Abstract
Human polymeric immunoglobulin receptor (pIgR) is present on the surface of glandular epithelium, and it plays a crucial role in the mucosal immune defence. pIgR expression in HT-29 cells is up-regulated by one of the proinflammatory cytokines, tumour necrosis factor (TNF)-α. However, the mechanism used by the TNF-α-mediated signalling pathway has not been examined exclusively. To elucidate this mechanism in detail, HT-29 cells were cotreated with TNF-α and mitogen-activated protein kinase kinase (MAPKK, also called MEK1) inhibitor, PD98059, and the amount of free secretory component (SC) secreted into the culture medium was measured. The amount of free SC stimulated by TNF-α was increased by addition of PD98059. This up-regulation occurred at the transcriptional level. The amount of SC was also up-regulated by addition of TNF-α with U0126, an inhibitor of MEK1 and MEK2. Nuclear factor (NF)-κB activity and NF-κB binding to the κB2 site localized upstream of the pIgR gene did not change after coincubation of HT-29 cells with TNF-α and PD98059. The expression level of pIgR by TNF-α was decreased by LY294002, an inhibitor of phosphatidylinositol-3-kinase (PI3K), at the transcriptional level. Extracellular signal-regulated kinase (ERK)1/2 phosphorylation and NF-κB binding to the κB2 site were not affected by LY294002 treatment. These data suggest that TNF-α-mediated pIgR expression is negatively regulated by ERK pathway, which is independent of NF-κB. In addition, decrease of SC production by Ly294002 suggests that the presence of PI3K mediated regulation of SC production.
Keywords: pIgR, TNF-α, MAPK, PI3K, NF-κB
Introduction
Tumour necrosis factor-α (TNF-α) has pleiotropic functions that include roles in cell growth, differentiation, inflammatory effects, tumorigenesis, and viral replication.1 TNF-α has been identified as the most important cytokine in the pathogenesis of inflammatory bowel disease (IBD).2 The effect of TNF-α is mediated through two types of receptors, TNF-receptor (TNFR) 1 (also known as p55TNFR) and TNFR2 (also known as p75TNFR). TNF-α binding to these receptors activates transcription factors such as nuclear factor (NF)-κB or AP-1 that control multiple gene expressions of inflammatory cytokines such as interleukin (IL)-1, IL-6, and IL-8. TNF-α signals mediated through the TNFRs are followed by activation of mitogen-activated protein kinase (MAPK) pathways, including extracellular signal-regulated kinases (ERKs), p38, and c-Jun NH2-terminal kinases.3
Human polymeric immunoglobulin receptor (pIgR) is a 120 000 MW glycoprotein present on glandular epithelial cells that functions as a receptor for polymeric immunoglobulin (pIg). pIgR transports polymeric immunoglobulin A (IgA) into external secretions as secretory IgA (S-IgA), which is critical for the defence of mucosal tissues.4 Free secretory component (SC) is important for the enhancement of immune responses. For example, degranulation of eosinophils caused by binding of S-IgA or SC is mediated through the 15 000 MW SC receptor expressed in eosinophils.5,6 Degranulation of IL-3-primed basophils is also mediated by SC.7 Free SC can bind to several bacterial proteins, such as colonization factor antigen,8Clostridium difficile toxin A,9 and Streptococcus pneumoniae protein SpsA.10–12 Interferon-γ (IFN-γ),13 TNF-α,14 IL-415 and IL-1β16 can up-regulate the release of SC into the culture supernatant of the human colonic adenocarcinoma cell line, HT-29. Production of SC by IFN-γ, TNF-α, or IL-1β is regulated by IFN-γ regulatory factor-1 (IRF-1) binding to an element in exon 1 of the pIgR gene that is induced by Janus kinase/signal transducer and activator of transcription (STAT) cascade or NF-κB.17–19 In addition, IL-4 and IFN-γ synergistically increased the release of SC in HT-29 cells.15 These data indicate that pIgR expression is regulated by cytokines.
We hypothesized that the activation of ERK contributes to the TNF-α–induced pIgR gene expression. Treatment of HT-29 cells with TNF-α up-regulates the SC production at the transcriptional level, as previously described.14,20 However, cotreatment of HT-29 cells with TNF-α and MAPK kinase (MAPKK, also called MEK1) inhibitor, PD98059, enhanced the TNF-α–induced SC production. Our data suggest that the production of SC in HT-29 cells is also regulated by a negative effect that is involved in ERK activation.
Materials and methods
cDNA
Human pIgR21–23 cDNA was kindly provided by Prof. P. Brandtzeag (LIIPAT, Institute of Pathology, National Hospital, University of Oslo, Oslo, Norway). As a probe for Northern blot analysis, Pst I (668-bp) fragments were used for the detection of pIgR mRNA.
Reagents
Recombinant human TNF-α (specific activity = 2 × 107 U/mg) was purchased from Genzyme Corp. (Cambridge, MA). 2′-amino-3′-1,4-diamino-2,3-dicyano-1 methoxyflavone (PD98059),24 4-bis[2-aminophenylthio]butadiene (U0126),25 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002)26 and antibodies against phosphoERK1/2 (anti-ACTIVE MAPK) and ERK1/2 were purchased from Promega Corp. (Madison, WI).
