Abstract
During neuronal-induced inflammation, mast cells may respond to stimuli such as neuropeptides in an FcεRI-independent manner. In this study, we characterized human mast cell responses to substance P (SP), nerve growth factor (NGF), calcitonin gene-related peptide (CGRP) and vasoactive intestinal polypeptide (VIP) and compared these responses to human mast cell responses to immunoglobulin E (IgE)/anti-IgE and compound 48/80. Primary cultured mast cells, generated from CD34+ progenitors in the presence of stem cell factor and interleukin-6 (IL-6), and human cultured mast cells (LAD2) were stimulated with these and other stimuli (gastrin, concanavalin A, radiocontrast media, and mannitol) and their degranulation and chemokine production was assessed. VIP and SP stimulated primary human mast cells and LAD cells to degranulate; gastrin, concanavalin A, radiocontrast media, mannitol, CGRP and NGF did not activate degranulation. While anti-IgE stimulation did not induce significant production of chemokines, stimulation with VIP, SP or compound 48/80 potently induced production of monocyte chemoattractant protein-1, inducible protein-10, monokine induced by interferon-γ (MIG), RANTES (regulated on activation, normal, T-cell expressed, and secreted) and IL-8. VIP, SP and compound 48/80 also activated release of tumour necrosis factor, IL-3 and granulocyte–macrophage colony-stimulating factor, but not IL-4, interferon-γ or eotaxin. Human mast cells expressed surface neurokinin 1 receptor (NK1R), NK2R, NK3R and VIP receptor type 2 (VPAC2) but not VPAC1 and activation of human mast cells by IgE/anti-IgE up-regulated expression of VPAC2, NK2R, and NK3R. These studies demonstrate the pattern of receptor expression and activation of mast cell by a host of G-protein coupled receptor ligands and suggest that SP and VIP activate a unique signalling pathway in human mast cells. These results are likely to have direct relevance to neuronally induced inflammatory diseases.
Keywords: mast cells, IgE, substance P, VIP, chemokines, neuropeptides
Introduction
Mast cells are effector cells in allergic and anaphylactic reactions. In some cases, mast cells are activated when IgE bound to the Fc epsilon receptor I (FcεRI) on the cell surface is crosslinked by specific antigen, triggering mast cell degranulation and de novo synthesis of arachidonic metabolites, cytokines and chemokines. Mast cell production of these numerous vasoactive, nociceptive, and proinflammatory molecules facilitates their interaction with nearby cells and initiates the allergic response.
However, mast cells can also respond to stimuli that are independent of FcεRI, such as neuropeptides, during inflammatory responses. Mast cells are ubiquitous in the body, located primarily in perivascular spaces and often close to neurons and blood vessels; as such they are uniquely positioned to respond to neuropeptides produced by nearby neurons.1 Acute stress can trigger mast cell degranulation and this process is blocked by depletion of sensory nerves of their content of substance P (SP), an important neuropeptide.2 In rodents, mast cells express receptors for SP and other neuropeptides such as nerve growth factor (NGF), calcitonin gene-related peptide (CGRP) and vasoactive intestinal polypeptide (VIP). These neuropeptides are believed to activate rodent mast cells either by direct G protein binding or by ligating specific surface receptors.3 Low concentrations of SP induce electrical responses in rodent mast cells without degranulation,4 but high concentrations of SP activate degranulation and lead to mast cell-dependent granulocyte infiltration directly through the synthesis of tumour necrosis factor (TNF) or interleukin-8 (IL-8) by mast cells.5 Furthermore, responsiveness to substance P has been used to differentiate connective tissue and mucosal mast cells in rodents. Mouse bone marrow derived mast cells cultured in stem cell factor (SCF) and IL-4 are considered to have a connective tissue phenotype, express the neurokinin 1 receptors (NK1R) for substance P6 and degranulate in response to substance P.7 Human intestinal mast cells, considered to be of the mucosal type, do not respond to substance P and do not constitutively express any of the three NK receptors.8 In fact, other neuropeptides such as CGRP and VIP at micromolar concentrations also fail to induce human intestinal mast cell degranulation or production of leukotrienes and TNF.8 However, upon stimulation by immunoglobulin E (IgE) receptor-crosslinking, which induces an extensive mediator release reaction, a subpopulation of intestinal mast cells were induced to express NK-1, the SP receptor,8 suggesting that allergic inflammation may prime mast cells to respond to neuropeptides. Curiously, SP activates specific gene transcription pathways in human skin mast cells causing them to produce TNF but not IL-4 or IL-5.5 Although SP activation of rodent mast cells is clearly NK1R mediated,9,10 it has not been established whether SP activation of human mast cells is a receptor-mediated event.
In this study, we characterized human mast cell responses to SP, NGF, CGRP, and VIP and compared them to other stimuli such as IgE/anti-IgE and compound 48/80. We show that human CD34± derived mast cells (HuMC) and the LAD mast cell line differ from rodent and human intestinal mast cells in their response to SP and VIP. We demonstrate that SP and VIP induce human mast cells to degranulate and release cytokines and chemokines. Furthermore, we show that activation of human mast cells via FcεRI up-regulates VIP receptor type 2 (VPAC2) and NK2R and NK3R suggesting that exposure to allergen may enhance neuronal inflammation. This is the first report to our knowledge to show that human mast cells respond to neuropeptides by producing chemokines, to directly show that human mast cells constitutively express neuropeptide receptors and to demonstrate that FcεRI activation modifies SP and VIP receptor expression in connective tissue type human mast cells. These observations suggest that SP and VIP activate a unique signalling pathway in human mast cells, which may be relevant to stress-induced or neuronal inflammatory diseases.
Materials and methods
Growth of human mast cells
LAD211 were cultured in serum free media (StemPro-34 SFM, Life Technologies, Gaithersburg, MD) supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 50 µg/ml streptomycin and 100 ng/ml SCF. The LAD2 human mast cell line was originally obtained from a patient with severe systemic mast cell disease and they have many features in common with primary CD34± derived human mast cells.11 Specifically, these cells stain metachromatically with toluidine blue and contain histamine, tryptase and chymase.12 The LAD2 cells were periodically tested for expression of Kit and FcεRI by flow cytometry and the expression of these receptors was equal to that of blood derived CD34+ cells (HuMC). To generate HuMC, human peripheral blood-derived CD34+ cells were cultured in StemPro-34 SFM supplemented with 2 mm l-glutamine, 50 µg/ml streptomycin, 100 IU/ml penicillin, 100 ng/ml SCF, and 100 ng/ml recombinant human IL-6 (PeproTech, Inc., Rocky Hill, NJ). Recombinant human IL-3 (30 ng/ml) was added for the first week. At 8–10 weeks, cultures consisted of greater than 99% huMC.13 HuMC express tryptase, chymase and stain metachromatically with toluidine blue.14 HuMC express higher levels of chymase and surface Kit than cord blood derived mast cells (15,16 and personal communication). For experiments requiring overnight incubation, both LAD2 and HuMC were cultured in serum free media (StemPro-34 SFM) supplemented with 2 mm l-glutamine, 100 U/ml penicillin, 50 µg/ml streptomycin and 100 ng/ml SCF. Except in the case of anti-IgE, preincubation of LAD2 with IgE overnight did not alter their response to the stimuli listed.
