were incubated for 30 min at 4° with saturating concentrations of phycoerythrin (PE)-conjugated hamster anti-mouse CD69 plus fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD8 (Ly-2) monoclonal antibody, FITC-conjugated rat anti-CD4, or FITC-conjugated goat anti-mouse IgM (BD Pharmingen, San Diego, CA). After two washes with PBS/2% FCS, the cells were analysed by flow cytometry in a FACScan equipped with cellquest Pro software (Becton Dickinson, San Jose, CA). Dead cells were excluded from all samples by propidium iodide labelling.
Enzyme-linked immunosorbent assays (ELISAs) for cytokines
Cytokine production was determined by two site sandwich ELISAs in the supernatants of spleen cells or isolated B cells from BALB/c mice, stimulated with ConA (6 µg/ml), LPS (10 µg/ml) or rLiTXN1 (5 or 50 µg/ml) and collected after 24 hr (IL-2), 48 hr (IL-10 and IL-4) or 72 hr (IFN-γ) of incubation at 37° and 5% CO2. Ninety-six-well flat-bottom microtitre plates (Maxisorp Immunoplates; Nunc, Roskilde, Denmark) were coated overnight at 4° with unlabelled rat antibodies to the cytokines IL-2 (JES6-1A12 cell line), IL-4 (BVD4-1D11 cell line), IFN-γ (R4-6A2 cell line) and IL-10 (Mouse IL-10 ELISA Set from BD Pharmingen, San Diego, CA). IL-10 measurement was performed following the manufacturer's instructions. For the others ILs, the plates were washed with PBS/0·1% Tween-20 (PBS-T) and blocked with PBS/1% gelatine for 1 hr at room temperature. After 2 hr of incubation with serial dilutions of each supernatant, plates were washed with PBS-Tween 20 and incubated for another 1 hr with biotinylated rat antibodies to IL-2 (JES6-5H4 cell line), IL-4 (BVD6-24G2 cell line) and IFN-γ (XMG1·2 cell line), from BD Pharmingen. The plates were then washed with PBS-T and incubated for 1 hr with streptavidin-peroxidase (Sigma, St Louis, MO) and developed with 0·5 mg/ml of o-phenylenediamine dihydrochloride (OPD; Sigma) and H2O2 in citrate buffer. Reactions were stopped by the addition of 10% SDS. Optical densities were measured at 450 nm in an automatic ELISA reader (Power Wave™XS; Bio-Tek, Winooski, VT). Cytokine concentrations were determined by comparison with a standard curve generated with the different recombinant cytokines: rIL-2, rIL-4 and rIFN-γ (BD Pharmingen).
ELISA for immunoglobulins
Ninety-six-well flat-bottom plates (Greiner Labortachnik, Solingen, Germany) were coated overnight at 4° in carbonate buffer, pH 8·2, with unlabelled goat anti-mouse immunoglobulin (5 µg/ml), total L. infantum protein extract (10 µg/ml), rLiTXN1 (5 µg/ml), recombinant L. infantum cytosolic tryparedoxin peroxidase (rLicTXNPx; 5 µg/ml), bovine serum albumin (BSA; 5 µg/ml), whale skeletal muscle type II myoglobulin (Myo; 5 µg/ml), or native double stranded DNA (DNA; 5 µg/ml). The plates were washed with PBS-T and blocked with PBS/1% gelatine for 1 hr at 37°. Plates were then incubated for 2 hr with serial dilutions (for total titres) or 1 : 900 dilutions (for specific antibody reactivity) of each serum sample (from PBS or rLiTXN1 injected mice). After washing with PBS-T, the plates were incubated for 30 min at 37° with peroxidase-labelled goat anti-mouse immunoglobulin isotypes (anti-IgM, anti-IgG, anti-IgG1, anti-IgG2a, anti-IgG2b and anti-IgG3) and developed with 0·5 mg/ml OPD (Sigma) and H2O2 in citrate buffer. Reactions were stopped by the addition of 3 N HCl. Unlabelled purified isotypes were used in serial dilutions as standards. Absorbance values were read at 492 nm in an automatic ELISA reader (Power Wave™XS).
Reverse transcription–polymerase chain reaction (RT-PCR)
Isolated spleen B cells were stimulated or not in vitro with rLiTXN1 (5 and 50 µg/ml) and incubated at 37° in 5% CO2. After 12 hr of stimulation, total RNA (from 4 × 106 cells per stimulus) was isolated using a guanidium thiocyanate-phenol-chloroform single step method.21 cDNA synthesis was achieved with 1 μg of total extracted RNA using Superscript II RT (Gibco BRL, New York, NY) and random primers (Promega, Madison, WI). PCR amplification was performed with 1 µg of cDNA using primers for IL-10 and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). After 35 PCR cycles consisting of DNA denaturation at 94° for 30 seconds, primer annealing at 55° for 30 seconds, and DNA extension at 72° for 30 seconds, the reaction was terminated. Then 25 µl of each PCR product was analysed by electrophoresis and visualized and photographed on a UV transilluminator (Vilber Lourmat, Eberhardzell, Germany). PCR band densities were determined using bio-pofil bio-1d software (Vilber Lourmat).
Western blot
Parasite cell lysates (30 µg/lane) or rLiTXN1 (2 µg/lane) was electrophoresed on 15% SDS-PAGE gels and transferred onto a nitrocellulose membrane. After saturation for 1 hr with a PBS solution containing 5% fat dehydrated milk, the membranes were incubated with different dilutions of polyclonal sera against rLiTXN1 and sera from control mice (PBS injected mice) overnight at 4°. After washing with PBS-T, the membranes were incubated with horseradish peroxidase (HRP)-labelled goat anti-mouse IgG antibody (Southern Biotechnologies, Birmingham, AL) for 1 hr and revealed with the ECL western blotting analysis system (Amersham).