Cell culture
A human colonic adenocarcinoma cell line, HT-29, was maintained in MaCoy's 5 A medium (Life Technologies Inc., Gaithersburg, MD) supplemented with 10% fetal calf serum (Cansera International Inc., Ontario, Canada), 1 mm glutamine (Life Technologies Inc., Gaithersburg, MD), amphotericin B, and penicillin/streptomycin (Life Technologies Inc., Gaithersburg, MD) at 37° in an atmosphere of humidified 5% CO2. We selected HT-29 cell line because this cell line has been often used to examined the mechanisms of SC production by TNF-α20,30,31,32 and we considered that HT-29 is a very good pIgR expression model. In addition and there is no appropriate cell line to use this study. Prior to treatment, cells (1 × 106 cells/ml) were plated with fresh medium and cultured. On the second day after plating, 10 ng/ml of human recombinant TNF-α, with or without PD98059, U0126, or LY294002, was added to the medium of the serum-free condition, and cultivation continued for 48 hr. Then, cultured cells were harvested, and 2 ml of Solution D was added for RNA extraction.27 The supernatants were also collected and subjected to enzyme-linked immunosorbent assay (ELISA), as previously described.16,28 All experiments were performed in triplicate, and differences between means were calculated using Student's t-test.
Northern blot analysis
Ten µg of total RNA was mixed with loading buffer {50% formamide, 6% formalin, 1 × Goldberg buffer [40 mm Na-MOPS pH 7·2, 5 mm sodium acetate, 0·5 mm ethylenediamine N,N,N′,N′tetra-acetic acid (EDTA)]}, denatured at 65° for 15 min, and cooled on ice. Then, RNA was electrophoresed through a 1·0% agarose gel containing 6% formalin. After electrophoresis, the gel was neutralized by incubation with 0·25 m ammonium acetate solution for 5 min, and the RNA was transferred onto Hybond-N (Amersham, Little Chalfont, UK). The RNA was fixed to the membrane by using a UV crosslinker. Before hybridization, the membrane was incubated in prehybridization solution [50% formamide, 0·65 m NaCl, 5 mm EDTA (pH 7·5), 0·1% sodium dodecyl sulphate (SDS), 0·1 m piperazine-N,N′-bis-[2-ethanesulfonic acid] (PIPES)[pH 6·8], 5× Denhardt's solution] at 42° for 6 hr. The hybridization probe was prepared by using a random prime DNA labelling kit (TAKARA Shuzo Co., Shiga, Japan) and purified by Sephadex G-50 (Pharmacia Biotech, Uppsala, Sweden). For hybridization, dextran sulfate (Pharmacia Biotech) was added to the prehybridization solution at a final concentration of 10%. After hybridization, the membrane was washed four times with washing buffer (2× standard sodium citrate, 0·1% SDS, 0·2% sodium pyrophosphate) at 55° for 30 min and exposed to X-OMAT film (Kodak, Rochester, NY) at −80°.
Reverse trranscription–polymerase chain reaction (RT–PCR)
Cells treated with LY294002 and/or TNF-α were collected and total RNA was extracted by RNeasy (Qiagen, Valencia, CA). After extraction of total RNA, 500 ng of total RNA was used for cDNA synthesis using SuperScript III reverse transcriptase (Invitrogen, San Diego, CA) as manufacture's instruction. Then, 2 µl of synthesized cDNA was used as a template for RT–PCR. The sequence of primer is as following; human pIgR hSC3 5′-TTGTCTCCCTGACCCTGAAC-3′, hSC4; 5′-CAAATTCCCTGGAGTTCTCG-3′ (product size is 501 bp), β-actin BACT F 4TH; 5′-TTCCAGCCTTCCTTCCTGG-3′, β-actin BACT R 4TH; 5′-TTGCGCTCAGGAGGAGCAA-3′ (product size is 225 bp).Amplification of human SC and β-action was performed using HotMasterMix (Eppendorf) as following condition; 94° for 3 min, then, 94° for 30 s, 60° for 30 s, 72° for 30 s for 35 cycles. Then PCR product was incubated for 8 min at 72° and stored at 4°.
Electrophoretic mobility shift assay (EMSA)
Nuclear extracts were prepared by the method as described previously.20 For preparation of the probe, synthetic oligomers ([κB2, corresponding to −461∼–440; 5′-GATCGTCGGAGGGGATTCCAGAGCAA-3′, 3′-CAGCCTCCCCTAAGGTCTCGTTCTAG-5′) were annealed and radiolabelled with the Klenow fragment of DNA polymerase I and [α-32P] dCTP (Amersham). For the binding reaction, 10 µg of nuclear extract was incubated in 22 µl of a buffer containing 25 mm HEPES (pH 7·9), 2 mm EDTA (pH 8·0), 50 mm KCl, 10% glycerol, 2 µg of poly dI-dC (Pharmacia Biotech), 1% bovine serum albumin (BSA), and 104 counts per minute (c.p.m.) of radiolabelled probe for 30 min at 30°. After the reaction, 1 µl of loading buffer (5% glycerol, 50 mm EDTA [pH 8·0], 0·05% bromophenol blue, 0·05% xylene cyanol) was added and separated through a 6% native polyacrylamide gel in TAE buffer (67 mm Tris, 33 mm sodium acetate [pH 6·0], 10 mm EDTA) at room temperature. The gel was then dried and exposed to Kodak X-OMAT film at −80° for 12 hr.