Degranulation assay
Cells were sensitized overnight with 0·5 µg/ml of myeloma IgE (BioDesign, Saco, ME). Cells were stimulated with rabbit anti-IgE (Dako, Carpinteria, CA) or other agonists and incubated at 37° for 0·5 hr. The β-hexosaminidase released into the supernatants and in cell lysates was quantified by hydrolysis of p-nitrophenyl N-acetyl-β-d-glucosamide (Sigma-Aldrich, St Louis, MO) in 0·1 m sodium citrate buffer (pH 4·5) for 90 min at 37°. The percentage of β-hexosaminidase release was calculated as a percent of total content. Agonists tested were A23187 (Sigma), compound 48/80 (Sigma), SP (Sigma), SP constructs (Phoenix Pharmaceuticals, Belmont, CA), VIP (Sigma), NGF (Sigma), CGRP (Sigma), gastrin (Sigma), concanavalin A (Sigma), osmotic media (iohexol; Amersham Biosciences, Piscataway, NJ), mannitol (Sigma) and TMA-HSA (Fisher, Hampton, NH).
In some cases, LAD2 cells were pretreated with vehicle (0·1% DMSO), H89 (0·1–10 µm), SQ222536 (0·1–10 µm), Ro-31-8220 (0·1–10 µm), forskolin (0·1–10 µm), or wortmannin (0·1–10 µm) for 10 min or pertussis toxin (3·5 nm) for 2 hr. Cells were then stimulated with either VIP or SP for 30 min and β-hexosaminidase release was measured. The inhibitory concentrations (IC50) for these inhibitors in cell-based assays are as follows: H89 (100 nm),17 SQ 22536 (10 µm), Ro-31–8220 (10 nm for PKC, 1 µm for PKA),17 Forskolin (10 µm), wortmannin (10 nm),7 and pertussis toxin (0·1 nm). In antagonist studies, mast cells were pretreated with NK1R antagonists spantide or SP (4–11) at 0·1 µm for 30 min, stimulated with 0·1 µm SP for another 30 min and β-hexosaminidase release was measured (n = 3, P < 0·01).
Real-time polymerase chain reaction (PCR) analysis
Total RNA was isolated from each preparation using the RNeasy Mini Kit (Qiagen Inc. Valencia, CA). Five µg of total cellular RNA was reverse transcribed using the Taqman Reverse Transcription reagents and Random Hexamer primer (Perkin–Elmer Applied Biosystems, Foster City, CA). Gene expression was analysed using real-time PCR on an ABI7500 SDS system. Fifty ng of cDNA was used in each quantitative PCR assay. Primer sets for PCR amplifications were designed using the Primer Express software (Perkin-Elmer Applied Biosystems). All reactions were performed in triplicate for 40 cycles as per the manufacturer's recommendation. Results are expressed as relative mRNA corrected with reference to glyceraldehyde 3-phosphate dehydrogenase mRNA as an internal control.18
Flow cytometric analysis
Cells were washed and resuspended at 5 × 105 cells/ml in phosphate-buffered saline (PBS)/0·1% bovine serum albumin (BSA) and incubated with anti-VIP1, anti-VIP2, anti-NK1, anti-NK3, anti-NKB (all from Abcam, Cambridge, MA), anti-Kit-PE (BD Biosciences, San Jose, CA), anti-FcεRI-PE (eBiosciences, San Diego, CA) or appropriate isotype control antibody (BD Biosciences) for 30 min at 4°. Cells were washed twice and in the cases where the primary antibody was unconjugated, anti-rabbit-phycoerythrin (PE, BD Biosciences) or anti-mouse-PE (BD Biosciences) was added for 30 min at 4°. Cells were washed twice, resuspended in PBS/0·1% BSA and analysed on a FACSArray (BD Biosciences).
CBA assay for cytokines and chemokines
Cells were washed with media and suspended at 1 × 106 cells per well, then stimulated with agonists for 24 hr. Cell free supernatants were isolated and analysed for human cytokine or chemokine expression using the following commercial cytometric bead array (CBA) kits; Human Chemokine Kit, Human T helper 1 (Th1)/Th2 Cytokine KitII and Human Allergy Mediators KitII (BD Biosciences). The minimum detection levels for the chemokines and cytokines are IL-8, 0·2 pg/ml, RANTES (regulated on activation, normal, T-cell expressed, and secreted), 1·0 pg/ml, monokine induced by interferon-γ (MIG), 2·5 pg/ml, monocyte chemoattractant protein-1 (MCP-1), 2·7 pg/ml, inducible protein-10 (IP-10), 2·9 pg/ml, eotaxin, 9·7 pg/ml, IL-2, 2·6 pg/ml, IL-4, 2·6 pg/ml, IL-6, 3·0 pg/ml, IL-10, 2·8 pg/ml, TNF, 2·8 pg/ml, interferon-γ (IFN-γ), 7·1 pg/ml, IL-3, 1·31 pg/ml, granulocyte–macrophage colony-stimulating factor (GM-CSF), 1·61 pg/ml.
Statistical analysis
Each experiment was performed at least three separate times and in quadruplicate and values displayed represent mean ± standard error of the mean. P-values were determined by Student's t-test (between groups) or one-way anova (comparing more than two groups).
Results
Human mast cells degranulate in response to substance P and VIP but not NGF or CGRP
Because the LAD2 cell line is relatively new, we screened several stimuli that have been previously reported to stimulate human or rodent mast cells for their ability to degranulate LAD2 cells. These stimuli included non-receptor-mediated activators (A23187 and compound 48/80), neuropeptides (SP, VIP, NGF and CGRP), hormones (gastrin), lectins (concanavalin A; Con A), osmotic media (iohexol and mannitol) and FcεRI-mediated activation (IgE/anti-IgE). LAD2 cells were incubated with these stimuli for various times (1–60 min; data not shown) and over a range of concentrations (highest effective concentration shown in Table 1). As shown in Table 1, A23187 and compound 48/80 were potent activators of LAD2 cells. VIP and SP activated LAD2 to degranulate but CGRP and NGF did not. Although gastrin, Con A, radiocontrast media (iohexol), and mannitol had previously been shown to cause rodent mast cell degranulation, they had no effect on LAD2 mast cells even at relatively high concentrations (see Table 1). Preincubation of LAD2 with IgE overnight to up-regulate FcεRI did not alter their response to A34187, compound 48/80, SP, VIP, NGF, CGRP, gastrin, Con A, iohexol or mannitol (data not shown). However, LAD2 preincubated with IgE overnight and stimulated with anti-IgE released more β-hexosaminidase (27 ± 2%) than LAD2 that had only been preincubated with IgE for 3 hr and stimulated with anti-IgE (15 ± 3%; data not shown).