Statistical analysis
The data were analysed using Student's t-test.
Results
rLiTXN1 induces CD69 expression in murine B cells
In a previous study, we examined the expression pattern of LiTXN1 during the Leishmania life cycle and found it to be up-regulated during the stationary promastigote phase and the intracellular amastigote stage of the parasite.18 Moreover, in these stages, LiTXN1 is abundantly excreted/secreted from the parasite.19 Thus, we decided to examine the effect of LiTXN1 on the host immune system cells. Hence, we subcloned the LiTXN1 enconding gene into an expression vector and the corresponding recombinant protein (rLiTXN1) was purified.
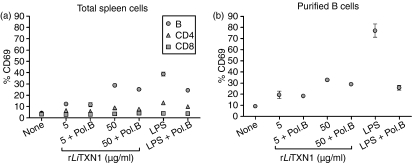
To identify the cell populations responding to rLiTXN1, we searched for the cell membrane expression of an early activation marker (CD69) in CD4+, CD8+ and B-cell populations after 20 hr of in vitro rLiTXN1 stimulation (Fig. 1a). The fluorescence-activated cell sorting (FACS) analysis showed a dose-dependent increase in CD69+ B cells after rLiTXN1 stimulation (12·2 ± 1·2% (mean ± standard deviation) and 28·8 ± 0·44% positive B cells upon stimulation with 5 and 50 µg/ml of rLiTXN1, respectively) compared with unstimulated cells (4·3 ± 1·4% positive B cells). However, no significant differences were found for the CD4+ and CD8+ T-cell populations. As a positive control we used a known B-cell mitogen, LPS, which induces high expression of CD69 on B cells. In order to rule out the possibility that rLiTXN1 biological activity resulted from contaminating bacterial components present in our recombinant protein, additional experiments were performed with the addition of polymyxin B. In these conditions, the LPS activity was partially abrogated, while no significant differences were observed in B-cell activation induced by rLiTXN1.
Figure 1.
Recombinant Leishmania infantum tryparedoxin 1 (rLiTXN1) induces the expression of CD69 on total spleen and purified B cells from BALB/c mice. Expression of the CD69 activation marker in total spleen cells (a) and purified B cells (b) from BALB/c mice after in vitro stimulation with rLiTXN1 is shown. Cells (2·5 × 105 per well) were cultured in the presence of rLiTXN1 (5 or 50 µg/ml) or lipopolysaccharide (LPS) (10 µg/ml), both with and without 10 µg/ml of polymixin B (Pol.B). After 20 hr, percentages of CD69+ B cells and CD4+ and CD8+ T cells were determined by flow cytometry analysis. The results represent the means and standard deviations of three experiments carried out independently.
Furthermore, to elucidate whether T-cell dependent mechanisms are involved in the B-cell activation induced by rLiTXN1, we purified resting B cells from naïve BALB/c splenocytes. As shown in Fig. 1(b), the rLiTXN1 stimulus induced a significant increase in CD69 expression on isolated B cells (19·5 ± 3·2% and 32·3 ± 0·9% positive B cells upon stimulation with 5 and 50 μg/ml of rLiTXN1, respectively) when compared with the unstimulated cells (9·2 ± 1·2% positive B cells). Similar results were obtained when polymixin B was added to rLiTXN1-treated B cells, suggesting that the observed effects are a result of rLiTXN1 biological activity and not bacterial contamination. Thus, it is reasonable to assume that rLiTXN1 behaves as a B-cell activator, in the presence or absence of T cells.
In vitro effects of rLiTXN1 on B-lymphocyte proliferation
To further examine the effect of rLiTXN1 on spleen cell populations, we measured proliferation using the incorporation of [3H]thymidine in total spleen and isolated B cells submitted to in vitro rLiTXN1 stimulus. Spleen cells from BALB/c mice were cultured in the presence or absence of rLiTXN1 for 48 and 72 hr. As shown in Fig. 2(a), total spleen cells from BALB/c mice responded in a dose dependent manner to rLiTXN1 in vitro stimulus, as revealed by increased thymidine incorporation compared with unstimulated cells.
Figure 2.
Lymphocyte proliferation of BALB/c mice spleen cells induced by recombinant Leishmania infantum tryparedoxin 1 (rLiTXN1). Total spleen cells (a) or purified spleen B cells (b) (2·5 × 105 cells per well) from BALB/c mice were stimulated in vitro with increasing concentrations of rLiTXN1 (5, 10 or 50 µg/ml) for 48 or 72 hr. Cells were pulsed with [3H]thymidine for the last 8 hr. Data [counts per minute (c.p.m.)] represent the mean and standard deviation for three animals analysed individually and are representative of three independent experiments. Statistically significant differences between non-stimulated and stimulated cells are indicated: **P < 0·01.
These results suggest that B cells are a target for rLiTXN1. B-cell proliferation can occur in a T-cell dependent or independent manner. We found that, even in the absence of a T-cell population, isolated resting B cells proliferated in response to rLiTXN1 in vitro stimulus (Fig. 2b). Moreover, the rLiTXN1 induced proliferation was significantly enhanced in isolated B cells compared with the results obtained for total spleen cells, as confirmed by an increased stimulatory index (32·62 versus 16·63 after 72 hr of stimulation) (Table 1). In order to rule out the possibility that LPS contaminating the rLiTXN1 preparation was responsible, at least in part, for the cell stimulation, spleen cells from C3H/HeJ mice (LPS non-responder) were cultured with or without rLiTXN1. The proliferation of C3H/HeJ spleen cells induced by rLiTXN1 stimulus reached similar levels to those observed with BALB/c cells, as revealed by the corresponding stimulatory index: 2·42, 5·96 and 9·09 for C3H/HeJ mice and 2·57, 6·64 and 10·98 for BALB/c mice after 48 hr of stimulation with 5, 10 and 50 µg/ml of rLiTXN1, respectively. These observations suggest that B cells are a putative rLiTXN1 target.