Preparation of cell lysate and SDS–Polyacrylamide gele electrophoresis (PAGE)
For analysis of phosphorylated ERK, HT-29 cells were treated with TNF-α with or without PD98059 at 37° for 15 min and 48 hr. Then, cells were washed with PBS and lysed in cell lysis buffer (20 mm Tris, pH 7·5, 150 mm NaCl, 0·25 m sucrose, 5 mm EDTA, 5 mm ethylene glycole bis-(β-aminoethyl ether)-N,N,N′-N′-tetraacetic acid (EGTA), 0·5% Triton-X-100, 25 mm NaF, 5 mm Na3VO4, 5 mmβ-glycerophosphate, 1·5 mm phenylmethylsulphonyl fluoride, 5 µg/ml leupeptin, 10 µg/ml aprotinin),29 and the soluble fraction was collected by centrifugation at 20 000 g at 4° for 15 min. Protein concentration was measured by a Bio-Rad Protein assay kit (Bio-Rad Laboratories, Hercules, CA). Fifty µg of protein was separated by 10% SDS–PAGE.
Western blot analysis
After electrophoresis, the gel was incubated with transfer buffer (24·8 mm Tris, 192·5 mm glycine, 0·05% methanol) for 30 min and transferred onto Immobilon P (Millipore, Bedford, MA) by electroblot. The membrane was incubated in blocking buffer (1% BSA in TBST [20 mm Tris-HCl pH 7·5, 150 mm NaCl, 0·05% Tween 20]) at room temperature for 12 hr. Detection of MAPKs was performed using anti-active MAPK antibody as a primary antibody, donkey anti-rabbit IgG conjugated with alkaline phosphatase as a secondary antibody, and CDP-Star reagent for signal detection (Amersham). For reprobing, the membrane was incubated with stripping buffer (100 mm 2-mercaptoethanol, 2% SDS, 62·5 mm Tris-HCl, pH 6·8) at 50° for 30 min and washed twice with TBST at room temperature for 10 min. Then, the membrane was incubated with blocking buffer at room temperature for 16 h and subjected to immunodetection using anti-ERK1/2 antibody.
Results
Production of SC by TNF-α is enhanced by PD98059
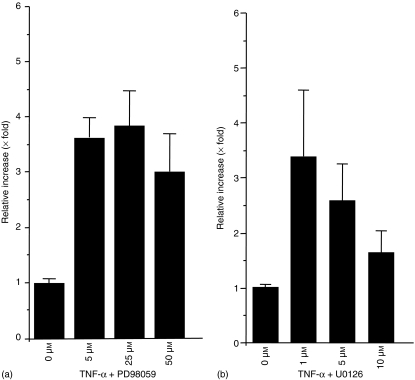
To elucidate whether the MAPK is involved in TNF-α signalling and SC production, HT-29 cells were incubated with TNF-α for 48 h with 0, 5, 25, or 50 µm of PD98059. Then, ELISA was performed on culture supernatants to quantify the amount of SC. The amount of SC is enhanced by TNF-α as compared to the control, as described previously (data not shown). This effect was enhanced significantly by addition of PD98059 (P < 0·002 when concentration is 50 µm) (Fig. 1a). The same effect was also observed by pretreatment of HT-29 with PD98059 for 30 min followed by stimulation of TNF-α for 48 hr (data not shown). SC production by TNF-α was also significantly up-regulated by cotreatment with U0126, an inhibitor of MEK1/2 (P < 0·01 when concentration is 1 µm) (Fig. 1b).
Figure 1.
Production of SC by TNF-α is up-regulated by addition of MEK1 inhibitor, PD98059 (a) or MEK1/2 inhibitor U0126 (b) in a concentration-dependent manner. HT-29 cells were treated with 10 ng/ml of TNF-α and 0, 5, 25, or 50 µm of PD98059 (a) or 0, 1, 5, or 10 µm of U0126 (b) and cultured for 48 hr. Then, supernatants were subjected to ELISA, and the amounts of SC were measured.
Enhancement of SC production by PD98059 is regulated at the transcriptional level
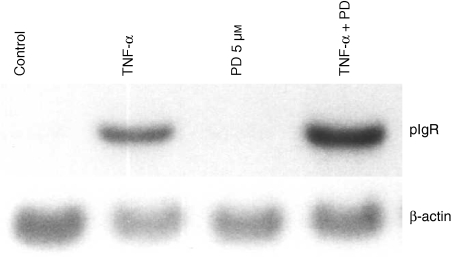
To determine if the enhancement of SC production by PD98059 is regulated at the transcriptional level or translational level, HT-29 cells were treated with TNF-α and 5 µm of PD98059 for 48 hr, and northern blot analysis was performed. The expression of pIgR mRNA induced by TNF-α was increased by PD98059 (Fig. 2).