Table 1.
Degranulation of LAD2 cells by various stimuli
| Agonist | %β-hex release | Concentration (µg/ml) |
|---|---|---|
| Not receptor mediated | ||
| A23187 | 82 ± 3 | 0·5 |
| Compound 48/80 | 76 ± 7 | 1 |
| Neuropeptides | ||
| Substance P | 78 ± 2 | 10 |
| Vasoactive intestinal peptide (VIP) | 54 ± 1 | 1 |
| Nerve growth factor (NGF) | 5 ± 1 | 10 |
| Calcitonin gene related peptide (CGRP) | 5 ± 1 | 10 |
| Hormone | ||
| Gastrin | 4 ± 1 | 10 |
| Lectin | ||
| Concanavalin A (Con A) | 4 ± 1 | 1 |
| Osmotic | ||
| Radiocontrast media (iohexol) | 4 ± 1 | 100 |
| Mannitol | 5 ± 1 | 10 |
| FcεRI-mediated | ||
| Non-specific IgE/anti-IgE | 27 ± 2 | 10 |
Results shown are from highest concentration tested.
Substance P and VIP activate both LAD2 and primary cultured human mast cells to degranulate
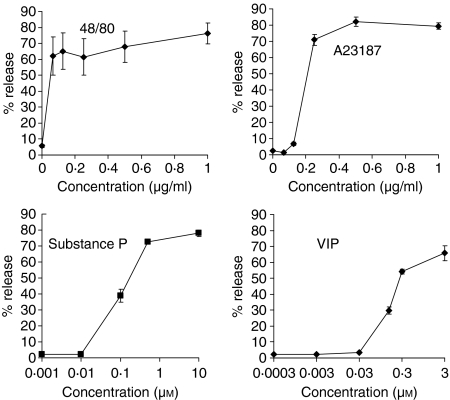
LAD2 cells were stimulated with different concentrations of compound 48/80, A23187, SP and VIP and their concentration response curves were compared (Fig. 1). Compound 48/80 stimulated LAD2 cell degranulation with an IC50 of 0·03 µg/ml and reached maximum release at 0·07 µg/ml. A23187 stimulated LAD2 cell degranulation with an IC50 of 0·2 µg/ml and reached maximum release at 0·5 µg/ml. SP stimulated LAD2 cell degranulation with an IC50 of 0·1 µm and reached maximum release at 0·4 µm. VIP stimulated LAD2 cell degranulation with an IC50 of 0·15 µm and reached a maximum release at 0·3 µm.
Figure 1.
Neuropeptides activate LAD2 human mast cell degranulation. LAD2 cells were activated with compound 48/80, A23187, substance P and VIP for 30 min and β-hexosaminidase release was measured (n = 3, P < 0·01).
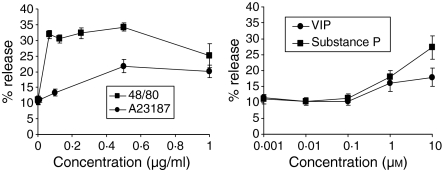
To determine if neuropeptide responsiveness was similar in primary cultured human mast cells (HuMC), we cultured human mast cells from peripheral blood CD34+ progenitors in the presence of SCF and IL-6 for 8 weeks and measured their degranulation in response to the same stimuli (Fig. 2). Compound 48/80 stimulated HuMC degranulation with an IC50 of 0·03 µg/ml, similar to the LAD2 cells. However, compound 48/80 stimulated 34 ± 2% maximum degranulation which was lower than that observed with LAD2 cells. Similarly, A23187 stimulated HuMC degranulation with an IC50 of 0·3 µg/ml and induced 22 + 2% maximum degranulation. VIP and SP also degranulated HuMC, with maximum degranulation of 18 ± 3% and 27 ± 4%, respectively.
Figure 2.
Neuropeptides activate CD34+ progenitor-derived human mast cell degranulation. Human mast cells were cultured from CD34+ progenitor cells as described in Materials and methods. Cultured mast cells were activated by compound 48/80, A23187, substance P and VIP for 30 min and β-hexosaminidase release was measured (n = 3, P < 0·01). c.p.s., counts per second.
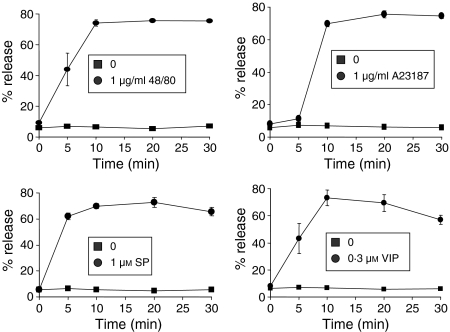
Time course analysis showed that maximum degranulation occurred within 10 min of stimulation of human mast cells with VIP, SP, compound 48/80 and A23187. VIP activated a similar profile of degranulation to that of compound 48/80 because both secretagogues stimulated approximately 50% degranulation by 5 min (Fig. 3).
Figure 3.
Time course of degranulation. LAD2 cells were stimulated with compound 48/80 (a), A23187 (b), substance P and VIP (at 1 µm and 0·3 µm, respectively; circles) or untreated (squares) for indicated times and β-hexosaminidase release was measured (n = 3, P < 0·01).
For purposes of comparison, we characterized human mast cell degranulation in response to FcεRI crosslinking with anti-IgE. Mast cells were sensitized prior to stimulation using either purified myeloma IgE or control medium. In response to anti-IgE stimulation, sensitized LAD2 cells released 27 + 2% of their total β-hex content (data not shown).
Activation by neuropeptides is sensitive to inhibitors of G proteins and phosphoinositol-3 (PI3) kinase
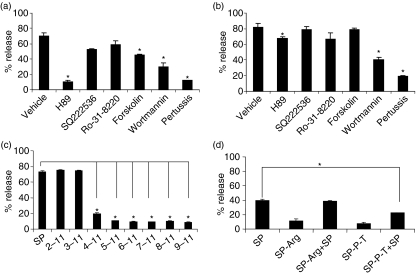
To characterize signal transduction pathways involved in VIP and SP mediated activation of human mast cell degranulation, we pretreated LAD2 cells with inhibitors of PI3 kinase (wortmannin), PKA (H89), adenylate cyclase (SQ 22536), Gαi proteins (pertussis toxin), PKC (Ro-31–8220) and an activator of cAMP (forskolin). All of these molecules have been hypothesized to be important in G protein receptor activation of mast cells. Wortmannin, H89 and pertussis toxin inhibited both VIP and SP activated degranulation (Fig. 4). Forskolin inhibited degranulation induced by VIP but not SP. None of the other compounds had any significant effect on VIP- or SP-activated LAD2 degranulation.
Figure 4.
