Abstract
We earlier demonstrated that nitric oxide (NO) is a fungicidal molecule against Sporothrix schenckii in vitro. In the present study we used mice deficient in inducible nitric oxide synthase (iNOS–/–) and C57BL/6 wild-type (WT) mice treated with Nω-nitro-arginine (Nitro-Arg-treated mice), an NOS inhibitor, both defective in the production of reactive nitrogen intermediates, to investigate the role of endogenous NO during systemic sporotrichosis. When inoculated with yeast cells of S. schenckii, WT mice presented T-cell suppression and high tissue fungal dissemination, succumbing to infection. Furthermore, susceptibility of mice seems to be related to apoptosis and high interleukin-10 and tumour necrosis factor-α production by spleen cells. In addition, fungicidal activity and NO production by interferon-γ (IFN-γ) and lipopolysaccharide-activated macrophages from WT mice were abolished after fungal infection. Strikingly, iNOS–/– and Nitro-Arg-treated mice presented fungal resistance, controlling fungal load in tissues and restoring T-cell activity, as well as producing high amounts of IFN-γ Interestingly, macrophages from these groups of mice presented fungicidal activity after in vitro stimulation with higher doses of IFN-γ. Herein, these results suggest that although NO was an essential mediator to the in vitro killing of S. schenckii by macrophages, the activation of NO system in vivo contributes to the immunosuppression and cytokine balance during early phases of infection with S. schenckii.
Keywords: fungus, nitric oxide, virulence, macrophages, phagocytosis, killing activity
Introduction
Sporotrichosis is a chronic infection of humans and animals caused by the dimorphic saprophytic fungus Sporothrix schenckii. If left untreated, the infection may spread along lymphatic channels, causing a chronic, slowly progressive lymphocutaneous reaction. The risk factors to sporotrichosis exist both to a healthy host and to an immunosuppressed one, although immunosuppression predisposes to a more severe form of sporotrichosis. However, the extent of the disease varies with host immune status.1,2 The fact that sporotrichosis is more severe and usually disseminated in nude mice3 and in patients with acquired immune deficiency syndrome4–6 suggests that T-cell mediated immunity is important in limiting the extent of infection.
We had previously characterized a model of systemic sporotrichosis in which, depending on the time of fungal cultivation, a severe or a chronic form of the disease could be induced in different mice strains, using a single fungal strain.7 Later, we described that nitric oxide (NO) is essential to fungal killing by interferon-γ (IFN-γ) and lipopolysaccharide (LPS)-activated macrophages. The fungicidal activity of macrophages was more effective against the less virulent conidia compared to the more virulent fungal forms.8 However, the mechanisms that determine the modulation of fungal virulence in vivo remain poorly understood.
The role of reactive nitrogen intermediates (RNIs) in the immune system is extremely varied.9 To date, there is no doubt that NO is an integral part of important immunological signaling pathways, regulating cytokines responses, T-cell responsiveness, cell survival and contributing to tissue damage as seen in some forms of parasitic infections.10–12 Despite the generation of RNI by phagocytes being thought to be critical to fungal killing, it has been described that other antifungal factors either compensate or are sufficient for the killing of phagocytosed pathogens. Among these alternative fungicidal pathways is the IFN-γ-induced antimicrobial effects mediated by degradation of l-tryptophan by indoleamine 2,3-dioxygenase (IDO).13,14
In the present study we have investigated whether inhibition of NO production in vivo alters the susceptibility of mice to the infection with S. schenckii yeast cells. We report here that inhibition of NOS system in vivo enhances the resistance of mice to S. schenckii in early phases of infection, which is related mainly with T-cell function and cytokine balance between IFN-γ and interleukin-10 (IL-10). The findings should have important implications in our understanding of the role of NO in immunosuppression induced by S. schenckii.
Materials and methods
Culture medium and reagents
Brain heart infusion (BHI) and agar were purchased from Oxoid Reagents (Cambridge, UK). RPMI-1640 medium; HEPES; streptomycin; penicillin, lipopolysaccharide (LPS; Escherichia coli serotype O26:B6); thyoglicollate, 2-β-mercaptoethanol (2-ME); l-glutamine; sodium pyruvate; non-essential amino acids; sodium nitrite ( ); Nω-mono-methyl-l-arginine (L-NMMA); Nω-nitro-l-arginine (Nitro-Arg); concanavalin A (Con A); Tween-20; bovine serum albumin (BSA) and O-phenylenediamine dihydrochloride (OPD) were purchased from Sigma Chemical Co (St Louis, MO). Fetal bovine serum (FBS) was purchased from Cultilab (Sao Paulo, Brazil). [3H]-thymidine (3HTd) was purchased from Amersham Co. (Arlington Heights, IL). Recombinant murine interferon-gamma (IFN-γ) was obtained from Genzyme (San Diego, CA). To perform enzyme-linked immunosorbent assay (ELISA), all specific recombinant cytokines and antibodies were purchased from BD Pharmingen (San Diego, CA). Avidin–horseradish peroxidase (HRP) complex was purchased from Vector (Burlingame, CA).
); Nω-mono-methyl-l-arginine (L-NMMA); Nω-nitro-l-arginine (Nitro-Arg); concanavalin A (Con A); Tween-20; bovine serum albumin (BSA) and O-phenylenediamine dihydrochloride (OPD) were purchased from Sigma Chemical Co (St Louis, MO). Fetal bovine serum (FBS) was purchased from Cultilab (Sao Paulo, Brazil). [3H]-thymidine (3HTd) was purchased from Amersham Co. (Arlington Heights, IL). Recombinant murine interferon-gamma (IFN-γ) was obtained from Genzyme (San Diego, CA). To perform enzyme-linked immunosorbent assay (ELISA), all specific recombinant cytokines and antibodies were purchased from BD Pharmingen (San Diego, CA). Avidin–horseradish peroxidase (HRP) complex was purchased from Vector (Burlingame, CA).
Animals
Five- to 8-week-old male C57BL/6 wild type (WT) and iNOS knockout (iNOS–/–) mice were maintained in the animal house of the Division of Immunology, School of Medicine of Ribeirão Preto (University of São Paulo, Sao Paulo, Brazil) under specific-pathogen-free conditions. iNOS–/– mice were obtained from Jackson Laboratories (Bar Harbor, ME). All procedures were approved by our Institutional Ethics Committee and are in accordance with the National Institutes of Health Animal Care Guidelines.
Fungal cells and experimental infection
The virulent Sporothrix schenckii, strain 1099-18 (Columbia University, New York, USA) was used throughout this study. A yeast-like form of S. schenckii was cultured at 37° for 7 days in BHI medium, as described earlier.7 Mice were infected intravenously with 5 × 106 yeast cells suspended in 0·2 ml of sterile phosphate-buffered saline (PBS). The viability of the S. schenckii inocula were ascertained by colony-forming units (CFU) counts after 7 days of incubation in BHI-agar plates at 37°. Spleen cells, peritoneal macrophages, and serum were collected at appropriate times after infection. Survival of mice was observed for up to 30 days after infection.
Treatment of animals with Nitro-Arg
Infected mice were treated daily with an intraperitoneal administration of PBS (pH 7·4) or with the specific inhibitor of NO synthase, Nitro-Arg at a dose of 50 mg/kg according to body weight.