Table 1.
Stimulation index for total spleen cells and purified spleen B cells from BALB/c mice, stimulated in vitro with recombinant Leishmania infantum tryparedoxin 1 (rLiTXN1) (50 μg/ml)
| Total spleen cells | Purified B cells | 48 hr | 72 hr | |
|---|---|---|---|---|
| LPS | + | – | 89·93 | 97·66 |
| – | + | 93·65 | 100·15 | |
| rLiTXN1 | + | – | 10·98 | 16·63 |
| – | + | 27·93 | 32·62 |
LPS, lipopolysaccharide.
rLiTXN1 up-regulates IL-10 production in spleen cells
Complementary investigations were performed for the cellular responses induced by rLiTXN1, namely cytokine production. Thus, spleen cells from BALB/c mice were cultured in vitro in the presence or absence of ConA, as a positive control, or rLiTXN1, and the supernatant levels of IL-2, IL-4, IL-10 and IFN-γ were measured by ELISA. As shown in Table 2, a significant dose-dependent increase in IL-10 secretion by spleen cells stimulated with rLiTXN1 was detected compared with unstimulated cells (P < 0·01 for 5 and 50 µg/ml of rLiTXN1). No significant variations were observed for IL-2, IL-4 and IFN-γ secretion after rLiTXN1 stimulus. Moreover, we determined the effect of in vivo rLiTXN1 treatment on ex vivo cytokine secretion by spleen cells after ConA or rLiTXN1 restimulation. Total spleen cells from rLiTXN1-treated mice also increased IL-10 secretion when restimulated with rLiTXN1. However, no significant differences were found between the IL-10 production by spleen cells of rLiTXN1 treated and untreated mice after rLiTXN1 ex vivo stimulus, suggesting that no B-cell memory was created.
Table 2.
Increase in interleukin (IL)-10 production by spleen cells from BALB/c mice in response to recombinant Leishmania infantum tryparedoxin 1 (rLiTXN1) stimulation
| Cytokine level (ng/ml) in supernatants1 of spleen cells from untreated and rLiTXN1 treated BALB/c mice | |||||
|---|---|---|---|---|---|
| Cytokine | rLiTXN1 treatment | Control | ConA (5 μg/ml) | rLiTXN1 (5 μg/ml) | rLiTXN1 (50 μg/ml) |
| IFN-γ | – | 1·66 ± 0·32 | 7·45 ± 0·77 | 1·58 ± 0·50 | 1·48 ± 0·25 |
| + | 2·16 ± 0·44 | 9·08 ± 2·54 | 1·76 ± 0·59 | 2·85 ± 1·01 | |
| IL-2 | – | 0·26 ± 0·05 | 5·09 ± 0·27 | 0·22 ± 0·03 | 0·21 ± 0·05 |
| + | 0·27 ± 0·05 | 5·48 ± 1·01 | 0·25 ± 0·02 | 0·23 ± 0·04 | |
| IL-10 | – | 0·03 ± 0·00 | 1·05 ± 0·09 | 0·162 ± 0·04 | 0·582 ± 0·19 |
| + | 0·04 ± 0·02 | 1·26 ± 0·20 | 0·212 ± 0·05 | 0·762 ± 0·09 | |
| IL-4 | – | 0·22 ± 0·05 | 1·08 ± 0·11 | 0·23 ± 0·05 | 0·16 ± 0·05 |
| + | 0·27 ± 0·09 | 1·27 ± 0·24 | 0·20 ± 0·05 | 0·20 ± 0·04 | |
The cytokine levels in the supernatants of spleen cells (2·5 × 105 cells per well) from untreated or rLiTXN1 treated BALB/c mice were cultured with concanavalin A (ConA) or rLiTXN1 for 24 hr (IL-2), 48 hr (IL-4 and IL-10) or 72 hr [interferon (IFN)-γ]. These levels were determined by enzyme-linked immunosorbent assay (ELISA) in comparison with a standard curve using the recombinant interleukins. The data represent the means and standard deviations for three animals analysed individually and are representative of three independent experiments.
Significant differences (P < 0·01) in IL-10 levels were observed between non-stimulated and rLiTXN1-stimulated cells, from both untreated and rLiTXN1 treated BALB/c mice.
B cells secrete IL-10 after rLiTXN1 stimulus
B cells are one of the cellular sources of IL-10.22 Thus, given that rLiTXN1 triggered B-cell activation, we examined the splenic B-cell population as a possible source of the IL-10 production induced by the rLiTXN1 stimulus. RT-PCR analysis was performed using specific primers for IL-10 and GAPDH, as an internal control, with mRNA purified from isolated B cells after incubation for 12 hr with or without rLiTXN1. rLiTXN1 stimulus induced a 1·3 fold increase (1·32 ± 0·07; P < 0·01) in IL-10 mRNA expression compared with the non-stimulated cells. Because IL-10 mRNA expression was increased, we measured IL-10 secretion by isolated B cells after 48 hr of rLiTXN1 stimulation. In these conditions, we detected a dose-dependent increase in IL-10 concentration in the supernatant of isolated B cells compared with unstimulated cells (P < 0·01 for 5 and 50 µg/ml of rLiTXN1) (data not shown). These observations suggest that B cells are involved in IL-10 secretion in response to the rLiTXN1 stimulus.