Mechanisms of neuropeptide activation. (a, b) Activation by neuropeptides is sensitive to inhibitors of G proteins and PI3 kinase. (a) LAD2 cells were pretreated with vehicle (0·1% DMSO), H89 (1 µm), SQ222536 (10 µm), Ro-31–8220 (100 nm), forskolin (10 µm), wortmannin (10 µm) or pertussis toxin (3·5 nm), stimulated with either VIP (0·3 µm) or (b) SP (1 µm) for 30 min and β-hexosaminidase release was measured (n = 3, P < 0·01; P-value calculated relative to vehicle alone). (c) Truncated substance P peptides activate human mast cell degranulation. LAD2 cells were activated with 1 µg/ml of indicated peptides (see also Table 2) for 30 min and β-hexosaminidase release was measured (n = 3, P < 0·01; P-value calculated relative to entire SP peptide). (d) NK1R peptide inhibitors block substance P activation of human mast cells. LAD2 cells were pretreated with 1 µg/ml of inhibitor (SP-Arg or SP-P-T, see Table 2) for 30 min, then activated with substance P (0·1 µm) for 30 min and β-hexosaminidase release was measured (n = 3, P < 0·01; P-value calculated relative to SP alone).
To further characterize the SP effects, we stimulated LAD2 with eight SP peptide constructs missing amino acids from the C-terminus (Table 2). The constructs 2–11 and 3–11 that are missing the arginine and proline amino acids, respectively, caused mast cell degranulation similarly to full length SP (Fig. 4c). However, the constructs missing the lysine residue (4–11, 5–11, 6–11, 7–11, 8–11 and 9–11) did not cause LAD2 degranulation. To determine if the SP effect was mediated via NK1R, we pretreated human mast cells with two peptide antagonists of NK1R, SP (4–11) [D-Pro4 D-Trp7.9] (called SP-P-T in this study), and spantide I (called SP-Arg). Neither antagonist induced significant LAD2 (Fig. 4c). Although SP-Arg had no effect on SP-induced LAD2 degranulation, SP-P-T inhibited SP-induced degranulation by approximately 50%.
Table 2.
Amino acid sequences of substance P and analogues
| Substance P (SP) | Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
| SP-Arg (spantide I) | D-Arg-Pro-Lys-Pro-Gln-Gln-D-Trp-Phe-D-Trp-Leu-Leu-NH2 |
| SP-P-T (4–11) | D-Pro-Gln-Gln-D-Trp-Phe-D-Trp-Leu-Met-NH2 |
| SP(2–11) | Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
| SP(3–11) | Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
| SP(4–11) | Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
| SP(5–11) | Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
| SP(6–11) | Gln-Phe-Phe-Gly-Leu-Met-NH2 |
| SP(7–11) | Phe-Phe-Gly-Leu-Met-NH2 |
| SP(8–11) | Phe-Gly-Leu-Met-NH2 |
| SP(9–11) | Gly-Leu-Met-NH2 |
Chemokine and TNF production induced by agonists
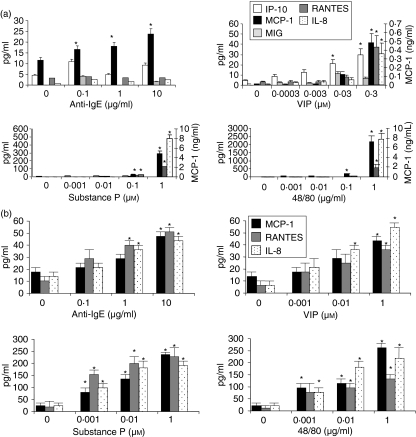
Although some reports have shown that human skin mast cells produce TNF in response to SP,5 it is unknown whether human mast cells produce chemokines or other cytokines in response to neuropeptides, particularly chemokines involved in the recruitment of inflammatory cells such as lymphocytes and monocytes. We therefore stimulated LAD2 and HuMC cells with IgE/anti-IgE, VIP, SP and compound 48/80 and measured production of the chemokines IP-10 (CXCL10), MCP-1 (CCL2), MIG (CXCL9), RANTES (CCL5) and IL-8 (CXCL8; Fig. 5a). While anti-IgE stimulation induced LAD2 to produce relatively small amounts of MCP-1, stimulation with VIP, SP and compound 48/80 potently induced production of MCP-1 (Fig. 5a). Although anti-IgE stimulation did not induce any production of IP-10, MIG, RANTES or IL-8, stimulation with VIP, SP and compound 48/80 induced significant production of these chemokines with the exception of MIG. None of the stimuli tested induced production of eotaxin (CCL11; data not shown). By comparison, HuMC stimulated with anti-IgE, VIP, SP and compound 48/80 produced MCP-1, RANTES and IL-8 (Fig. 5b). Stimulation with SP and compound 48/80 induced HuMC to produce the highest quantities of these cytokines.
Figure 5.
Neuropeptides activate LAD2 cells to produce chemokines. (a) LAD2 cells were stimulated with IgE/anti-IgE (see legend, Fig. 4), substance P, VIP, or compound 48/80 for 24 hr and IP-10, MCP-1, MIG, RANTES and IL-8 production was measured in cell-free supernatants (n = 3, P < 0·01). (b) HuMC were stimulated as above and IP-10, MCP-1, MIG, RANTES and IL-8 production was measured in cell free supernatants (n = 3, P < 0·01).
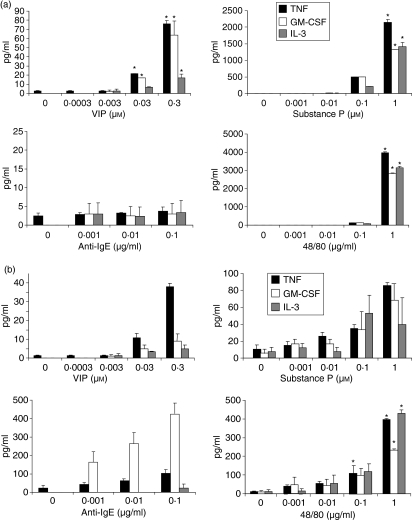
We next measured the production of the cytokines IL-2, IL-3, IL-4, IL-6, IL-10, TNF, GM-CSF and IFN-γ(Fig. 6a). VIP, SP and compound 48/80 induced LAD2 production of TNF, GM-CSF and IL-3 but not IL-2, IL-4, IL-6, IL-10 or IFN-γ. SP and compound 48/80 were the most effective inducers of TNF, GM-CSF and IL-3. Anti-IgE stimulation did not induce LAD2 to produce significant levels of any of the cytokines tested (<7 pg/ml). By comparison, HuMC stimulated with VIP produced small quantities of TNF but no significant levels of GM-CSF or IL-3 (Fig. 6b). SP and compound 48/80 stimulated HuMC to produce small quantities of TNF, GM-CSF and IL-3 whereas anti-IgE stimulated HuMC to produce GM-CSF and small amounts of TNF.
Figure 6.