Tissue fungal burden
The fungal burden in tissues of infected mice was determined by quantitative counts of CFU on the 14th day of infection. The lungs and spleen from iNOS–/– and WT infected mice, which had or had not been treated with Nitro-Arg, were weighed and homogenized in 2 ml of cold sterile PBS. The suspension was adjusted to 10 mg of tissue/ml and aliquots of 100 µl of each homogenate were plated onto BHI-agar containing antibiotics. The number of colonies per plate was counted after the plates had been incubated for 7 days at 37°. Values were expressed as the number of viable S. schenckii per gram of tissue.
RNA extraction and real time reverse transcription–polymerase chain reaction (qRT–PCR)
Peritoneal cells were collected from WT and iNOS–/– thioglycollate-elicited mice, added to six-well plates, at a cell density of 1 × 107 cells/ml, activated with IFN-γ (20 U/ml) and LPS (20 ng/ml) for 6 hr at 37° in an atmosphere of 5% CO2 in complete medium (RPMI-1640 containing 10% heat-inactivated FBS and 100 U/ml penicillin, 100 µg/ml streptomycin). After this time, total RNA was extracted using 1 ml of Trizol reagent according to manufacturer's recommendations (Life Technologies, Inc, Gaithersburg, MD). The cDNA was synthesized using Superscript II reverse transcriptase according to the supplier's specifications (Life Technologies, Inc). Briefly, the qRT–PCR assay was carried out as described earlier.15 The pairs of primer sequences used were as follows: β-actin forward GTGGGCCGCTCTAGGCACCAA, reverse CTCTTTGATGTCACGCACGATTTC, which results in a 550 base-pair (bp) amplification product. For iNOS forward TGGGAATGGAGACTGTCCCAG, reverse GGGATCTGAATGTGATGTTTG, which results in a 306 base-pair (bp) amplification product. Reaction conditions were 35 cycles, for β-actin primers, and 40 cycles, for iNOS primers, of 1 min at 94°, 1 minute at 54°, and 2 min at 72°. For each set of primers, a negative sample (water) was run in parallel. The results were determined as number of copies relative to β-actin expression.
Measurement of serum nitrite
The concentration of nitrite ( ) in culture supernatants of peritoneal macrophages and spleen cells were measured by a microplate Griess assay as described previously.16 Briefly, 100 µl of the samples were incubated with an equal volume of the Griess reagent at room temperature. The A550 was determined with an ELISA reader (Molecular Devices Co, Sunnyvale, CA). The
) in culture supernatants of peritoneal macrophages and spleen cells were measured by a microplate Griess assay as described previously.16 Briefly, 100 µl of the samples were incubated with an equal volume of the Griess reagent at room temperature. The A550 was determined with an ELISA reader (Molecular Devices Co, Sunnyvale, CA). The  concentration was determined using a standard curve of 1–200 µm.
concentration was determined using a standard curve of 1–200 µm.
Measurement of serum nitrate
The serum nitrate (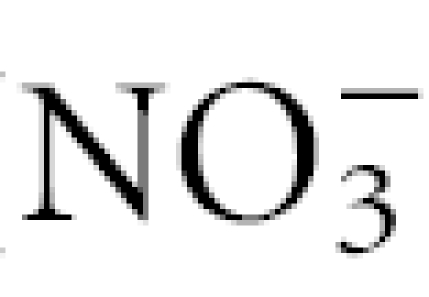 ) concentration was determined by enzymatically reducing nitrate to nitrite with nitrate reductase, as described previously;17 the total amount of nitrite was then determined using the Griess method. The results are reported as µm of
) concentration was determined by enzymatically reducing nitrate to nitrite with nitrate reductase, as described previously;17 the total amount of nitrite was then determined using the Griess method. The results are reported as µm of 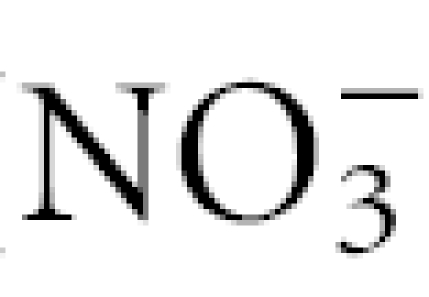 .
.
Macrophages cultures and fungal killing assay
Nitrite production and fungicidal activity of murine peritoneal macrophages were evaluated, as described earlier.8 On the 14th day of infection the peritoneal cavity was washed with 5 ml of cold Hanks balanced salt solution (HBSS). The resulting suspension was pelleted at 4° by centrifugation for 10 min at 300 g, and the supernatant was removed. The cells were suspended in complete RPMI-1640 and added onto 24-well plates at a cell density of 106 cells/ml, incubated for 30 min at 37° in an atmosphere of 5% CO2 and washed three times with prewarmed medium to remove any adherent cells. Macrophages were suspended in fresh complete RPMI and then activated with IFN-γ (20–60 U/ml) plus LPS (20 ng/ml) for 4 hr, then yeast cells of S. schenckii (105/ml) were added to the cultures and incubated for 18 h at 37° in an atmosphere of 5% CO2. Some wells were filled with medium alone and inoculated with the fungus as a control for the fungal growth. Cell-free supernatants were collected and stored at −70° for nitrite determination. Cells were then lysed with sterile distilled water, wells were scrapped and cell lysates were diluted with sterile PBS. To determine fungal viability, aliquots were plated on BHI-agar containing antibiotics, and CFU was determined as described above. The macrophage fungicidal activity was evaluated as the percentage of killing, calculated as: [(1 − CFU samples)/CFU control] × 100.
Spleen cell proliferation assay
The T-cell proliferation induced by Con A or fungal antigen (heat-killed yeast cells from S. schenckii) was evaluated by [3H]-thymidine ([3H]TdR) incorporation. Spleens of non-infected and infected WT and iNOS–/– mice were harvested after 14 days of infection. Briefly, spleen cells were disrupted in HBSS, centrifuged, washed twice, and erythrocytes were lysed in a lysing buffer (1 part of 0·17 mol/l Tris and 9 parts of 0·16 mol/l ammonium chloride, pH 7·5) for 2 min. The erythrocyte-free cells were washed three times in HBSS, counted and added onto 96-well plates at a cell density of 5 × 106 cells/ml in RPMI-1640 that had been supplemented with 2 mm l-glutamine, 1 mm sodium pyruvate, 5% non-essential amino acids, 50 µm 2-ME, 100 µg/ml streptomycin, 100 U/ml penicillin, and 5% FBS. The cells were subsequently stimulated with 2 µg/ml Con A or fungal antigen at a 10 : 1 ratio of yeast cells per spleen cell at 37° in an atmosphere of 5% CO2 for 36 hr. The experiments were performed in five duplicates at a final volume of 200 µl/well. Lymphocyte proliferation was determined by liquid scintillation counting after the addition of 0·5 µCi/well [3H]-TdR in the last 18 hr of incubation. The culture supernatants were collected after 12 hr to evaluate the accumulation of  by the Griess method.
by the Griess method.