rLiTXN1 injection promotes a specific humoral response
In order to explore the in vivo effects of rLiTXN1 on the B-cell response, we immunized BALB/c mice i.p. without adjuvant, as described in the Materials and methods. Mice injected with PBS were used as a control group. Although rLiTXN1 induced B-cell activation and proliferation in vitro, we did not find significant increases in the spleen B-cell population after in vivo rLiTXN1 treatment (data not shown). However, a strong humoral response was developed in mice that received the rLiTXN1 immunization compared with control PBS-injected mice. As shown in Table 3, total serum levels of both IgM and IgG were found to be significantly increased (1·5 fold, P = 0·01, and 2·7 fold, P < 0·001, respectively) in the rLiTXN1-treated mice compared with the untreated mice, as determined by ELISA, 15 days after the last i.p. administration. Moreover, analysis of IgG antibodies showed that only IgG1 (1·9 fold, P < 0·01) and IgG3 (2·2 fold, P < 0·01) serum levels were significantly increased in immunized mice compared with controls (Fig. 3).
Table 3.
Total serum immunoglobulins in BALB/c mice, 14 days after recombinant Leishmania infantum tryparedoxin 1 (rLiTXN1) administration
| Total serum immunoglobulin (mg/ml) | ||
|---|---|---|
| BALB/c | IgM | IgG |
| PBS-treated | 0·85 ± 0·11 | 1·22 ± 0·23 |
| rLiTXN1-treated | 1·28 ± 0·23 | 3·33 ± 0·25 |
| P = 0·01 | P = 1 × 10−6 | |
PBS, phosphate-buffered saline.
Figure 3.
The humoral immune response induced by the intraperitoneal (i.p.) administration of recombinant Leishmania infantum tryparedoxin 1 (rLiTXN1) in BALB/c mice. Titres of immunoglobulin G (IgG) isotypes (IgG1, IgG2a, IgG2b and IgG3) in the sera of BALB/c mice were determined by enzyme-linked immunosorbent assay (ELISA), 14 days after the last rLiTXN1 i.p. administration (50 µg/mouse). The results are the arithmetic means of three animals analysed individually and are representative of three independent experiments. Statistically significant differences in IgG1 and IgG3 levels were observed between sera from rLiTXN1-treated and control mice: **P < 0·01.
Further, we tested the specificity of these antibodies. The levels of rLiTXN1 specific serum antibodies of all tested isotypes were increased in previously immunized mice. However, this increase was higher for IgG1 and IgG3 than for the other isotypes studied (data not shown). In addition, no significant reactivity was found against heterologous antigens (i.e. Myo, BSA and DNA) or another recombinant Leishmania excreted/secreted protein, namely Leishmania infantum cytosolic tryparedoxin peroxidase (LicTXNPx), in rLiTXN1 treated mice (Fig. 4). Moreover, a significant increase in L. infantum total extract IgG levels was found in an ELISA test (data not shown). This is not surprising given that tryparedoxin proteins are known to occur in high amounts in the cell.23 However, to confirm the specificity of rLiTXN1 treated mice serum among all parasitic antigens, we analysed by western blot their reactivity against the Leishmania total protein extract. Sera from mice immunized with rLiTXN1 recognized specifically the corresponding antigen (molecular weight approximately 16·6 kDa) in L. infantum protein extracts, whereas no reactivity was found for serum from PBS-injected mice (data not shown). Thus, rLiTXN1 when injected into BALB/c mice is capable of inducing B-cell differentiation leading to a specific humoral response.
Figure 4.
A specific humoral immune response is induced by recombinant Leishmania infantum tryparedoxin 1 (rLiTXN1) intraperitoneal (i.p.) administration in BALB/c mice. Immunoglobulin G1 (IgG1) and IgG3 antibody levels in the sera of rLiTXN1 treated BALB/c mice reacting against rLicTXNPx, bovine serum albumin (BSA), whale skeletal muscle type II myoglobulin (Myo) and native double-stranded DNA (DNA) were determined by enzyme-linked immunosorbent assay (ELISA). Mice sera were diluted 1/900 and optical density was recorded at 492 nm. The data represent the means and standard deviations for three mice analysed individually and are representative of three independent experiments.
Discussion
Leishmania is a typical intracellular pathogen. Shortly after inoculation in the dermis, metacyclic promastigotes infect mononuclear phagocytic cells in the skin and differentiate intracellularly into non-motile amastigotes. For successful establishment of the infection, Leishmania must be capable of surviving the acidic and protease rich environment inside the phagolysosome but also deal with oxygen and nitrogen reactive species (ROS and RNS, respectively), which form part of the host antimicrobial machinery. We have recently identified and cloned a novel L. infantum gene that encodes a cytosolic tryparedoxin homologue to previously known tryparedoxins of Crithidia fasciculata, Trypanosoma brucei and Trypanosoma cruzi. It was suggested that LiTXN1 may be implicated in the resistance to ROS and RNS, as it is a probable component of parasite cytoplasmatic hydroperoxide metabolism.18LiTXN1 is expressed throughout the parasite life cycle. However, northern and western blot analysis demonstrated that LiTXN1 is up-regulated (approximately 10-fold) in the stationary phase promastigotes that are enriched in the infective metacyclic form and in axenic amastigotes.18 Recently, using different approaches such as a highly sensitive radiolabelled immunoprecipitation technique19 and two dimensional electrophoresis (unpublished data), we detected LiTXN1 among the parasite excreted/secreted antigens. These observations suggest that LiTXN1 may interfere with host immune cells, playing an important role during the infective process.