Neuropeptides activate LAD2 cells to produce cytokines. (a) LAD2 cells were stimulated with IgE/anti-IgE (see legend, Fig. 4), substance P, VIP, or compound 48/80 for 24 hr and TNF, GM-CSF and IL-3 production were measured in cell free supernatants (n = 3, P < 0·01). (b) HuMC were stimulated as above and TNF, GM-CSF and IL-3 production were measured in cell-free supernatants (n = 3, P < 0·01).
Receptor expression by human mast cells
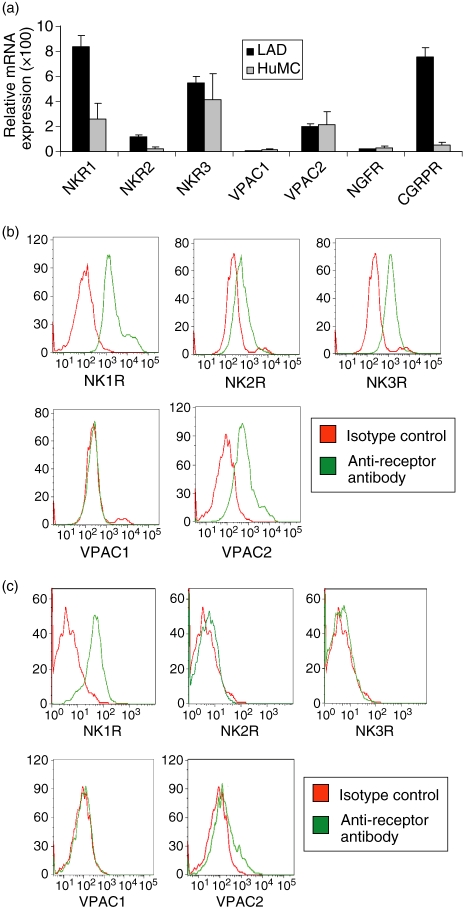
Because LAD2 were shown to be activated by SP and VIP, we tested whether LAD2 expressed the receptors for these ligands (Fig. 7). Real-time PCR analysis showed that LAD2 cells expressed mRNA for the SP receptors, NK1R, NK2R and NK3R, CGRP receptor (CGRPR) and the VIP receptor type 2 (VPAC2) but not VPAC1 or the NGF receptor (NGFR). HuMC expressed mRNA for NKR1, NKR3 and VPAC1. Protein expression of these receptors was tested by flow cytometry and showed that LAD2 expressed cell surface NK1R, NK2R, NK3R and VPAC2 but not VPAC1 confirming results with real-time PCR (Fig. 7b). Flow cytometry also showed that HuMC expressed NK1R, but not NK2R, NK3R, or VPAC1 and low levels of VPAC2 (Fig. 7c).
Figure 7.
LAD2 cells express receptors for VIP and substance P. (a) Receptor mRNA expression was measured by real-time PCR analysis of LAD2 and HuMC total RNA. (b) Surface expression of NK1R, NK2R, NK3R, VPAC1 and VPAC2 by LAD2 cells (b) and HuMC (c) was measured by flow cytometry.
Effect of stimulation on human mast cell neuropeptide receptor expression
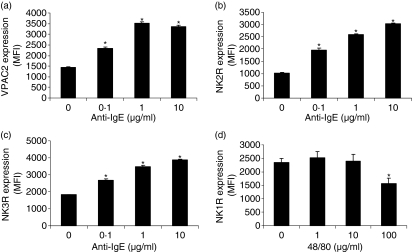
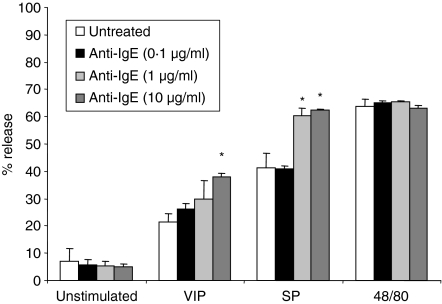
As it has been suggested that activation of human mast cells can alter receptor expression and alter mast cell responsiveness to non-IgE-mediated stimuli,19 we next measured neuropeptide and anaphylatoxin receptor expression by LAD2 following activation by IgE/anti-IgE, VIP, SP, and compound 48/80 (Fig. 8). LAD2 cells were treated with different concentrations of stimuli and expression of NK1R, NK2R, NK3R, VIPR2, and VIPR1 expression was measured by flow cytometry. IgE/anti-IgE stimulation up-regulated expression of VPAC2, NK2R and NK3R (Fig. 8a–c) and did not alter NK1R expression (data not shown). Compound 48/80 (100 ng/ml) stimulation down-regulated human mast cell expression of NK1R (Fig. 8d).
Figure 8.
Stimulation with anti-IgE or compound 48/80 modifies neuropeptide receptor expression by LAD2 cells. (a–c) LAD2 cells were stimulated with IgE/anti-IgE or for 24 hr and expression of VPAC2, NK2R and NK3R was measured by flow cytometry. (d) LAD2 cells were stimulated with compound 48/80 for 24 hr and expression of NK1R was measured by flow cytometry. Expression is presented as mean fluorescence intensity (MFI; n = 3, P < 0·01).
To determine if IgE/anti-IgE-mediated up-regulation of the NK and VPAC receptors renders the human mast cells more sensitive to SP or VIP stimulation, we pretreated LAD2 cells with IgE/anti-IgE for 24 hr then stimulated with SP, VIP or compound 48/80 and measured degranulation (Fig. 9). Cells pretreated with IgE/anti-IgE were more sensitive to both VIP and SP stimulation, but not compound 48/80.
Figure 9.
Preincubation of human mast cells with IgE/anti-IgE renders them more sensitive to SP and VIP stimulation. Sensitized LAD2 cells were preincubated with anti-IgE (10 µg/ml) for 24 hr, then stimulated with either SP (1 µm), VIP (0·3 µm) or compound 48/80 (1 µg/ml) and degranulation was measured. (n = 3, P < 0·01; P-value calculated relative to agonist alone).
Discussion
FcεRI-independent activation of human mast cells is thought to contribute to the pathophysiology of asthma,20 migraines,21 bladder inflammation,22 allergic rhinitis,23 hair growth,9 and conditions worsened by stress,24 such as arthritis or psoriasis.25 The central nervous system can activate mast cells through classical conditioning,26 and induce activation during times of stress.22,27,28 In diseases such as experimental allergic encephalomyelitis (EAE), mast cells may produce chemotactic factors that can recruit nearby inflammatory cells such as T cells.29,30 In the present report, we show that human mast cells degranulate and produce chemokines in response to neuropeptides SP and VIP but not CGRP or NGF. Chemokines produced included MCP-1, IP-10, RANTES and IL-8 which can recruit monocytes, eosinophils and neutrophils. Furthermore, activation of human mast cells by SP or VIP modulated expression of some important mast cell receptors such as FcεRI and Kit.