Cytokine determination by ELISA
Spleen cells at a cell density of 2 × 106 cells/ml) were cultured in 24-well plates with 2 µg/ml Con A or fungal antigen at a 10 : 1 ratio of yeast cell per spleen cell or medium alone at 37° in an atmosphere of 5% CO2 for 48 hr. The supernatants were collected and stored at −70° until assayed for IFN-γ, tumour necrosis factor-α (TNF-α) and IL-10, using a two-sandwich ELISA. Flat-bottomed, 96-well microdilution plates (Nalge Nunc Int., Rochester, NY) were coated with 50 µl of a capture monoclonal anti-mouse IFN-γ (0·5 µg/ml) or IL-10 (2 µg/ml) or the monoclonal rat anti-mouse TNF-α (XT22.11) (2 µg/ml), incubated overnight at 4°, and then washed four times with PBS containing 0·1% Tween-20. The wells were filled with a blocking buffer consisting of PBS plus 0·1% Tween-20 and 1% BSA, incubated for 1 hr at 4° and then washed four times with PBS–Tween. Samples were diluted in blocking buffer and 50 µl were added to each well. The plates were incubated overnight at 4°. The wells were washed four times with PBS–Tween, and 100 µl of a monoclonal biotin-conjugated anti-mouse IL-10 (1 µg/ml) or anti-IFN-γ (1 µg/ml) or the polyclonal antibodies from sheep anti-mouse TNF-α serum (H/92) (1 : 1000) in blocking buffer were added to each well. The plates were incubated for 1 hr at room temperature. After four washes with PBS–Tween, the reaction products were detected with an avidin–HRP complex, and the colour of the reaction was developed with OPD. The A492 was determined with an ELISA reader (Molecular Devices Co, Sunnyvale, CA). Cytokine concentration [pg/ml] was calculated from a standard curve of murine recombinant cytokines.
Apoptosis measurement
To evaluate populations of cells undergoing apoptosis in WT and iNOS–/– infected mice, we performed a flow cytometry (FCM) analysis of spleen cells using ApoScreen™ Annexin V Apoptosis Kit (Southern Biotech Inc, Birmingham, AL), as per the manufacturer's instructions. Briefly, disrupted spleen cells were washed twice in cold PBS, suspended in cold binding buffer at a cell density of 1 × 107 cells/ml into 12- by 75-mm polypropylene tubes (Becton Dickinson, San Jose, CA). Aliquots of 100 µl were incubated in ice for 15 min in the presence or not of Annexin V–fluoroscein isothiocyanate (FITC) protected from light. Without washing, 380 µl of cold binding buffer was added to each tube. Necrotic cells were evaluated by staining with 1 µg/ml propidium iodide (PI). Fluorescence of exposed cell surface phospholipid phosphatidylserine labelled with Annexin V–FITC and individual nuclei labelled with PI was measured in a fluorescence-activated cell sorting flow cytometer (Becton Dickinson). Percentile of apoptosis was determined by gating cells to exclude debris and necrotic cells and analysing Annexin V-labelled mice populations.
Statistical analysis
The results are expressed as the mean ± SEM of the indicated number of animals or experiments. Statistical analysis was performed using anova followed by Bonferronis's t-test and differences were considered significant when P < 0·05. Cumulative mortality curves were compared by log rank test.
Results
Fate of yeast cell-infected iNOS–/– mice: increase of survival, control of tissue fungal dissemination and abrogation of T-cell suppression
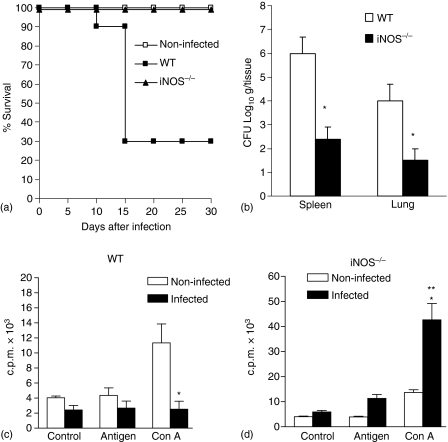
To determine the role of endogenous NO in the resistance to infection with S. schenckii, we infected iNOS knockout mice (iNOS–/–) with yeast cells and their survival was compared with that of littermate controls. As described earlier by Fernandes et al.8, yeast cell-infected WT mice presented a sharp decrease from 90% in survival rate on the 10th day of infection, decreasing to 30% on the 15th day, this was maintained until the 30th day of infection. Surprisingly, infected iNOS–/– mice were totally resistant to fungal infection (Fig. 1a). The absence of iNOS gene RNA transcripts in LPS and IFN-γ activated macrophages was confirmed in the iNOS–/– knockout mice genotype by real-time qRT–PCR, because amplification of iNOS was not detected. Furthermore, infected WT mice presented an increase in serum nitrate levels during the first 14 days of infection, being 15 times higher than in controls 7 days after infection (255 ± 53 and 17 ± 6·7 µm, n = 6, respectively), continuing to increase until the 14th day (360 ± 59 and 24 ± 9·6 µm, n = 6, respectively). On the 14th day of infection, the CFU counts in spleen and lung from infected WT mice were significantly higher when compared to iNOS–/– mice (Fig. 1b). Moreover, spleen cells of WT mice at 14th day of infection proliferated less in response to Con A than cells of non-infected mice (Fig. 1c). Besides this, these cells presented impairment in NO production after Con A or antigenic stimuli, when compared to non-infected ones (data not shown). Interestingly, the T-cell unresponsiveness observed in infected WT mice was abrogated in infected iNOS–/– mice, which presented a vigorous Con A-induced T-cell proliferation, when compared to infected WT mice (Fig. 1d). In our experimental conditions, the antigen-induced proliferative response did not achieve significant levels after infection with S. schenckii.
Figure 1.
Absence of iNOS leads to increased resistance and T cell responsiveness after infection with yeast cells. (a) Survival of WT mice (n = 30) and iNOS–/– mice (n = 10) following i.v. infection with 5 × 106 yeast cells. Deficient mice had an increased survival rate compared with wild-type controls (*, P < 0·05, log rank test). These data are representative of two independent experiments. (b) CFU counts of S. schenckii in organs of WT and iNOS–/– mice at 14th day of infection are expressed as means of CFU counts per g/tissue ± SD for groups of five animals each. P < 0·05 (ANOVA followed by Bonferroni's t-test) when compared to WT mice (*). (c) At 14th day of infection, spleen cells from WT (c) and iNOS–/– (d) mice were harvested and cultured with medium alone, fungal antigen or Con A for 36 hr as described in Methods. The values represent the mean ± SD of three experiments with groups of three mice for each one. P < 0·05 (ANOVA followed by Bonferroni's t-test) when compared to non-infected mice (*) or to WT infected mice (**).
Absence of NOS2 gene prevents apoptosis induced by yeast cell infection
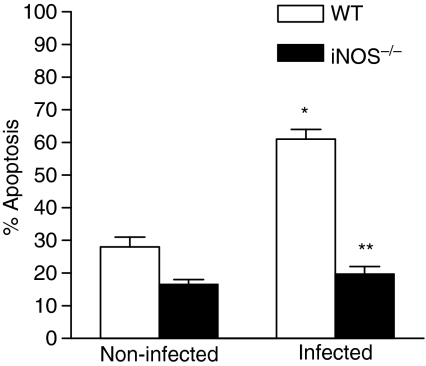
To evaluate whether T-cell proliferative response of S. schenckii-infected mice was related to apoptosis index, spleen cells were harvested from of WT and iNOS–/– mice after 14 days of infection with yeast cells, and apoptosis levels were evaluated. The results showed that 60% of cells from infected WT mice were apoptotic, whereas the percentage of iNOS–/– apoptotic cells was 20%(Fig. 2), being not significantly different from non-infected mice.