Figure 2.
Enhancement of SC by TNF-α and PD98029 is controlled at the transcriptional level. HT-29 cells were treated with TNF-α and 5 µm of PD98059 or 1 µm of U0126 or DMSO for 48 hr. Then, total RNA was extracted and 10 µg of total RNA was subjected to northern blot analysis using human pIgR cDNA.
ERK is activated by TNF-α in HT-29 and inhibited by PD98059, a MEK inhibitor
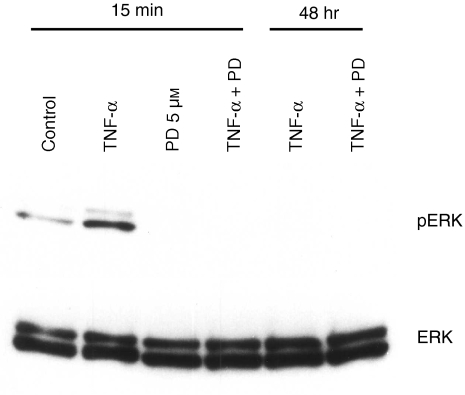
To check whether TNF-α-activated ERK1/2 was inhibited by PD98059, HT-29 cells were treated with TNF-α for 15 min and 48 hr with or without PD98059. Cell lysates were collected, and phosphorylated ERK1/2 was detected by western blotting analysis. When HT-29 cells were treated with TNF-α for 15 min, both ERK1 and ERK2 were phosphorylated as previously reported33 and after 48 hr, the amount of phosphorylated ERK1/2 was decreased. Activation of ERK1/2 by TNF-α was completely inhibited by treatment with 5 µm of PD98059 (Fig. 3). The amount of ERK protein did not change by treatment of TNF-α with or without PD98059 (Fig. 3).
Figure 3.
Phosphorylation of ERK1/2 by TNF-α is blocked by 5 µm of PD98059. HT-29 cells were treated with 10 ng/ml of TNF-α and 5 µm of PD98059 or DMSO for 15 min. Then, protein was extracted and phosphorylated ERK1/2 was detected by Western blot using antiactive ERK antibody. To check the amount of total ERK1/2, anti-ERK1/2 antibody was used.
SC production by TNF-α is inhibited by phosphatidylinositol-3-kinase (PI3K) inhibitor LY294002
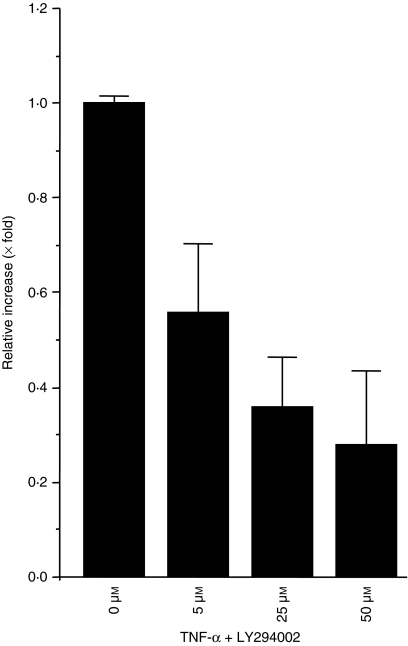
PI3K is also activated by extracellular signals such as growth factors, cytokines, integrin-mediated signals, and other survival signals.34 To elucidate whether PI3K is involved in TNF-α–induced SC production, HT-29 cells were costimulated with TNF-α and LY294002 for 48 hr, and then the amount of SC protein in culture supernatants was quantified. The production of SC was inhibited by PI3K inhibitor as previously reported35,36 at any concentration, especially at the 50 µm concentration (Fig. 4).
Figure 4.
SC production by TNF-α is inhibited by LY294002.HT-29 cells were treated with 10 ng/ml of TNF-α with 0, 5, 25, or 50 µm of LY294002 for 48 hr. Then, supernatants were subjected to ELISA, and the amounts of SC were measured.
Inhibition of SC production by LY294002 is regulated at the transcriptional level
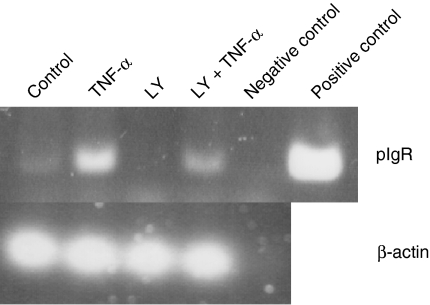
It has been indicated that PI3K inhibitor inhibit transcytosis of pIgR.35,36 To check whether inhibition of pIgR by LY294002 is regulated at the transcriptional or post-transcriptional level, RT–PCR was performed. As we showed the decrease of SC production by LY294002, expression level of pIgR mRNA was also decreased as compared with TNF-α stimulation. This data suggest that inhibition of SC production by LY294002 was regulated at the transcriptional level (Fig. 5). Treatment of HT-29 with only LY294002 completely inhibited the expression of SC mRNA as compared with control, which express free SC constitutively, suggesting that LY294002 inhibited mainly constitutively expressed pIgR mRNA.