SP is a member of the tachykinin family of neuropeptides that bind three neurokinin receptors (NK1R, NK2R and NK3R, although SP preferentially binds NK1R).31 Although SP has been described as a neuronal peptide, rodent studies have demonstrated that macrophages, eosinophils, lymphocytes and dendritic cells may also produce SP.32–35 In the spinal cord, SP participates in neurotransmission of pain and modulates autonomic reflexes.31 In the periphery, SP is localized in the primary sensory neurons and neurons intrinsic to the gastrointestinal, respiratory and genitourinary tracts.31 Centrally and peripherally released SP is involved in stress-induced bladder damage and inhibition of NK1R in mouse models of bladder damage prevents stress-induced inflammation,10,22 a process thought to be mediated by mast cells. The content of SP in human airways is increased in asthma, suggesting that SP may be involved in mast cell activation in asthma.33 SP-induced release of inflammatory mediates such as histamine may potentiate tissue injury, and SP-induced chemokine release may stimulate leucocyte recruitment, thereby further amplifying the inflammatory response. Our study shows that SP is the most potent of the neuropeptide agonists and induces production of nanogram quantities of chemokines and TNF from human mast cells. This observation is significant for at least two reasons. First, human mast cells, both CD34+ derived and those isolated from tissue have not been found to produce such high quantities of mediators in vitro in response to any other stimulus heretofore tested, including antigen.19 In a study by Guhl et al. SP degranulated human skin mast cells but did not induce the production of TNF or IL-8.36 In fact, it had been proposed by some groups that human mast cells, unlike their rodent counterparts, required an unknown tissue-specific factor in order to produce large quantities of TNF.37–39 Our studies suggest that SP may contribute to the activation of mast cells to produce large quantities of TNF in human subjects stimulated with antigen in vivo.40 Second, SP activates human mast cells to produce chemokines that can potentially recruit other effector cells such as T cells and eosinophils that mediate the inflammatory process during the late phase response associated with experimental antigen challenge.41 It is thus possible that nerves are activated to release SP in responses to antigen and feedback to trigger mast cell cytokine and chemokine generation. Activation of nerves could result in part from exposure to mast-cell derived histamine and leukotrienes.42,43
Interestingly, LAD2 do not produce large quantities of cytokines or chemokines when stimulated by IgE/anti-IgE. This is likely caused by: (1) the IgE/anti-IgE stimulus crosslinks relatively few FcεRI and therefore delivers a mild stimulus and (2) LAD2 are intermediately differentiated mast cells and therefore may not be mature enough to produce large quantities of cytokines in response to FcεRI stimulation. In this context, we believe that LAD2 may represent a subset of mast cells, perhaps reflecting a mast cell phenotype found in the brain or near nerves. As such, neuropeptide activation of LAD2 may be especially relevant to in vivo mast cell responses.
SP has been shown to bind to the mast cell surface, and an inactive analogue of SP has been shown to block SP induced activation of rat mast cells and mast cells in human skin.44 Our results showed that substance P (4-11)[D-Pro4 D-Trp7 9] (called SP-P-T in this study), a SP antagonist, blocked SP activation of human mast cells by approximately 50% while another inhibitor, SP-Arg (spantide I), had no affect (Fig. 5). SP-P-T has the same amino acid sequence as SP but has two modified D amino acids (see Table 2) and is an effective inhibitor in vivo.45,46 Although spantide I is also an effective inhibitor of SP effects in vivo, it is unclear if these are caused by mast cell activation.47,48 Our studies also showed that the first three C-terminus amino acids of SP (Arg-Pro-Lys) are unimportant for its activity on human mast cells because SP lacking these amino acids was still able to activate human mast cell degranulation (Fig. 5c). However, the loss of the C-terminus lysine caused SP to lose its stimulatory activity, suggesting that this lysine residue is either responsible for receptor binding or G protein activation. The C terminus nonapeptide segment folds into an α-helix and is important for interaction with and activation of the NK1R.49 Therefore, these studies suggest that SP may partly activate mast cells via NK1R. This is consistent with observations made by Guhl et al. who found that LAD2 production of TNF was only partially NK1R dependent even though LAD2 express mRNA for NK1R and NK2R.36 Consistent with rodent mast cells,50–52 we have shown that human mast cells express NK1R, NK2R and NK3R on their cell surface and SP may activate human mast cells via one or more of these receptors. However, because the SP-P-T inhibitor only partially blocked SP's effect, SP triggering of mast cells may also involve insertion of the amphiphilic SP molecule into the cell membrane, thus enabling direct activation of G proteins.53 It has been demonstrated that SP can translocate rapidly into rodent mast cells and is able to initiate secretion when introduced directly into the cytosol.54,55 It is likely that SP activation of human mast cells, like that of other basic secretogogues like compound 48/80, is receptor-independent and involves direction interaction with G proteins via the cell membrane.
Our data also show that VIP is not as potent a secretagogue as SP and appeared not to reduce Kit expression and only modestly reduced FcεRI expression. VIP is a prominent neuropeptide produced by nerves in the central and peripheral nervous system as well as lymphocytes and mast cells.56,57 However, rat peritoneal, intestinal and lung mast cells exclusively express a truncated form of VIP having a free carboxyl-terminal asparagine and the function of this peptide is unclear.56,57 VIP is preferentially produced by Th2 CD4+ cells after antigenic stimulation.58,59 VIP binds two receptors referred to as VPAC1 and VPAC2 expressed by lymphocytes, dendritic cells, NK cells and macrophages.59 The presence of functional VIP receptors on mast cells had been suggested by Groneberg et al. who reported VPAC2 mRNA expression in human skin mast cells,60 confirming early reports in a murine mastocytoma cell line.61 To our knowledge, the present study is the first to show VPAC protein expression in human mast cells. We have also shown that human mast cells express VPAC2 but not VPAC1. Because VPAC1 and VPAC2 have similar affinities for VIP and pituitary adenylate cyclase-activating polypeptide (PACAP),59 the significance of this differential expression is unclear.
Our data show that SP and VIP activated degranulation was blocked by H89, wortmannin and pertussis toxin. Although a range of H89 concentrations was tested (0·1–10 mm) only the highest concentration (10 mm) inhibited degranulation. H89 is a cell-permeable, selective, and potent inhibitor of protein kinase A (PKA, Ki = 48 nm) but it also inhibits other kinases at higher concentrations: CaM kinase II (Ki = 29·7 µm), casein kinase I (Ki = 38·3 µm), myosin light chain kinase (Ki = 28·3 µm), protein kinase C (Ki = 31·7 µm), and RhoA/Rho Kinase II (ROCK-II) (IC50 = 270 nm).17 Therefore, our data indicate that a kinase other than PKA may be involved in both SP and VIP signalling. Wortmannin (1 µm) inhibited SP-induced and VIP-induced β-hex release by approximately 50%, suggesting that PI3 kinase is involved in signalling for degranulation. Pertussis toxin inhibited SP-induced and VIP-induced β-hex release by approximately 80–90%, suggesting that Gαi protein activation is necessary for degranulation. Forskolin inhibited VIP-induced but not SP-induced degranulation, suggesting that VIP signalling is much more susceptible to inhibition by increases in intracellular cAMP levels.