Figure 2.
Absence of the iNOS gene reduces apoptosis induced by yeast cells in murine spleen cells. At 14th day of infection, percent apoptosis was determined by measuring Annexin V labelling of freshly explanted spleen cells from WT and iNOS–/– mice. P < 0·05 (ANOVA followed by Bonferroni's t-test) when compared to non-infected mice (*).
Different balance in cytokine production of spleen cells from infected WT and iNOS–/– mice
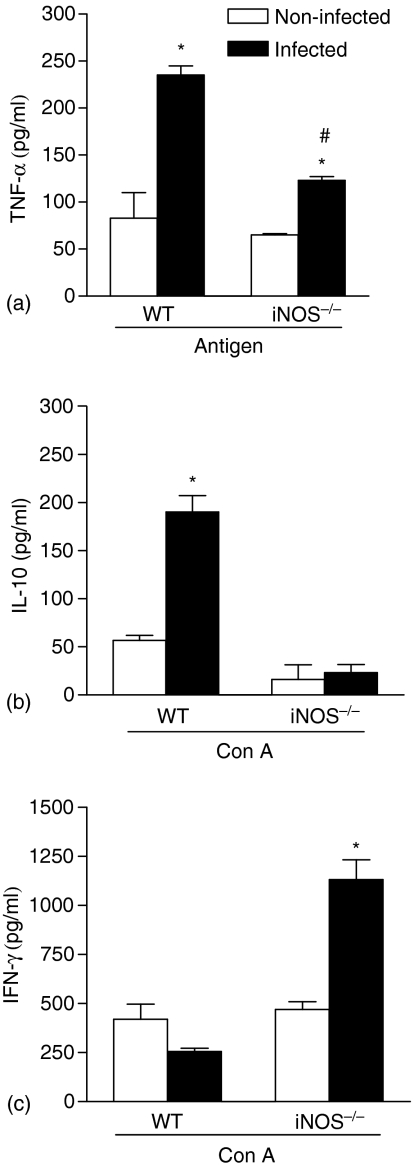
At 14th day of infection, the production of IL-10, IFN-γ and TNF-α were determined in supernatants of spleen cell cultures stimulated for 48 hr with fungal antigen or Con A. Figure 3(a) shows that infected WT mice produced significantly higher TNF-α levels after fungal antigen stimulus when compared to non-infected mice, reaching values of 230 ± 10 ng/ml (three times higher than controls). On the other hand, antigenic stimulus of spleen cell cultures from infected iNOS–/– mice induced just a moderate increase in TNF-α levels. Furthermore, we found that the higher yeast cell susceptibility was also related to a differential balance between IL-10 and IFN-γ production. While the susceptibility of infected WT mice was related to a vigorous IL-10 production by Con A-stimulated spleen cells (Fig. 3b), the resistance of infected iNOS–/– mice was related to higher IFN-γ production (Fig. 3c).
Figure 3.
Different cytokine production by spleen cells of WT and iNOS–/– infected mice. At 14th day of infection, TNF-α (a), IL-10 (b) and IFN-γ (c) production were determined in culture supernatants of spleen cells from WT and iNOS–/– mice cultured with fungal antigen (a) or Con A (b and c) by 48 hr. Values represent the mean ± SD of three mice in one experiment representative of three performed separately. P < 0·05 (ANOVA followed by Bonferroni's t-test) when compared to non-infected mice (*) or to WT infected mice (#).
Exacerbation of IL-10 and TNF-α production may account for failure in fungicidal activity of macrophages
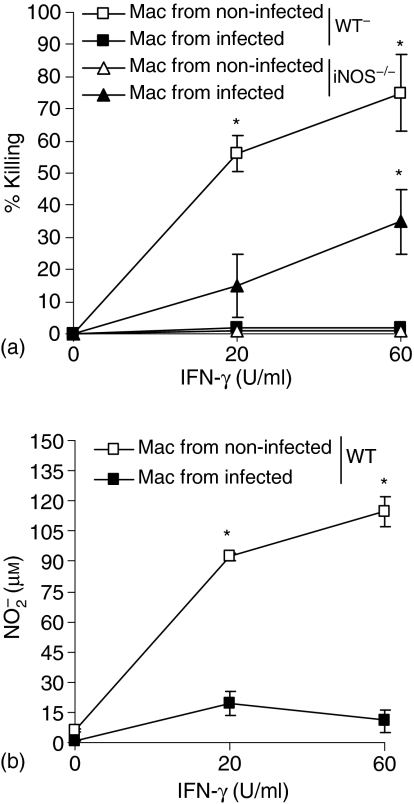
The IL-10 and TNF-α production by WT and iNOS–/– 18 hr-cultured peritoneal macrophages were investigated after infection with S. schenckii. Interestingly, as shown in Table 1, macrophages from infected WT mice spontaneously released significant IL-10 amounts (903·6 ± 43 pg/ml), when compared to the non-infected ones (224·2 ± 42·3 pg/ml). This increase significantly strengthened after antigen stimulation of infected WT macrophages, reaching values of 1266 ± 217·7 pg/ml when compared to non-infected mice (300 ± 7·2 pg/ml). On the other hand, macrophages isolated from infected iNOS–/– mice presented reduced spontaneous and antigen stimulated IL-10 production when compared to the non-infected ones (257·7 ± 1·2 pg/ml and 86·65 ± 14·4 pg/ml or 282·8 ± 11·9 pg/ml and 140·5 ± 1·15 pg/ml, respectively). Neither infected WT nor infected iNOS–/– macrophages presented an increase in spontaneous TNF-α production in relation to non-infected mice. However, WT and iNOS–/– infected macrophages were able to produce significantly higher TNF-α amounts after antigen stimulation when compared to the non-infected one, there being significantly higher levels in supernatants of infected WT mice when compared to infected iNOS–/– mice (1349 ± 19·8 pg/ml and 535·2 ± 5·5 pg/ml, respectively). In order to investigate whether host susceptibility to S. schenckii was related to fungicidal activity of macrophages, we determined the fungal growth in cultures of peritoneal macrophages from infected WT and iNOS–/– mice after activation with increasing doses of IFN-γ and LPS at an earlier established dose (20 ng/ml). As previously described by Fernandes et al.8, fungicidal activity of IFN-γ and LPS-activated murine macrophages is dependent on NO production. Again, WT macrophages were able to kill yeast cells of S. schenckii (Fig. 4a) and to produce nitrite (Fig. 4b) after IFN-γ and LPS activation when compared to unstimulated macrophages (values at 20 U/ml and values at 60 U/ml IFN-γ). After 14 days of infection, the fungicidal activity and nitrite production of WT macrophages were abrogated. Interestingly, despite the absence of nitrite production by iNOS–/– mice (undetected levels), it was observed that iNOS–/–-infected macrophages presented a significant increase in fungicidal activity after activation with higher doses of IFN-γ (60 U/ml) when compared to the non-infected ones (Fig. 4a).