Figure 5.
TNF-α mediated SC production inhibited by LY294002 is regulated at the transcriptional level. HT-29 cells were treated with LY294002 and TNF-α for 48 hr. Then total RNA was extracted and RT–PCR was performed to detect pIgR mRNA. For positive control of pIgR, 2 ng of full length pIgR cDNA was used.
Involvement of NF-κB in the enhancement of SC production by PD98059 and inhibition by Ly294002
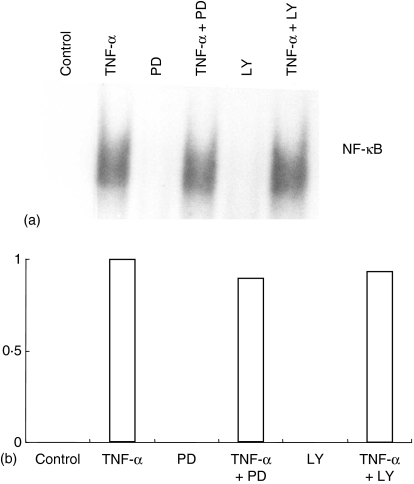
Treatment of glioblastoma cell line T98G with PD98059 and IL-1β augmented NF-κB activation.37 NF-κB is an important transcription factor for the pIgR gene.20,30–32 We checked whether the enhanced SC production by PD98059 is via enhanced NF-κB activation by using a gel shift assay to detect the NF-κB bound upstream of the pIgR gene (κB2). The amount of NF-κB binding to the κB2 site did not increase after PD98059 treatment (Fig. 6). We also checked whether inhibition of PI3K influences the effect of NF-κB activation. HT-29 cells were treated with 10 ng/ml of TNF-α with or without LY294002 for 90 min, and then nuclear extracts were prepared and the gel shift assay was performed using κB2 probe. Co-treatment of HT-29 cells with TNF-α and LY294002 did not change the binding of NF-κB to κB2 (Fig. 6).
Figure 6.
Binding of NF-κB to κB2 is not affected by PD98059 and LY294002. (a) HT-29 cells were treated with 10 ng/ml of TNF-α and 5 µm of PD98059 or 50 µm of LY294002 for 90 min, and then nuclear protein was extracted. Ten µg of nuclear protein was used for the gel shift assay. (b) Densitometric analysis of gel shift assay using Image J software. Y-axis shows the density of band compared to TNF-α stimulation (= 1) as a control.
Inhibition of PI3K by TNF-α is independent of ERK activation
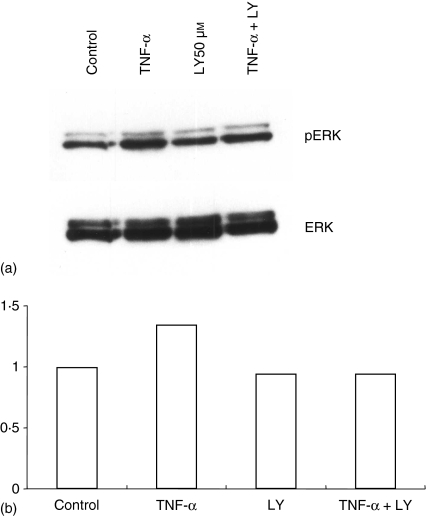
To determine if inhibition of PI3K affected ERK activation, HT-29 cells were treated with 50 µm of LY294002 for 15 min. Cell lysates were applied to western blots. The amount of phosphorylated ERK was unchanged by cotreatment with TNF-α and LY294002 (Fig. 7).
Figure 7.
Activation of ERK by TNF-α was not altered by LY294002. (a) HT-29 cells were treated with 10 ng/ml of TNF-α and 50 µm of LY294002 for 15 min. Then, protein was extracted and phosphorylated ERK1/2 was detected by Western blot using antiactive ERK antibody. (b) Densitometric analysis using Image J software.
Discussion
In IBD such as Crohn's disease and ulcerative colitis, the number of TNF-α-producing cells is increased in the small intestine.2 Some treatment strategies for IBD target TNF using anti-TNF monoclonal antibody (infliximab)38 or MAPK signalling pathway inhibitor (CNI-1493).39 On the other hand, the expression levels of pIgR and S-IgA were decreased in IBD.40 In lung epithelial cells, expression of SC is reduced in chronic obstructive pulmonary disease41 and in this condition SC is cleaved by serine protease produced by phorbol myristate-activated polynuclear neutrophil (PMN).42
In this study, we reported that production of SC by TNF-α was enhanced by MEK1/2 inhibitors (Fig. 1) without the redundant NF-κB activation (Fig. 6). Our data suggest that MAP kinase signalling pathway inhibitor is a beneficial treatment for IBD because there is a possibility that the decreased pIgR protein and S-IgA would be elevated and that might enhance the mucosal immunity without excessive NF-κB activation.