In this paper we demonstrated that rLiTXN1 can activate B cells of naïve BALB/c mice. The observed effect occurred even in the absence of accessory cells. Interestingly, rLiTXN1-induced activation seems to be B-cell specific among the lymphocyte population as no significant differences were found in the CD69 expression of CD4+ or CD8+ cells after stimulus. When cultured in the presence of rLiTXN1, BALB/c spleen cells underwent proliferation, as shown by increased thymidine incorporation. This effect was enhanced when a purified resting B-cell population was used. Because we used a recombinant protein produced in a bacterial expression system, it was essential to exclude the effect of a possible LPS contamination. Thus, complementary experiments were performed in the presence of polymixin B. No significant alteration of the responses of spleen or isolated B cells to rLiTXN1 was observed in the presence of polymixin B, whereas it partially abrogated the LPS effect. Also, experiments performed in C3H/HeJ, a strain characterized by its hyporesponsiveness to LPS,24 confirmed that contamination of rLiTXN1 with LPS, which might account for the biological effect observed, was highly unlikely. These results are direct evidence of the capacity of rLiTXN1 to induce in vitro B-cell proliferation by a T-cell independent mechanism. A link between B-cell expansion, circulating antibodies and virulence enhancement has been reported in models of Leishmania and T. cruzi infections. Mice depleted of B cells by continuous administration of anti-IgM, mice that were B-cell mutants, and mice lacking circulating antibodies infected with different Leishmania species showed enhanced resistance compared with control BALB/c mice. Moreover, susceptibility to leishmaniasis is recovered when the B-cell population is reconstituted.25,26 Recently, a T. cruzi trans-sialidase (TS) was shown to sensitize mice towards an increased susceptibility to infection.27 Also, TS-transgenic L. major was highly virulent.28 These effects have been suggested to be related to the TS activity as a T-cell independent B-cell mitogen.29 Thus, it is reasonable to assume that LiTXN1 can interfere with the host immune system before the onset of an adaptive response, playing a role in the outcome of the infection.
The functional dichotomy between Th1 and Th2 cell subsets is crucial in inducing resistance to cutaneous leishmaniasis in mice.4 However, in the case of visceral leishmaniasis, a counterproductive Th2 response is not apparent in mice, although it has been described in humans.30 In humans, it has been demonstrated that the capacity to mount a pro-inflammatory response, with critical roles for the Th1 cytokines IL-2 and IFN-γ, is essential for the control and resolution of the infection.30,31 While several studies supported the notion that the shift from IL-4 to IFN-γ would provide the key to recovery,32–34 recent findings have suggested that IL-10 is the most important regulatory cytokine involved in disease progression as it promotes parasite persistence.35,36 All these data prompted us to study the cytokine profile induced by rLiTXN1. Our results showed that in vitro treatment of spleen cells with rLiTXN1 induced dose-dependent secretion of IL-10 (increasing 5-fold with 5 μg/ml and 18 fold with 50 μg/ml of rLiTXN1, compared with unstimulated cells). In contrast, no significant differences were observed in the levels of the other cytokines measured (IL-2, IL-4 and IFN-γ) after rLiTXN1 stimulation. IL-10 is a pleiotropic Th1 suppressive cytokine which plays a pivotal role in the down regulation of macrophage activation, preventing antigen specific T-cell stimulation, and in limiting IFN-γ production.16 Acting as an immunomodulator, IL-10 promotes the attenuation of host defence mechanisms against the pathogenic invasion. In addition to findings in Leishmania,37,38 other infection models for intracellular pathogens such as Mycobacterium avium and Klebsiella pneumoniae have shown an increase in IL-10 secretion to be a common mechanism facilitating infection. Moreover, blocking of IL-10 activity with anti-IL-10 antibodies induced resistance against infection.39,40 Further experiments were carried out in spleen cells from mice previously immunized with rLiTXN1. The immunization did not alter the amount of secreted IL-10 in rLiTXN1 treated mice in comparison to untreated naïve mice upon ex vivo rLiTXN1 stimulus, suggesting that the observed effect was attributable to a direct effect on the naïve B-cell population, rather than the formation of memory. This suggestion is supported by observations that T-cell independent antigens are poor inducers of immunological memory.41 A recent review suggests the existence of B regulatory cells (Breg).42 Their regulatory functions are mediated by the production of the anti-inflammatory cytokine IL-10 or TGF-β, as shown in a wide range of chronic inflammation models. Also, alterations of B-cell cytokine production were reported in various parasitic infections. In Schistosoma mansoni, two parasite-derived antigens target B cells, triggering the secretion of IL-10, which leads to down-regulation of the Th1 inflammatory response and exacerbation of disease.43,44 Thus, rLiTXN1, through its capacity to stimulate IL-10, may generate an inappropriate cellular response that will contribute to the pathogenesis.