Table 1.
IL-10 and TNF-α production by peritoneal macrophages isolated from yeast cell-infected WT and iNOS–/– mice
| Macrophage treatment | IL-10 (pg/ml) | TNF-α (pg/ml) |
|---|---|---|
| No antigen added | ||
| Non-infected WT1 | 224·2 ± 29·9 | 26·9 ± 0·07 |
| Infected WT | 903·6 ± 302 | 56·9 ±46·42 |
| Non-infected iNOS–/– | 257·7 ± 1·2 | N.D. |
| Infected iNOS–/– | 86·65 ± 14·4 2,3 | N.D. |
| Antigen added | ||
| Non-infected WT | 300 ± 7·2 | 587·3 ± 37·25 |
| Infected WT | 1,266 ± 217·72 | 1,349 ± 19·82 |
| Non-infected iNOS–/– | 282·8 ± 11·9 | 535·2 ± 5·5 |
| Infected iNOS–/– | 140·5 ± 1·152,3 | 793·3 ± 72·22,3 |
Peritoneal cells were harvested from WT and iNOS–/– mice infected or not with yeast cells for 14 days. Macrophages were incubated for 18 hr in the presence or absence of antigen (heat-killed yeast cells) and cytokine production was determined in supernatants by ELISA as described in Methods.
P < 0·05 compared to noninfected mice and
P < 0·05 when compared to WT infected mice.
Figure 4.
Fungicidal activity of macrophages in the absence of iNOS gene is related to IFN-γ induced IDO activity. Peritoneal cells were harvested from WT and iNOS–/– mice infected (closed symbols) or not (open symbols) with yeast cells for 14 days. Macrophages were activated or not with different doses of IFN-γ and LPS (20 ng/ml) for 4 hr before addition of yeast cells. The percentage of killing (a) and nitrite concentration (b) in supernatants were determined after 18 hr of culture as described in Material and methods. No detected amounts of nitrite accumulation in supernatant of iNOS–/– mice were detected. *P < 0·05 when compared to non-infected WT mice (ANOVA followed by Bonferroni's t-test).
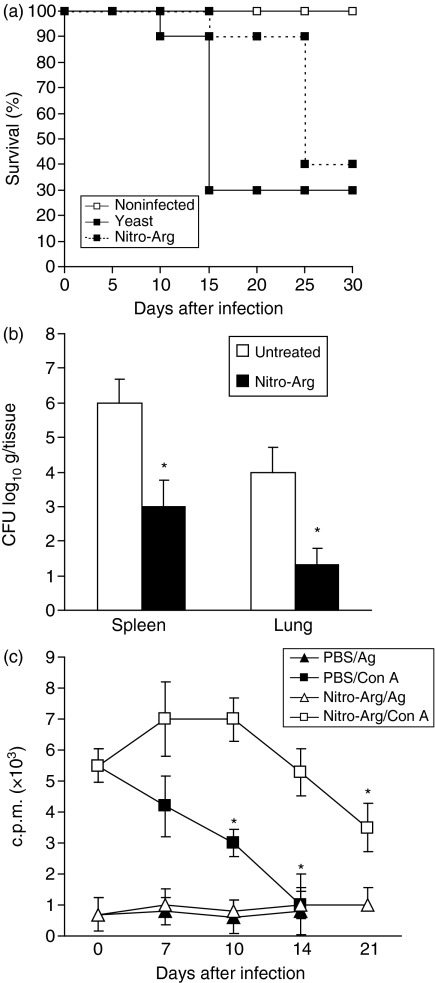
Effect of daily treatment of infected mice with Nitro-Arg on yeast cell-induced mortality, tissue fungal dissemination and T-cell proliferative responses
To confirm the hypothesis that endogenous NO production is determined by modulation of host defence against infection with yeast cells, we treated WT infected mice with Nitro-Arg, an inhibitor of NO production. Figure 5 shows the survival curves (Fig. 5a), fungal load in tissues (Fig. 5b) and T-cell responsiveness (Fig. 5c) of WT mice infected with 5 × 106 yeast cells of S. schenckii and treated daily with Nitro-Arg. As shown in Fig. 1, the increase of mortality of WT infected mice was followed by an increase in CFU counts in tissues and by a progressive decrease in spleen cell proliferation. The treatment in vivo with Nitro-Arg promoted a delay in mortality (Fig. 5a) in the acute phase of fungal infection (until 25th day), which was accompanied by a decrease in tissue CFU counts (Fig. 5b) and also prevented T-cell unresponsiveness to Con A (Fig. 5c), when compared to untreated ones. At late phases of infection, T cells were again unresponsive to Con A stimulus and mice succumbed to infection, despite Nitro-Arg treatment. As shown in Table 2, the serum nitrate levels have hugely increased in yeast cell-infected mice when compared with non-infected mice, decreasing significantly after nitro-Arg treatment (17 ± 6·7 µm, 255 ± 53 µm and 27 ± 9·5 µm at the 7th day, respectively, n = 6) (24 ± 9·6 µm, 360 ± 59 µm and 37·7 ± 10·2 µm at 14th day, respectively, n = 6) and (15 ± 9·7 µm, not detected and 33·6 ± 15 µm at 21st day, respectively, n = 6). Herein, it was confirmed that endogenous NO production was immunosuppressant after fungal infection, once the addition of L-NMMA to spleen cell cultures did not change the immunosuppressive response (data not shown).
Figure 5.
Treatment of S. schenckii-infected mice with a NO inhibitor also prevents T-cell unresponsiveness and improves fungal disease. (a) Survival of WT mice (n = 30) following i.v. infection with 5 × 106 yeast cells. One group of mice (n = 30) was treated daily with 50 mg/kg of Nitro-Arg to abrogate NO production in vivo. Curves of mortality rate were followed for 30 days after infection. All groups of non-infected mice were inoculated with PBS and presented no mortality and no CFU counts throughout the time. Similar results were obtained in a second experiment. Treated mice had an increased mortality compared with WT controls (*, P < 0·05, log rank test). (b) CFU counts in organs of untreated and Nitro-Arg-treated mice at 14th day of infection are expressed as means of CFU counts per g/tissue ± SD for groups of five animals each. P < 0·05 (a, log rank test; b and c, ANOVA followed by Bonferroni's t-test), when compared to untreated mice (*). (c) At the 0, 7th, 10th, 14th, and 21st day of infection, spleen cells were harvested from WT mice that were treated with PBS or Nitro-Arg. Cultures were incubated for 36 hr in the presence of fungal antigen or Con A as described in Methods. Lymphocyte proliferation was determined by [3H]-thymidine (3HTdR) incorporation during the last 12 hr. Spleen cells incubated with medium alone presented background 3HTdr uptakes of 600 ± 205 and 800 ± 105. P < 0·05 (a, log rank test; b and c, ANOVA followed by Bonferroni's t-test), when compared to non-infected mice (*).
Table 2.