There are several reports about the signalling mechanisms that enhance the expression level of pIgR by various cytokines. When HT-29 cells are treated with IFN-γ and IL-4, the expression level of pIgR mRNA is up-regulated, and protein tyrosine kinase is involved in up-regulation of pIgR.43 In addition, the STAT-6 binding site located at intron 1 is important for the up-regulation of pIgR gene expression by IL-444 and co-operation of STAT-6 and tissue-specific factor, hepatocyte NF-1, is also required.45 PIgR gene expression induced by TNF-α has also been suggested to be regulated by NF-κB located upstream of the transcription initiation site20 and another binding site located at intron 145 is strongly suggested to be involved. However, signalling mechanisms by TNF-α were not well elucidated.
ERKs were activated by treatment of HT-29 cells with TNF-α (Fig. 3), and production of SC by TNF-α was enhanced by inhibition of ERKs (Fig. 1). These data indicate the existence of a negative regulation pathway by TNF-α stimulation. In addition, PI3K inhibition by LY294002 decreased the production of SC by TNF-α (Fig. 4) without NF-κB inactivation (Fig. 6) and ERK activation (Fig. 7), suggesting that the PI3K pathway positively regulates SC production at the transcriptional level. As it has been reported that PI3K is involved in transcytosis of pIgR35,36 inhibition of SC production by PI3K inhibitor is controlled at the transcriptional level (Fig. 5). We could not detect SC production by ELISA from the control sample but could detect by RT–PCR, probably depending on the different sensitivities of detection system. These data suggest that PI3K is involved in trancytosis of pIgR in the HT-29 cells as previously indicated8,35,36 and our data confirm previous data. Because we have indicated that expression of pIgR mRNA was not completely inhibited by NF-κB inhibitor20 it is possible that there is also an NF-κB-independent pathway. In bronchial epithelial cells, up-regulation of SC production by activated PMN was also inhibited by NF-κB and p38 inhibitor. However, activation of NF-κB and p38 is independent of SC production by serine proteinase, which means that SC cleavage and expression are independently regulated.42 Our data indicate that there are several kinds of signalling pathways, such as NF-κB-dependent and NF-κB-independent pathways, which are involved in SC production by TNF-α. Our data also indicate the existence of negative and positive regulators of the NF-κB-independent pathway in HT-29 cells. Further investigations are needed to elucidate the complicated mechanisms of signalling pathways for SC production.
Acknowledgments
Financial support was obtained from the Nihon University Research Grant for Assistants and Young Researchers (2003), the Grant from the Ministry of Education, Culture, Sports, Science and Technology to promote multidisciplinary research projects, and Academic Frontier Project for Private Universities: matching fund subsidy from MEXT (to Dr Hiroki Nagase).
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- IBD
inflammatory bowel disease
- IL-1β
interleukin-1 β
- IL-6
interleukin-6
- IL-8
interleukin-8
- IFN-γ
interferon-γ
- IRF-1
IFN-γ regulatory factor-1
- MAPK
mitogen-activated protein kinase
- MEK
MAPK/ERK kinase
- NF-κB
nuclear factor-κB
- PI3K
phosphatidylinositol-3-kinase
- pIgR
polymeric immunoglobulin receptor
- SC
secretory component
- S-IgA
secretory immunoglobulin A
- TNF-α
tumor necrosis factor-α
- TNFR
TNF-receptor.