Mice and humans have phenotypically distinct populations of B cells, termed B-1 and B-2, which differ with respect to lineage origins, phenotype and anatomical origins.45 It is well established that B-1 cells represent the main B-cell population in the peritoneal cavity of mice, while B-2 cells constitute the major fraction of the spleen B-cell population. IL-10 production is a well known feature of peritoneal CD5+ B1 cells.46,47 However, in this work, during the B-cell isolation process, the B-1 population was eliminated by depletion of CD43 expressing B cells. This suggests that B-2 cells are direct targets for rLiTXN1 and are responsible for the IL-10 secretion. Moreover, several studies have indicated that the phenotype of IL-10 producing Breg is similar to that of B-2 but not B-1 cells.42 However, we cannot discount the possibility of an effect on the B-1 population, as T-cell-independent immune responses generally involve these cells.48
Leishmania spp. uses mechanisms common to other intracellular pathogens to escape the host immune system. It has been reported that Leishmania possesses a large number of molecules characterized by their immunosuppressive and mitogenic like activities.49,50 During infection, hyporesponsiveness and polyclonal activation lead to suppressed parasite specific humoral and cellular responses, which help the parasite to establish itself in the host. The secreted/excreted proteins are among the parasitic molecules that play a major role during the establishment of infection and later in its maintenance.51 Indeed, soluble parasite derived antigens from L. major and Leishmania donovani were reported to induce polyclonal activation with the production of autoantibody activity.16 Also, an excreted factor derived from the culture medium of L. major was found to suppress ConA induced polyclonal activation of mouse T cells.52 Similar findings were reported in T. cruzi, where excreted/secreted antigens were found to induce high levels of non-specific antibodies, such as T. cruzi transialidase and the flagellar Ca2+-binding protein (Tc24).29,53 These findings contrast with those of a recent report, in which we described a secreted Leishmania cytosolic nicotinamide adenine dinucleotide-dependent deacetylase, Leishmania major silence information regulatory protein 2 (LmSIR2), that induced high levels of SIR2 specific antibodies. These antibodies were able to promote complement mediated killing of promastigotes and amastigotes and to inhibit their multiplication inside macrophages. Indeed, SIR2 specific antibodies, which during natural and experimental leishmaniasis are only detected in the chronic phase,19,54 were suggested to play a role in the early protective immune responses induced by immunization. Here, we showed that in vivo administration of rLiTXN1 without adjuvant induces a strong humoral response, predominantly involving the IgM, IgG1 and IgG3 isotypes, which is a typical characteristic of T-cell independent antigens.55 Moreover, the antibodies produced reacted specifically against rLiTXN1 and not against other heterologous antigens, including DNA, Myo, BSA and other excreted/secreted Leishmania antigens such as LicTXNPx.56 Interestingly, during natural human and canine leishmaniasis and experimental murine leishmaniasis, rLiTXN1 specific antibodies were present at low levels or absent.57 Several lines of evidence have suggested that excreted/secreted proteins may play a role in the initial steps of infection. These proteins have been selected by evolution to be immunologically ‘silent’, allowing them to perform functions that are vital for the success of parasite infection,58,59 However, in contrast to findings with LmSIR2, no anti-parasitic activity was found with LiTXN1-specific antibodies (data not shown). Further, preliminary BALB/c immunization experiments were conducted with rLiTXN1 protein. However, no significant difference in the parasite load was observed in the liver or the spleen of rLiTXN1 immunized mice compared with non-immunized mice at 6 weeks postinfection (data not shown). Nevertheless, in a recent study a tryparedoxin peroxidase, which is present in the same hydroperoxide cascade as tryparedoxin, was shown to induce long lasting protection against murine leishmaniasis when heterologous prime boost immunization was used, but not when it was delivered alone in DNA.60 This suggests that a different vaccination approach should be investigated for rLiTXN1 to improve efficacy.
As an obligate intracellular parasite, Leishmania has evolved complex strategies for evading host defence mechanisms before (complement mediated killing), during (oxygen reactive species), and after (lysosomal hydrolases or nitric reactive species) entry into host cells. Overall, our present results show that LiTXN1 is a potent modulator of B-cell activity, inducing IL-10 release. We propose that LiTXN1 can play a dual role in protecting the parasite against the host immune system during the infection. In the early phase it induces immunosuppressive IL-10 secretion, which can facilitate the establishment of infection. In the later phase it may be involved in the ROS and RNS elimination cascade, facilitating maintenance of the parasite.
Acknowledgments
This work was supported by Fundação para a Ciência e Tecnologia (FCT) and FEDER, project number POCI/CVT/59840/2004. RS, JT and AS were supported by fellowships from FCT and FEDER, numbers SFRH/BD/13120/2003, SFRH/BD/18137/2004 and SFRH/BD/28316/2006, respectively. SC and NS were supported by fellowships with project numbers POCI/CVT/59840/2004 and POCI/SAU-FCT/59837/2004, respectively. LiTXN1 used in this work was purified from clones provided by A. Tomás's team.
Abbreviations
- ConA
concanavalin A
- FCS
fetal calf serum
- i.p.
intraperitoneally
- LPS
lipopolysaccharide
- PBS
phosphate-buffered saline
- rLiTXN1
recombinant Leishmania infantum tryparedoxin 1.