Serum nitrate levels of mice infected with of S. schenckii treated or not with NOS inhibitor
| Infection/treatment |
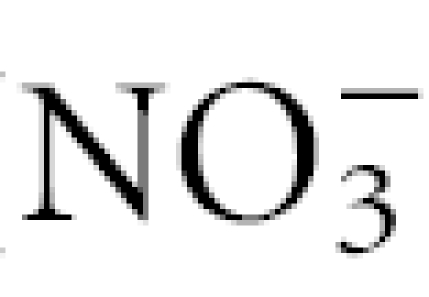 (μm)1 7th day (μm)1 7th day |
14th day | 21st day |
|---|---|---|---|
| Non-infected/– | 17 ± 6·7 | 24 ± 9·6 | 15 ± 9·7 |
| Non-infected/N-Arg | 12 ± 9·1 | 13 ± 8·7 | 12 ± 8·6 |
| Yeast infected/– | 255 ± 531 | 360 ± 59* | – |
| Yeast infected/N-Arg | 27 ± 9·5 | 37·7 ± 10·2 | 33·6 ± 15 |
Data are represented as nitrate (μm). Nitrate levels were assayed on 7th, 14th and 21st day after infection with S. schenckii. Animals were daily treated with 50 mg/kg of N-Arg.
P < 0·05 compared to non-infected mice.
Discussion
It was demonstrated that CD4+ T-cell, macrophages and IFN-γ are essential to acquired protective immunity against S. schenckii infection,18 and that NO is essential to macrophage fungicidal activity against S. schenckii.8 Despite of the host protective role of NO as fungicidal molecule, we prompted the current investigation of the contribution of NO produced endogenously to immune response against S. schenckii yeast cells, using animals treated with NOS inhibitor and animals genetically deficient in iNOS activity (iNOS–/– mice).
In the present study, we observed that C57/BL6 (WT) mice infected with yeast cells presented a severe outcome of disease with high mortality and tissue fungal load. The infection was also associated with suppression of T cell activity, once spleen cells from this group of mice were unresponsive to Con A stimulation, when compared to non-infected ones. This deficit of T-cell response had also been described in sporotrichosis in different murine models using the same S. schenckii strain. The authors had shown that spleen cells did not respond to mitogen or to S. schenckii antigenic stimuli in the early phases of infection, which was followed by depressed delayed-type hypersensitivity response in paws, high mortality rates and high fungal dissemination in tissues.19–21 However, the mediators and mechanisms involved in immunosuppression after infection with S. schenckii have not been recognized.
It has been shown that cell wall components of S. schenckii might induce high TNF-α and NO production by murine macrophages and also are able to inhibit fungal phagocytosis.22 Herein our data has shown that spleen cell as well as macrophage cultures from WT infected mice produced huge amounts of TNF-α after antigen stimulation, besides being detected high nitrate serum levels after yeast infection. Therefore, the in vivo dissemination of yeast cells and consequent continuous antigenic stimulation could be important in inducing NO and TNF-α production. In addition, it has been described that detection of high serum TNF-α levels after S. schenckii infection was related with the susceptibility pattern of a genetically selected mice strain, whereas lower amounts of this cytokine was detected in serum of a resistant mouse strain.23 This is reasonable because shock, tissue injury, apoptosis are all conditions recognizably induced by large amounts of TNF-α released into vascular compartment,24,25 whereas a controlled TNF-α production is essential to a wide spectrum of biological responses such as cell migration, differentiation and granuloma formation at the inflammatory site, thus making animals targeted by the TNF-α receptor gene more susceptible to fungal infections.26,27
Evidence has shown that after an intense activation and expansion, T cells achieve a tolerant or anergic state. It has been demonstrated that cytotoxic lymphocyte antigen-4 (CTLA-4), a T-cell receptor, induces T-cell anergy by inhibition of IL-2 production and cell cycle blockage.28,29 In addition, Fas/Fas-L-induced apoptosis is involved in T-cell suppression, being exacerbated by TNF-α production.30–32 In this context, we observed that CTLA-4, FasL and TNF-α are among the molecules involved in the suppression of T-cell responses after infection with S. schenckii, once antibodies against those molecules were able to restore T-cell proliferation of yeast-infected WT mice (data not shown).
It has been described that nuclear factor-κB activators induce IL-10 synthesis.33 Therefore, early TNF-α amounts detected in yeast cell-infected WT mice could be inducing a huge and spontaneous IL-10 production by peritoneal macrophages. This cytokine has been extensively recognized as a suppressor of pro-inflammatory mediators,34–36 being associated with iNOS inhibition37 and also with antifungal defects in murine macrophages.38 Therefore, the inhibition observed in vitro of NO production by yeast cell-infected macrophages could be resultant of IL-10 production. In addition, IL-10 inhibits T-cell proliferation and IFN-γ and IL-2 production,39 being also involved in immunosuppression in many fungal infections.40–43 Therefore, these results indicate that IL-10 could be directly related to impairment of macrophage fungicidal activity and to T-cell unresponsiveness in WT-infected mice.
Although NO was extensively recognized as a microbicidal molecule, its production can be also detrimental, even lethal, to the host. Indeed, it has been described that Trypanosoma gondii infection of iNOS–/– mice or aminoguanidine (an NOS inhibitor)-treated mice results in delay of death, control of inflammatory response, decreased hepatic degeneration and bowel necrosis when compared to controls.44 Other reports have pointed out to NO as an immunosuppressive molecule in conditions in which the host is in continuous pathogenic stimulus.10,12,45–48 The concept is that the high NO output, as a first innate immunity step against pathogens, invokes a delay in developing acquired immunity through T-cell unresponsiveness.49 In fact, it was known that NO prevent the overexpansion of T helper 1 (Th1) cells relative to Th2 cells.50 Our results have shown that systemic infection of iNOS–/– mice resulted in longer survival, improved fungal clearance in tissues and high T-cell proliferation. Furthermore, iNOS–/– infected mice prevented IL-10 production, besides producing moderate TNF-α and high IFN-γ levels. It has been described that T. cruzi infected iNOS–/– mice produced significantly more IFN-γ than the infected control mice and also presented reduced apoptosis.32
In the present study, fungal clearance observed in yeast cell-infected iNOS–/– mice could involve NO-independent mechanisms elicited by IFN-γ It was known that immunoreactive IFN-γ has been detected in granulomatous skin lesions of sporotrichosis patients,51 and that GKO mice are more susceptible to S. schenckii infection than their parental strain (unpublished data). In addition, the results have shown that macrophages from iNOS–/– mice presented fungicidal activity after activation with high doses of IFN-γ, despite not producing NO. One possible microbicidal mechanism could be the improvement of tryptophan degradation pathway metabolism in phagocytes by activation of indoleamine 2,3-dioxygenase (IDO). It has been well recognized that NO inhibits IDO activity in IFN-γ-primed mononuclear phagocytes,52 and that tryptophan degradation is involved in host resistance to early infection with various parasites.13,53,54 Therefore, improvement of T-cell function and IFN-γ production in iNOS–/– mice could be responsible early in S. schenckii infection for the activation of other fungicidal mechanisms, such as the IDO-mediated tryptophan degradation.