Expression of VIP and VPAC is increased in inflammatory disease, particularly in conditions mediated by mast cells, suggesting that autonomic mucosal innervation may mediate mast cell activation in vivo. For example, a study of neuropeptide expression in mucosal nerve fibres in patients with seasonal allergic rhinitis showed a significant increase in the numbers of VIP- and NPY-immunoreactive nerve fibres in biopsies of rhinitis patients in comparison with sections of normal human nasal mucosa.23 In a separate study, quantitative immunohistochemistry for VPAC2 in acute atopic dermatitis lesions showed a significant decrease in VPAC2 immunoreactivity in mast cells.60 These studies were interpreted to suggest that mast cell activation, either by antigen (via FcεRI) or by neuropeptides such as VIP, may down-regulate VPAC2 receptors. Our studies, however, have shown that stimulation of human mast cells with SP, VIP, and compound 48/80 had no effect on VPAC1 or VPAC2 expression and stimulation with anti-IgE up-regulated VPAC2 expression, leaving this interpretation open to question. In fact, we observe that anti-IgE activation up-regulated both the NK2R and NK3R, supporting similar observations in human intestinal mast cells in which anti-IgE activation induced expression of NK1R in a subpopulation of cells.8 These data suggest that antigen-mediated activation may prime mast cells to respond to neurogenic stimuli and thereby amplify their ability to mediate inflammation. Such an effect could contribute to airway hyperreactivity such as occurs in asthma.62
We found that VIP and SP, but not NGF or CGRP, caused human mast cells to produce chemokines such as RANTES and MCP-1, suggesting that these mediators may be involved in neurogenic inflammation. Injections of RANTES in the skin of humans and of rats caused eosinophil and macrophage recruitment.63,64 This suggests that neuropeptide activation of human mast cells may indirectly cause inflammation via cellular recruitment and may thus contribute to the late phase response in asthma.65 We also found that VIP and SP caused human mast cells to produce GM-CSF and IL-3, both of which can promote the survival of eosinophils, the main inflammatory cell type that persists throughout the late and chronic phases of the allergic reaction.
In conclusion, our studies show that neuropeptide activation of human mast cells not only activates degranulation and release of preformed granule contained mediators, but can also induce the production of cytokines and chemokines including GM-CSF, IL-3, MCP-1, IP-10, RANTES and IL-8. These mediators can recruit and activate T lymphocytes, eosinophils and other inflammatory cells. On the basis of these observations, changes in neuropeptide expression in the respiratory tract may represent an important link between the nervous system and mast cells in the mediation of neuroinflammation. This link may prove useful in the treatment of mast cell mediated diseases, especially those exacerbated by stress.
Acknowledgments
This work was supported by The Ernest S. Bazely Trust and grants R01HL068546 and R01HL078860 from the National Institutes of Health.
Abbreviations
- LAD
Laboratory of Allergic Diseases cells
- FcεRI
Fc epsilon receptor 1
- SP
substance P
- VIP
vasoactive intestinal peptide
- NGF
nerve growth factor
- CGRP
calcitonin gene-related peptide.
References
- 1.Bienenstock J, MacQueen G, Sestini P, Marshall JS, Stead RH, Perdue MH. Mast cell/nerve interactions in vitro and in vivo. Am Rev Respir Dis. 1991;143:S55–S58. doi: 10.1164/ajrccm/143.3_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 2.Singh LK, Pang X, Alexacos N, Letourneau R, Theoharides TC. Acute immobilization stress triggers skin mast cell degranulation via corticotropin releasing hormone, neurotensin, and substance P. A link to neurogenic skin disorders. Brain Behav Immun. 1999;13:225–39. doi: 10.1006/brbi.1998.0541. [DOI] [PubMed] [Google Scholar]
- 3.Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507–15. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 4.Janiszewski J, Bienenstock J, Blennerhassett MG. Picomolar doses of substance P trigger electrical responses in mast cells without degranulation. Am J Physiol – Cell Physiol. 1994;267:C138–C145. doi: 10.1152/ajpcell.1994.267.1.C138. [DOI] [PubMed] [Google Scholar]
- 5.Okayama Y, Ono Y, Nakazawa T, Church MK, Mori M. Human skin mast cells produce TNF-alpha by substance P. Int Arch Allergy Immunol. 1998;117:48–51. doi: 10.1159/000053571. [DOI] [PubMed] [Google Scholar]
- 6.van der Kleij HP, Ma D, Redegeld FA, Kraneveld AD, Nijkamp FP, Bienenstock J. Functional expression of neurokinin 1 receptors on mast cells induced by IL-4 and stem cell factor. J Immunol. 2003;171:2074–9. doi: 10.4049/jimmunol.171.4.2074. [DOI] [PubMed] [Google Scholar]
- 7.Karimi K, Redegeld FA, Heijdra B, Nijkamp FP. Stem cell factor and interleukin-4 induce murine bone marrow cells to develop into mast cells with connective tissue type characteristics in vitro. Exp Hematol. 1999;27:654–62. doi: 10.1016/s0301-472x(98)00083-6. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff SC, Schwengberg S, Lorentz A, et al. Substance P and other neuropeptides do not induce mediator release in isolated human intestinal mast cells. Neurogastroenterol Motil. 2004;16:185–93. doi: 10.1111/j.1365-2982.2004.00502.x. [DOI] [PubMed] [Google Scholar]
- 9.Arck PC, Handjiski B, Kuhlmei A, et al. Mast cell deficient and neurokinin-1 receptor knockout mice are protected from stress-induced hair growth inhibition. J Mol Med. 2005;83:386–96. doi: 10.1007/s00109-004-0627-z. [DOI] [PubMed] [Google Scholar]
- 10.Erin N, Ersoy Y, Ercan F, Akici A, Oktay S. NK-1 antagonist CP99994 inhibits stress-induced mast cell degranulation in rats. Clin Exp Dermatol. 2004;29:644–8. doi: 10.1111/j.1365-2230.2004.01613.x. [DOI] [PubMed] [Google Scholar]
- 11.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–82. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 12.Kulka M, Fukuishi N, Rottem M, Mekori YA, Metcalf D. Mast cells which interact with Escherichia coli upregulate genes associated with innate immunity and become less responsive to FcεRI-mediated activation. J Leukoc Biol. 2006;79:339–50. doi: 10.1189/jlb.1004600. [DOI] [PubMed] [Google Scholar]
- 13.Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34 (+), c-kit (+), and expresses aminopeptidase N (CD13) Blood. 1999;94:2333–42. [PubMed] [Google Scholar]
- 14.Kirshenbaum AS, Kessler SW, Goff JP, Metcalfe DD. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol. 1991;146:1410–5. [PubMed] [Google Scholar]