References
- 1.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–43. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 2.Badaro R, Jones TC, Carvalho EM, Sampaio D, Reed SG, Barral A, Teixeira R, Johnson WD., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986;154:1003–11. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- 3.Kaye PM. Costimulation and the regulation of antimicrobial immunity. Immunol Today. 1995;16:423–7. doi: 10.1016/0167-5699(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 4.Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–5. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie AN. IL-13 is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164:1458–62. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- 6.Barral-Netto M, Barral A, Brodskyn C, Carvalho EM, Reed SG. Cytotoxicity in human mucosal and cutaneous leishmaniasis. Parasite Immunol. 1995;17:21–8. doi: 10.1111/j.1365-3024.1995.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 7.Galvao-Castro B, Sa Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Polyclonal B cell activation, circulating immune complexes and autoimmunity in human American visceral leishmaniasis. Clin Exp Immunol. 1984;56:58–66. [PMC free article] [PubMed] [Google Scholar]
- 8.Launois P, Ohteki T, Swihart K, MacDonald HR, Louis JA. In susceptible mice, Leishmania major induces very rapid interleukin-4 production by CD4+ T cells which are NK1.1. Eur J Immunol. 1995;25:3298–307. doi: 10.1002/eji.1830251215. [DOI] [PubMed] [Google Scholar]
- 9.Cordeiro-Da-Silva A, Borges MC, Guilvard E, Ouaissi A. Dual role of the Leishmania major ribosomal protein S3a homologue in regulation of T- and B-cell activation. Infect Immun. 2001;69:6588–96. doi: 10.1128/IAI.69.11.6588-6596.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frankenburg S, Leibovici V, Mansbach N, Turco SJ, Rosen G. Effect of glycolipids of Leishmania parasites on human monocyte activity. Inhibition by lipophosphoglycan. J Immunol. 1990;145:4284–9. [PubMed] [Google Scholar]
- 11.Suffia I, Ferrua B, Stien X, Mograbi B, Marty P, Rousseau D, Fragaki K, Kubar J. A novel Leishmania infantum recombinant antigen which elicits interleukin 10 production by peripheral blood mononuclear cells of patients with visceral leishmaniasis. Infect Immun. 2000;68:630–6. doi: 10.1128/iai.68.2.630-636.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevens JR, Noyes HA, Schofield CJ, Gibson W. The molecular evolution of Trypanosomatidae. Adv Parasitol. 2001;48:1–56. doi: 10.1016/s0065-308x(01)48003-1. [DOI] [PubMed] [Google Scholar]
- 13.Wilson ME, Young BM, Davidson BL, Mente KA, McGowan SE. The importance of TGF-beta in murine visceral leishmaniasis. J Immunol. 1998;161:6148–55. [PubMed] [Google Scholar]
- 14.Satoskar A, Bluethmann H, Alexander J. Disruption of the murine interleukin-4 gene inhibits disease progression during Leishmania mexicana infection but does not increase control of Leishmania donovani infection. Infect Immun. 1995;63:4894–9. doi: 10.1128/iai.63.12.4894-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goto H, Lindoso JA. Immunity and immunosuppression in experimental visceral leishmaniasis. Braz J Med Biol Res. 2004;37:615–23. doi: 10.1590/s0100-879x2004000400020. [DOI] [PubMed] [Google Scholar]
- 16.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 17.Bacellar O, Brodskyn C, Guerreiro J, Barral-Netto M, Costa CH, Coffman RL, Johnson WD, Carvalho EM. Interleukin-12 restores interferon-gamma production and cytotoxic responses in visceral leishmaniasis. J Infect Dis. 1996;173:1515–8. doi: 10.1093/infdis/173.6.1515. [DOI] [PubMed] [Google Scholar]
- 18.Castro H, Sousa C, Novais M, Santos M, Budde H, Cordeiro-da-Silva A, Flohe L, Tomas AM. Two linked genes of Leishmania infantum encode tryparedoxins localised to cytosol and mitochondria. Mol Biochem Parasitol. 2004;136:137–47. doi: 10.1016/j.molbiopara.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Santarem N, Silvestre R, Tavares J, Silva M, Cabral S, Maciel J, Cordeiro-Da-Silva A. Immune response regulation by Leishmania secreted and non secreted antigens. J Biomed Biotechnol. 2007;6:851–54. doi: 10.1155/2007/85154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 22.Burdin N, Rousset F, Banchereau J. B-cell-derived IL-10: production and function. Methods. 1997;11:98–111. doi: 10.1006/meth.1996.0393. [DOI] [PubMed] [Google Scholar]
- 23.Nogoceke E, Gommel DU, Kiess M, Kalisz HM, Flohe L. A unique cascade of oxidoreductases catalyses trypanothione-mediated peroxide metabolism in Crithidia fasciculata. Biol Chem. 1997;378:827–36. doi: 10.1515/bchm.1997.378.8.827. [DOI] [PubMed] [Google Scholar]
- 24.Morrison DC, Ryan JL. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- 25.Sacks DL, Scott PA, Asofsky R, Sher FA. Cutaneous leishmaniasis in anti-IgM-treated mice: enhanced resistance due to functional depletion of a B cell-dependent T cell involved in the suppressor pathway. J Immunol. 1984;132:2072–7. [PubMed] [Google Scholar]
- 26.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. J Exp Med. 2000;191:1063–8. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chuenkova M, Pereira ME. Trypanosoma cruzi trans-sialidase: enhancement of virulence in a murine model of Chagas' disease. J Exp Med. 1995;181:1693–703. doi: 10.1084/jem.181.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belen Carrillo M, Gao W, Herrera M, Alroy J, Moore JB, Beverley SM, Pereira MA. Heterologous expression of Trypanosoma cruzi trans-sialidase in Leishmania major enhances virulence. Infect Immun. 2000;68:2728–34. doi: 10.1128/iai.68.5.2728-2734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao W, Wortis HH, Pereira MA. The Trypanosoma cruzi trans-sialidase is a T cell-independent B cell mitogen and an inducer of non-specific Ig secretion. Int Immunol. 2002;14:299–308. doi: 10.1093/intimm/14.3.299. [DOI] [PubMed] [Google Scholar]
- 30.Kaye PM, Curry AJ, Blackwell JM. Differential production of Th1- and Th2-derived cytokines does not determine the genetically controlled or vaccine-induced rate of cure in murine visceral leishmaniasis. J Immunol. 1991;146:2763–70. [PubMed] [Google Scholar]