The deleterious role of NO to host defence was confirmed in studies with mice that had received treatment with Nitro-Arg. The treatment improved fungal disease, extending the survival until the 25th day of infection, when mice died abruptly. Indeed, it is well described in literature that inhibition of endogenous NO production is protective to the host in the first phases of infection, however, as NO is also important for the killing of the fungi, the inhibition of this molecule in vivo causes rough disease susceptibility.10
In summary, results of this study suggest that in addition to being a fungicidal molecule, NO may be implicated in immunosuppression in vivo. After infection, yeast cells seem to be resistant to intracellular fungicidal mechanisms of macrophages and promptly disseminate into the tissues. The severe infection promotes release of pro-inflammatory mediators, including TNF-α and NO and a subsequent induction of molecules, such as IL-10, Fas L and CTLA-4, that leads to suppression of T cell response. The transitory resistance of infected mice after Nitro-Arg treatment shows that NO is deleterious just at initial infection, being crucial some time after fungal inoculation. Thus, we suggest that the full resistance of mutant mice to S. schenckii is related to IFN-γ-induced alternative fungicidal pathways in phagocytes. In conclusion, it would appear that the beneficial effects caused by the loss of nitric oxide immunoregulation outweighed the detrimental effects associated with loss of nitric oxide fungicidal activity.
Acknowledgments
The authors are grateful to Giuliana Bertozi Francisco for their technical assistance. This work was supported by CNPq, CAPES, FAPESP, FAPERJ and SR-2 UERJ (Brazil).
References
- 1.Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427–31. doi: 10.1046/j.1365-2230.2002.01087.x. [DOI] [PubMed] [Google Scholar]
- 2.Lopes-Bezerra LM, Schubach A, Costa RO. Sporothrix schenckii and sporotrichosis. Ann Braz Acad Sci. 2006;78:1–16. doi: 10.1590/s0001-37652006000200009. [DOI] [PubMed] [Google Scholar]
- 3.Dickerson CL, Taylor RL, Drutz DJ. Susceptibility of congenitally athymic (nude) mice to sporotrichosis. Infect Immun. 1983;40:417–20. doi: 10.1128/iai.40.1.417-420.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aarestrup FM, Guerra RO, Vieira BJ, Cunha RM. Oral manifestation of sporotrichosis in AIDS patients. Oral Dis. 2001;7:134–6. [PubMed] [Google Scholar]
- 5.Carvalho MT, de Castro AP, Baby C, Werner B, Filus Neto J, Queiroz-Telles F. Disseminated cutaneous sporotrichosis in a patient with AIDS. report of a case. Rev Soc Bras Med Trop. 2002;35:655–9. doi: 10.1590/s0037-86822002000600018. [DOI] [PubMed] [Google Scholar]
- 6.Losman JA, Cavanaugh K. Cases from the Osler Medical Service at Johns Hopkins University. Diagnosis: P. carinii pneumonia and primary pulmonary sporotrichosis. Am J Med. 2004;117:353–6. doi: 10.1016/j.amjmed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes KS, Mathews HL, Lopes Bezerra LM. Differences in virulence of Sporothrix schenckii conidia related to culture conditions and cell-wall components. J Med Microbiol. 1999;48:195–203. doi: 10.1099/00222615-48-2-195. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes KS, Coelho AL, Lopes Bezerra LM, Barja-Fidalgo C. Virulence of Sporothrix schenckii conidia and yeast cells and the susceptibility to nitric oxide. Immunology. 2000;101:563–9. doi: 10.1046/j.1365-2567.2000.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdan C, Rollinghoff M, Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Curr Opin Immunol. 2000;12:64–76. doi: 10.1016/s0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 10.Bocca AL, Hayashi EE, Pinheiro AG, Furnaletto AB, Campanelli AP, Cunha FQ, Figueiredo F. Treatment of Paracoccidioides brasiliensis-infected mice with a nitric oxide inhibitor prevents the failure of cell-mediated immune response. J Immunol. 1998;161:3056–63. [PubMed] [Google Scholar]
- 11.Martins GA, Vieira LQ, Cunha FQ, Silva JS. Gamma interferon modulates CD95 (Fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypanosoma cruzi infection: a possible role in immune response control. Infect Immun. 1999;67:3864–71. doi: 10.1128/iai.67.8.3864-3871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nascimento FR, Calich VL, Rodriguez D, Russo MJ. Dual role for nitric oxide in paracoccidioidomycosis: essential for resistance, but overproduction associated with susceptibility. Immunology. 2002;168:4593–600. doi: 10.4049/jimmunol.168.9.4593. [DOI] [PubMed] [Google Scholar]
- 13.Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, Seishima M. 1-tryptophan-l-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice. cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun. 2002;70:779–86. doi: 10.1128/iai.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balish E, Warner TF, Nicholas PJ, Paulling EE, Westwater C, Schofield DA. Susceptibility of germfree phagocyte oxidase- and nitric oxide synthase 2-deficient mice, defective in the production of reactive metabolites of both oxygen and nitrogen, to mucosal and systemic candidiasis of endogenous origin. Infect Immun. 2005;73:1313–20. doi: 10.1128/IAI.73.3.1313-1320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garlet GP, Cardoso CR, Silva TA, Ferreira BR, Avila-Campos MJ, Cunha FQ, Silva JS. Cytokine pattern determines the progression of experimental periodontal disease induced by Actinobacillus actinomycetemcomitans through the modulation of MMPs, RANKL, and their physiological inhibitors. Oral Microbiol Immunol. 2006;21:12–20. doi: 10.1111/j.1399-302X.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 16.Green LC, Tannenbaum SR, Goldman P. Nitrate biosynthesis in germfree and conventional rat. Science. 1981;212:56–68. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt H, Wilke P, Evers B, Bohme E. Enzymatic formation of nitrogen oxides from 1-arginine in bovine brain cytosol. Biochem Biophys Res Commun. 1989;165:284–91. doi: 10.1016/0006-291x(89)91067-x. [DOI] [PubMed] [Google Scholar]
- 18.Tachibana T, Matsuyama T, Mitsuyama M. Involvement of CD4+ T cells and macrophages in acquired protection against Sporothrix schenckii in mice. Med Mycol. 1999;37:397–404. doi: 10.1046/j.1365-280x.1999.00239.x. [DOI] [PubMed] [Google Scholar]
- 19.Carlos IZ, Sgarbi DB, Angluster J, Alviano CS, Silva CL. Detection of cellular immunity with the soluble antigen of the fungus Sporothrix schenckii in the systemic form of the disease. Mycopathologia. 1992;117:139–44. doi: 10.1007/BF00442774. [DOI] [PubMed] [Google Scholar]
- 20.Carlos IZ, Zini MM, Sgarbi DB, Angluster J, Alviano CS, Silva CL. Disturbances in the production of interleukin-1 and tumor necrosis factor in disseminated murine sporotrichosis. Mycopathologia. 1994;127:189–94. doi: 10.1007/BF01102920. [DOI] [PubMed] [Google Scholar]
- 21.Carlos IZ, Sgarbi DB, Placeres MC. Host organism defense by a peptide-polysaccharide extracted from the fungus Sporothrix schenckii. Mycopathologia. 1999;144:9–14. doi: 10.1023/a:1006964516334. [DOI] [PubMed] [Google Scholar]