References
- 1.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45–65. doi: 10.1038/sj.cdd.4401189. [DOI] [PubMed] [Google Scholar]
- 2.Breese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, MacDonald TT. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–66. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 3.MacEwan DJ. TNF ligands and receptors – a matter of life and death. Br J Pharmacol. 2002;135:855–75. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mestecky J, McGhee JR. Immunoglobulin A (IgA). molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. 153. 245. [DOI] [PubMed] [Google Scholar]
- 5.Motegi Y, Kita H. Interaction with secretory component stimulates effector functions of human eosinophils but not of neutrophils. J Immunol. 1998;161:4340–6. [PubMed] [Google Scholar]
- 6.Lamkhioued B, Gounni AS, Gruart V, Pierce A, Capron A, Capron M. Human eosinophils express a receptor for secretory component. Role in secretory IgA-dependent activation. Eur J Immunol. 1995;25:117–25. doi: 10.1002/eji.1830250121. [DOI] [PubMed] [Google Scholar]
- 7.Iikura M, Yamaguchi M, Fujisawa T, et al. Secretory IgA induces degranulation of IL-3-primed basophils. J Immunol. 1998;161:1510–5. [PubMed] [Google Scholar]
- 8.de Oliveira IR, de Araujo AN, Bao SN, Giugliano LG. Binding of lactoferrin and free secretory component to enterotoxigenic Escherichia coli. FEMS Microbiol Lett. 2001;203:29–33. doi: 10.1111/j.1574-6968.2001.tb10816.x. [DOI] [PubMed] [Google Scholar]
- 9.Dallas SD, Rolfe RD. Binding of Clostridium difficile toxin A to human milk secretory component. J Med Microbiol. 1998;47:879–88. doi: 10.1099/00222615-47-10-879. [DOI] [PubMed] [Google Scholar]
- 10.Hammerschmidt S, Tillig MP, Wolff S, Vaerman JP, Chhatwal GS. Species-specific binding of human secretory component to SpsA protein of Streptococcus pneumoniae via a hexapeptide motif. Mol Microbiol. 2000;36:726–36. doi: 10.1046/j.1365-2958.2000.01897.x. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt S, Talay SR, Brandtzaeg P, Chhatwal GS. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol Microbiol. 1997;25:1113–24. doi: 10.1046/j.1365-2958.1997.5391899.x. [DOI] [PubMed] [Google Scholar]
- 12.Elm C, Rohde M, Vaerman JP, Chhatwal GS, Hammerschmidt S. Characterization of the interaction of the pneumococcal surface protein SpsA with the human polymeric immunoglobulin receptor (hpIgR) Indian J Med Res. 2004;119(Suppl.):61–5. [PubMed] [Google Scholar]
- 13.Sollid LM, Kvale D, Brandtzaeg P, Markussen G, Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987;138:4303–6. [PubMed] [Google Scholar]
- 14.Kvale D, Lovhaug D, Sollid LM, Brandtzaeg P. Tumor necrosis factor-alpha up-regulates expression of secretory component, the epithelial receptor for polymeric Ig. J Immunol. 1988;140:3086–9. [PubMed] [Google Scholar]
- 15.Phillips JO, Everson MP, Moldoveanu Z, Lue C, Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990;145:1740–4. [PubMed] [Google Scholar]
- 16.Hayashi M, Takenouchi N, Asano M, Kato M, Tsurumachi T, Saito T, Moro I. The polymeric immunoglobulin receptor (secretory component) in a human intestinal epithelial cell line is up-regulated by interleukin-1. Immunology. 1997;92:220–5. doi: 10.1046/j.1365-2567.1997.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piskurich JF, Youngman KR, Phillips KM, Hempen PM, Blanchard MH, France JA, Kaetzel CS. Transcriptional regulation of the human polymeric immunoglobulin receptor gene by interferon-gamma. Mol Immunol. 1997;34:75–91. doi: 10.1016/s0161-5890(96)00079-x. [DOI] [PubMed] [Google Scholar]
- 18.Kaetzel CS, Blanch VJ, Hempen PM, Phillips KM, Piskurich JF, Youngman KR. The polymeric immunoglobulin receptor: structure and synthesis. Biochem Soc Trans. 1997;25:475–80. doi: 10.1042/bst0250475. [DOI] [PubMed] [Google Scholar]
- 19.Blanch VJ, Piskurich JF, Kaetzel CS. Cutting edge: coordinate regulation of IFN regulatory factor-1 and the polymeric Ig receptor by proinflammatory cytokines. J Immunol. 1999;162:1232–5. [PubMed] [Google Scholar]
- 20.Takenouchi-Ohkubo N, Takahashi T, Tsuchiya M, Mestecky J, Moldoveanu Z, Moro I. Role of nuclear factor-kappaB in the expression by tumor necrosis factor-alpha of the human polymeric immunoglobulin receptor (plgR) gene. Immunogenetics. 2000;51:289–95. doi: 10.1007/s002510050622. [DOI] [PubMed] [Google Scholar]
- 21.Krajci P, Grzeschik KH, Geurts van Kessel AH, Olaisen B, Brandtzaeg P. The human transmembrane secretory component (poly-Ig receptor): molecular cloning, restriction fragment length polymorphism and chromosomal sublocalization. Hum Genet. 1991;87:642–8. doi: 10.1007/BF00201717. [DOI] [PubMed] [Google Scholar]
- 22.Krajci P, Kvale D, Tasken K, Brandtzaeg P. Molecular cloning and exon-intron mapping of the gene encoding human transmembrane secretory component (the poly-Ig receptor) Eur J Immunol. 1992;22:2309–15. doi: 10.1002/eji.1830220920. [DOI] [PubMed] [Google Scholar]