- 31.Engwerda CR, Smelt SC, Kaye PM. An in vivo analysis of cytokine production during Leishmania donovani infection in scid mice. Exp Parasitol. 1996;84:195–202. doi: 10.1006/expr.1996.0105. [DOI] [PubMed] [Google Scholar]
- 32.Fowell DJ, Bix M, Shinkai K, Lacy D, Locksley RM. Disease susceptibility and development of the cytokine repertoire in the murine Leishmania major model. Eur Cytokine Netw. 1998;9:102–6. [PubMed] [Google Scholar]
- 33.Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maekawa Y, Himeno K, Ishikawa H, et al. Switch of CD4+ T cell differentiation from Th2 to Th1 by treatment with cathepsin B inhibitor in experimental leishmaniasis. J Immunol. 1998;161:2120–7. [PubMed] [Google Scholar]
- 35.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–7. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 37.Murray HW, Lu CM, Mauze S, Freeman S, Moreira AL, Kaplan G, Coffman RL. Interleukin-10 (IL-10) in experimental visceral leishmaniasis and IL-10 receptor blockade as immunotherapy. Infect Immun. 2002;70:6284–93. doi: 10.1128/IAI.70.11.6284-6293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy ML, Wille U, Villegas EN, Hunter CA, Farrell JP. IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol. 2001;31:2848–56. doi: 10.1002/1521-4141(2001010)31:10<2848::aid-immu2848>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 39.Bermudez LE, Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993;61:3093–7. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–9. [PubMed] [Google Scholar]
- 41.Lesinski GB, Westerink MA. Novel vaccine strategies to T-independent antigens. J Microbiol Meth. 2001;47:135–49. doi: 10.1016/s0167-7012(01)00290-1. [DOI] [PubMed] [Google Scholar]
- 42.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–10. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 43.Palanivel V, Posey C, Horauf AM, Solbach W, Piessens WF, Harn DA. B-cell outgrowth and ligand-specific production of IL-10 correlate with Th2 dominance in certain parasitic diseases. Exp Parasitol. 1996;84:168–77. doi: 10.1006/expr.1996.0102. [DOI] [PubMed] [Google Scholar]
- 44.Velupillai P, Harn DA. Oligosaccharide-specific induction of interleukin 10 production by B220+ cells from schistosome-infected mice: a mechanism for regulation of CD4+ T-cell subsets. Proc Natl Acad Sci USA. 1994;91:18–22. doi: 10.1073/pnas.91.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–38. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 46.O'Garra A, Chang R, Go N, Hastings R, Haughton G, Howard M. Ly-1 B (B-1) cells are the main source of B cell-derived interleukin 10. Eur J Immunol. 1992;22:711–7. doi: 10.1002/eji.1830220314. [DOI] [PubMed] [Google Scholar]
- 47.O'Garra A, Howard M. Cytokines and Ly-1 (B1) B cells. Int Rev Immunol. 1992;8:219–34. doi: 10.3109/08830189209055575. [DOI] [PubMed] [Google Scholar]
- 48.Fagarasan S, Honjo T. T-independent immune response: new aspects of B cell biology. Science. 2000;290:89–92. doi: 10.1126/science.290.5489.89. [DOI] [PubMed] [Google Scholar]
- 49.Bunn-Moreno MM, Madeira ED, Miller K, Menezes JA, Campos-Neto A. Hypergammaglobulinaemia in Leishmania donovani infected hamsters: possible association with a polyclonal activator of B cells and with suppression of T cell function. Clin Exp Immunol. 1985;59:427–34. [PMC free article] [PubMed] [Google Scholar]
- 50.Lohoff M, Matzner C, Rollinghoff M. Polyclonal B-cell stimulation by L3T4+ T cells in experimental leishmaniasis. Infect Immun. 1988;56:2120–4. doi: 10.1128/iai.56.8.2120-2124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Olivier M, Gregory DJ, Forget G. Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin Microbiol Rev. 2005;18:293–305. doi: 10.1128/CMR.18.2.293-305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grimaldi G, Jr, Tesh RB. Leishmaniases of the New World: current concepts and implications for future research. Clin Microbiol Rev. 1993;6:230–50. doi: 10.1128/cmr.6.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Da Silva AC, Espinoza AG, Taibi A, Ouaissi A, Minoprio P. A 24,000 MW Trypanosoma cruzi antigen is a B-cell activator. Immunology. 1998;94:189–96. doi: 10.1046/j.1365-2567.1998.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cordeiro-da-Silva A, Cardoso L, Araujo N, et al. Identification of antibodies to Leishmania silent information regulatory 2 (SIR2) protein homologue during canine natural infections: pathological implications. Immunol Lett. 2003;86:155–62. doi: 10.1016/s0165-2478(03)00020-8. [DOI] [PubMed] [Google Scholar]
- 55.Bjorklund M, Coutinho A. Isotype commitment in the in vivo immune responses. II. Polyclonal plaque-forming cell responses to lipopolysaccharide in the spleen and bone marrow. Eur J Immunol. 1983;13:44–50. doi: 10.1002/eji.1830130111. [DOI] [PubMed] [Google Scholar]
- 56.Castro H, Sousa C, Santos M, Cordeiro-da-Silva A, Flohe L, Tomas AM. Complementary antioxidant defense by cytoplasmic and mitochondrial peroxiredoxins in Leishmania infantum. Free Radic Biol Med. 2002;33:1552–62. doi: 10.1016/s0891-5849(02)01089-4. [DOI] [PubMed] [Google Scholar]
- 57.Santarem N, Tomas A, Ouaissi A, et al. Antibodies against a Leishmania infantum peroxiredoxin as a possible marker for diagnosis of visceral leishmaniasis and for monitoring the efficacy of treatment. Immunol Lett. 2005;101:18–23. doi: 10.1016/j.imlet.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 58.Brittingham A, Chen G, McGwire BS, Chang KP, Mosser DM. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infect Immun. 1999;67:4477–84. doi: 10.1128/iai.67.9.4477-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rittig MG, Bogdan C. Leishmania–host-cell interaction: complexities and alternative views. Parasitol Today. 2000;16:292–7. doi: 10.1016/s0169-4758(00)01692-6. [DOI] [PubMed] [Google Scholar]
- 60.Stober CB, Lange UG, Roberts MT, Alcami A, Blackwell JM. Heterologous priming-boosting with DNA and modified vaccinia virus Ankara expressing tryparedoxin peroxidase promotes long-term memory against Leishmania major in susceptible BALB/c mice. Infect Immun. 2007;75:852–60. doi: 10.1128/IAI.01490-06. [DOI] [PMC free article] [PubMed] [Google Scholar]