- 22.Carlos IZ, Sgarbi DB, Santos GC, Placeres MC. Sporothrix schenckii lipid inhibits macrophage phagocytosis: involvement of nitric oxide and tumour necrosis factor-alpha. Scand J Immunol. 2003;57:214–20. doi: 10.1046/j.1365-3083.2003.01175.x. [DOI] [PubMed] [Google Scholar]
- 23.da Silva AC, Bezerra LM, Aguiar TS, Tavares D, Araujo LM, Pinto CE, Ribeiro OG. Effect of genetic modifications by selection for immunological tolerance on fungus infection in mice. Microbes Infect. 2001;3:215–22. doi: 10.1016/s1286-4579(01)01373-9. [DOI] [PubMed] [Google Scholar]
- 24.Vogels MT, Hermsen CC, Huys HL, Eling WM, van Der Meen JM. Roles of tumor necrose factor-alpha, granulocyte–macrophage colony-stimulating factor, platelet-activating factor, and arachidonic acid metabolites in interleukin-1-induced resistance to infection in neutropenic mice. Infect Immun. 1994;62:2065–70. doi: 10.1128/iai.62.5.2065-2070.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kappaB activation prevents cell death. Cell. 1996;87:565–76. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 26.Allendoerfer R, Deepe GS. Regulation of infection with Histoplasma capsulatum by TNFR1 and -2. J Immunol. 2000;165:2657–64. doi: 10.4049/jimmunol.165.5.2657. [DOI] [PubMed] [Google Scholar]
- 27.Souto JT, Figueiredo F, Furlanetto A, Pfeffer K, Rossi MA, Silva JS. Interferon-gamma and tumor necrosis factor-alpha determine resistance to Paracoccidioides brasiliensis infection in mice. Am J Pathol. 2000;156:1811–20. doi: 10.1016/s0002-9440(10)65053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–40. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvo CR, Amsen D, Kruisbeek AM. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and Jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J Exp Med. 1997;186:1645–53. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ashkenzi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–60. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 31.Loweth AC, Williams GT, James RF, Scarpello JH, Morgan NG. Human islets of Langerhans express Fas ligant and undergo apoptosis in response to interleukin -1 beta and Fas ligation. Diabetes. 1998;47:727–32. doi: 10.2337/diabetes.47.5.727. [DOI] [PubMed] [Google Scholar]
- 32.Martins GA, Petkova SB, MacHado FS, Kitsis RN, Weiss LM, Wittner M, Tanowitz HB, Silva JS. Fas–FasL interaction modulates nitric oxide production in Trypanosoma cruzi-infected mice. Immunology. 2001;103:122–9. doi: 10.1046/j.1365-2567.2001.01216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown CY, Lagnado CA, Vadas MA, Goodall GJ. Differential regulation of the stability of cytokine mRNAs in lipopolysaccharide-activated blood monocytes in response to interleukin-10. J Biol Chem. 1996;271:20108–12. doi: 10.1074/jbc.271.33.20108. [DOI] [PubMed] [Google Scholar]
- 34.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–51. [PubMed] [Google Scholar]
- 35.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 36.Bogdan C, Vodovotz Y, Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991;174:1549–55. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha FQ, Moncada S, Liew FY. Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun. 1992;182:1155–9. doi: 10.1016/0006-291x(92)91852-h. [DOI] [PubMed] [Google Scholar]
- 38.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–4. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 39.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–95. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Sero G, Mencacci A, Cenci E, et al. Antifungal type 1 responses are upregulated in IL-10-deficient mice. Microbes Infect. 1999;1:1169–80. doi: 10.1016/s1286-4579(99)00245-2. [DOI] [PubMed] [Google Scholar]
- 41.Michelin MA, Figueiredo F, Cunha FQ. Involvement of prostaglandins in the immunosuppression occurring during experimental infection by Paracoccidioides brasiliensis. Exp Parasitol. 2002;102:170–7. doi: 10.1016/s0014-4894(03)00053-5. [DOI] [PubMed] [Google Scholar]
- 42.Sisto F, Miluzio A, Leopardi O, Mirra M, Boelaert JR, Taramelli D. Differential cytokine pattern in the spleens and livers of BALB/c mice infected with Penicillium marneffei protective role of gamma interferon. Infect Immun. 2003;71:465–73. doi: 10.1128/IAI.71.1.465-473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Netea MG, Sutmuller R, Hermann C, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172:3712–8. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 44.Khan IA, Schwartzman JD, Matsuura T, Kasper LH. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc Natl Acad Sci U S A. 1997;94:13955–60. doi: 10.1073/pnas.94.25.13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu-Hsieh BA, Chen W, Lee H. Nitric oxide synthase expression in macrophages of Histoplasma capsulatum-infected mice is associated with splenocyte apoptosis and unresponsiveness. Infect Immun. 1998;66:5520–6. doi: 10.1128/iai.66.11.5520-5526.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bocca AL, Silva MF, Silva CL, Cunha FQ, Figueiredo F. Macrophage expression of class II major histocompatibility complex gene products in Paracoccidioides brasiliensis-infected mice. Am J Trop Med Hyg. 1999;61:280–7. doi: 10.4269/ajtmh.1999.61.280. [DOI] [PubMed] [Google Scholar]
- 47.MacFarlane AS, Schwacha MG, Eisenstein TK. In vivo blockage of nitric oxide with aminoguanidine inhibits immunosuppression induced by an attenuated strain of Salmonella typhimurium, potentiates Salmonella infection, and inhibits macrophage and polymorphonuclear leukocyte influx into the spleen. Infect Immun. 1999;67:891–8. doi: 10.1128/iai.67.2.891-898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van der Veen RC. Nitric oxide and T helper cell immunity. Int Immunopharmacol. 2001;1:1491–500. doi: 10.1016/s1567-5769(01)00093-5. [DOI] [PubMed] [Google Scholar]
- 49.Eisenstein TK. Implications of Salmonella-induced nitric oxide (NO) for host defense and vaccines: NO, an antimicrobial, antitumor, immunosuppressive and immunoregulatory molecule. Microbes Infect. 2001;3:1223–31. doi: 10.1016/s1286-4579(01)01482-4. [DOI] [PubMed] [Google Scholar]
- 50.Wei XQ, Charles IG, Smith A, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–11. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- 51.Koga T, Duan H, Furue M. Immunohistochemical detection of interferon-gamma-producing cells in granuloma formation of sporotrichosis. Med Mycol. 2002;40:111–4. doi: 10.1080/mmy.40.2.111.114. [DOI] [PubMed] [Google Scholar]
- 52.Thomas SR, Mohr D, Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem. 1994;269:14457–64. [PubMed] [Google Scholar]
- 53.Ramsey KH, Miranpuri GS, Sigar IM, Ouellette S, Byrne GI. Chlamydia trachomatis persistence in the female mouse genital tract: inducible nitric oxide synthase and infection outcome. Infect Immun. 2001;69:5131–7. doi: 10.1128/IAI.69.8.5131-5137.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silva NM, Rodrigues CV, Santoro MM, Reis LF, Alvarez-Leite JI, Gazzinelli RT. Expression of indoleamine 2,3-dioxygenase, tryptophan degradation, and kynurenine formation during in vivo infection with Toxoplasma gondii. induction by endogenous gamma interferon and requirement of interferon regulatory factor 1. Infect Immun. 2002;70:859–68. doi: 10.1128/iai.70.2.859-868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]