- 23.Krajci P, Solberg R, Sandberg M, Oyen O, Jahnsen T, Brandtzaeg P. Molecular cloning of the human transmembrane secretory component (poly-Ig receptor) and its mRNA expression in human tissues. Biochem Biophys Res Commun. 1989;158:783–9. doi: 10.1016/0006-291x(89)92790-3. [DOI] [PubMed] [Google Scholar]
- 24.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–94. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 25.Favata MF, Horiuchi KY, Manos EJ, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 26.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–8. [PubMed] [Google Scholar]
- 27.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 28.Kusama K, Takahashi T, Asano M, Iwase T, Saito T, Nagasaki Y, Moro I. Murine monoclonal antibody to secretory component. Shika Kiso Igakkai Zasshi. 1989;31:471–3. doi: 10.2330/joralbiosci1965.31.471. [DOI] [PubMed] [Google Scholar]
- 29.Ogier-Denis E, Pattingre S, El Benna J, Codogno P. Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem. 2000;275:39090–5. doi: 10.1074/jbc.M006198200. [DOI] [PubMed] [Google Scholar]
- 30.Ackermann LW, Denning GM. Nuclear factor-kappaB contributes to interleukin-4- and interferon-dependent polymeric immunoglobulin receptor expression in human intestinal epithelial cells. Immunology. 2004;111:75–85. doi: 10.1111/j.1365-2567.2003.01773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schjerven H, Brandtzaeg P, Johansen FE. A novel NF-kappa B/Rel site in intron 1 cooperates with proximal promoter elements to mediate TNF-alpha-induced transcription of the human polymeric Ig receptor. J Immunol. 2001;167:6412–20. doi: 10.4049/jimmunol.167.11.6412. [DOI] [PubMed] [Google Scholar]
- 32.Schjerven H, Tran TN, Brandtzaeg P, Johansen FE. De novo synthesized RelB mediates TNF-induced up-regulation of the human polymeric Ig receptor. J Immunol. 2004;173:1849–57. doi: 10.4049/jimmunol.173.3.1849. [DOI] [PubMed] [Google Scholar]
- 33.Jijon HB, Panenka WJ, Madsen KL, Parsons HG. MAP kinases contribute to IL-8 secretion by intestinal epithelial cells via a posttranscriptional mechanism. Am J Physiol Cell Physiol. 2002;283:C31–C41. doi: 10.1152/ajpcell.00113.2001. [DOI] [PubMed] [Google Scholar]
- 34.Fruman DA, Cantley LC. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002;14:7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- 35.Cardone M, Mostov K. Wortmannin inhibits transcytosis of dimeric IgA by the polymeric immunoglobulin receptor. FEBS Lett. 1995;376:74–6. doi: 10.1016/0014-5793(95)01251-8. [DOI] [PubMed] [Google Scholar]
- 36.Verges M, Sebastian I, Mostov KE. Phosphoinositide 3-kinase regulates the role of retromer in transcytosis of the polymeric immunoglobulin receptor. Exp Cell Res. 2007;313:707–18. doi: 10.1016/j.yexcr.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Funakoshi M, Tago K, Sonoda Y, Tominaga S, Kasahara T. A MEK inhibitor, PD98059 enhances IL-1-induced NF-kappaB activation by the enhanced and sustained degradation of IkappaBalpha. Biochem Biophys Res Commun. 2001;283:248–54. doi: 10.1006/bbrc.2001.4759. [DOI] [PubMed] [Google Scholar]
- 38.Stein RB, Hanauer SB. Medical therapy for inflammatory bowel disease. Gastroenterol Clin North Am. 1999;28:297–321. doi: 10.1016/s0889-8553(05)70058-3. [DOI] [PubMed] [Google Scholar]
- 39.Sandborn WJ. Strategies for targeting tumour necrosis factor in IBD. Best Pract Res Clin Gastroenterol. 2003;17:105–17. doi: 10.1053/bega.2002.0345. [DOI] [PubMed] [Google Scholar]
- 40.Marteau P, Colombel JF, Nemeth J, Vaerman JP, Dive JC, Rambaud JC. Immunological study of histologically non-involved jejunum during Crohn's disease: evidence for reduced in vivo secretion of secretory IgA. Clin Exp Immunol. 1990;80:196–201. doi: 10.1111/j.1365-2249.1990.tb05233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pilette C, Godding V, Kiss R, et al. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:185–94. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 42.Pilette C, Ouadrhiri Y, Dimanche F, Vaerman JP, Sibille Y. Secretory component is cleaved by neutrophil serine proteinases but its epithelial production is increased by neutrophils through NF-kappa B- and p38 mitogen-activated protein kinase-dependent mechanisms. Am J Respir Cell Mol Biol. 2003;28:485–98. doi: 10.1165/rcmb.4913. [DOI] [PubMed] [Google Scholar]
- 43.Denning GM. IL-4 and IFN-gamma synergistically increase total polymeric IgA receptor levels in human intestinal epithelial cells. Role of protein tyrosine kinases. J Immunol. 1996;156:4807–14. [PubMed] [Google Scholar]
- 44.Schjerven H, Brandtzaeg P, Johansen FE. Mechanism of IL-4-mediated up-regulation of the polymeric Ig receptor: role of STAT6 in cell type-specific delayed transcriptional response. J Immunol. 2000;165:3898–906. doi: 10.4049/jimmunol.165.7.3898. [DOI] [PubMed] [Google Scholar]
- 45.Schjerven H, Brandtzaeg P, Johansen FE. Hepatocyte NF-1 and STAT6 cooperate with additional DNA-binding factors to activate transcription of the human polymeric Ig receptor gene in response to IL-4. J Immunol. 2003;170:6048–56. doi: 10.4049/jimmunol.170.12.6048. [DOI] [PubMed] [Google Scholar]