- 15.Ahn K, Takai S, Pawankar R, et al. Regulation of chymase production in human mast cell progenitors. J Allergy Clin Immunol. 2000;106:321–8. doi: 10.1067/mai.2000.108107. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita T, Sawai N, Hidaka E, Yamashita T, Koike K. Interleukin-6 directly modulates stem cell factor-dependent development of human mast cells derived from CD34 (+) cord blood cells. Blood. 1999;94:496–508. [PubMed] [Google Scholar]
- 17.Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT–PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. RESEARCH0034.1–.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kulka M, Metcalfe DD. Chemokine production by human mast cells exposed to E. coli. J Allergy Clin Immunol. 2002;117 S65 (Abstract) [Google Scholar]
- 20.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy. 2005;59:1139–52. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 21.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Ercan F, Akici A, Ersoy Y, Hurdag C, Erin N. Inhibition of substance P activity prevents stress-induced bladder damage. Regul Pept. 2006;133:82–9. doi: 10.1016/j.regpep.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 23.Heppt W, Dinh QT, Cryer A, et al. Phenotypic alteration of neuropeptide-containing nerve fibres in seasonal intermittent allergic rhinitis. Clin Exp Allergy. 2004;34:1105–10. doi: 10.1111/j.1365-2222.2004.01990.x. [DOI] [PubMed] [Google Scholar]
- 24.Theoharides TC, Cochrane DE. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J Neuroimmunol. 2004;146:1–12. doi: 10.1016/j.jneuroim.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Maurer M, Theoharides T, Granstein RD, et al. What is the physiological function of mast cells? Exp Dermatol. 2003;12:886–910. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- 26.Russell M, Dark KA, Cummins RW, Ellman G, Callaway E, Peeke HV. Learned histamine release. Science. 1984;225:733–4. doi: 10.1126/science.6205449. [DOI] [PubMed] [Google Scholar]
- 27.Papadopoulou N, Kalogeromitros D, Staurianeas NG, Tiblalexi D, Theoharides TC. Corticotropin-releasing hormone receptor-1 and histidine decarboxylase expression in chronic urticaria. J Invest Dermatol. 2005;125:952–5. doi: 10.1111/j.0022-202X.2005.23913.x. [DOI] [PubMed] [Google Scholar]
- 28.Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–75. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- 29.Bebo BF, Jr, Yong T, Orr EL, Linthicum DS. Hypothesis: a possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J Neurosci Res. 1996;45:340–8. doi: 10.1002/(SICI)1097-4547(19960815)45:4<340::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Gregory GD, Robbie-Ryan M, Secor VH, Sabatino JJ, Jr, Brown MA. Mast cells are required for optimal autoreactive T cell responses in a murine model of multiple sclerosis. Eur J Immunol. 2005;35:3478–86. doi: 10.1002/eji.200535271. [DOI] [PubMed] [Google Scholar]
- 31.O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, Shanahan F. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201:167–80. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 32.Weinstock JV, Blum A, Walder J, Walder R. Eosinophils from granulomas in murine schistosomiasis mansoni produce substance P. J Immunol. 1988;141:961–6. [PubMed] [Google Scholar]
- 33.Lambrecht BN, Germonpre PR, Everaert EG, et al. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur J Immunol. 1999;29:3815–25. doi: 10.1002/(SICI)1521-4141(199912)29:12<3815::AID-IMMU3815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Lambert RW, Granstein RD. Neuropeptides and Langerhans cells. Exp Dermatol. 1998;7:73–80. doi: 10.1111/j.1600-0625.1998.tb00306.x. [DOI] [PubMed] [Google Scholar]
- 35.Marriott I, Bost KL. Expression of authentic substance P receptors in murine and human dendritic cells. J Neuroimmunol. 2001;114:131–41. doi: 10.1016/s0165-5728(00)00466-5. [DOI] [PubMed] [Google Scholar]
- 36.Guhl S, Lee HH, Babina M, Henz BM, Zuberbier T. Evidence for a restricted rather than generalized stimulatory response of skin-derived human mast cells to substance P. J Neuroimmunol. 2005;163:92–101. doi: 10.1016/j.jneuroim.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Steffen M, Abboud M, Potter GK, Yung YP, Moore MA. Presence of tumour necrosis factor or a related factor in human basophil/mast cells. Immunology. 1989;66:445–50. [PMC free article] [PubMed] [Google Scholar]
- 38.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;19:274–6. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 39.Okayama Y. Mast cell-derived cytokine expression induced via Fc receptors and Toll-like receptors. Chem Immunol Allergy. 2005;87:101–10. 101–10. doi: 10.1159/000087574. [DOI] [PubMed] [Google Scholar]
- 40.Walsh LJ, Trinchieri G, Waldorf HA, Whitaker D, Murphy GF. Human dermal mast cells contain and release tumor necrosis factor alpha, which induces endothelial leukocyte adhesion molecule 1. Proc Natl Acad Sci USA. 1991;88:4220–4. doi: 10.1073/pnas.88.10.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cieslewicz G, Tomkinson A, Adler A, et al. The late, but not early, asthmatic response is dependent on IL-5 and correlates with eosinophil infiltration. J Clin Invest. 1999;104:301–8. doi: 10.1172/JCI7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor-Clark TE, Kollarik M, MacGlashan DW, Jr, Undem BJ. Nasal sensory nerve populations responding to histamine and capsaicin. J Allergy Clin Immunol. 2005;116:1282–8. doi: 10.1016/j.jaci.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 43.Chen N, Restivo A, Reiss CS. Leukotrienes play protective roles early during experimental VSV encephalitis. J Neuroimmunol. 2001;120:94–102. doi: 10.1016/s0165-5728(01)00415-5. [DOI] [PubMed] [Google Scholar]
- 44.Piotrowski W, Devoy MA, Jordan CC, Foreman JC. The substance P receptor on rat mast cells and in human skin. Agents Actions. 1984;14:420–4. doi: 10.1007/BF01973842. [DOI] [PubMed] [Google Scholar]
- 45.Caranikas S, Mizrahi J, Escher E, Regoli D. Synthesis and biological activities of substance P antagonists. J Med. 1982;25:1313–6. doi: 10.1021/jm00353a008. [DOI] [PubMed] [Google Scholar]
- 46.Mathison R, Escher E, Huggel H, Mizrahi J, Regoli D. Tachykinin antagonists: substitutions in positions 5 and 6 with amino acids from the primary sequence of substance P homologues. Eur J Pharmacol. 1985;114:147–55. doi: 10.1016/0014-2999(85)90622-3. [DOI] [PubMed] [Google Scholar]
- 47.Andoh T, Nagasawa T, Satoh M, Kuraishi Y. Substance P induction of itch-associated response mediated by cutaneous NK1 tachykinin receptors in mice. J Pharmacol Exp Ther. 1998;286:1140–5. [PubMed] [Google Scholar]
- 48.Inoue H, Nagata N, Koshihara Y. Inhibition by actinomycin D of neurogenic mouse ear oedema. Inflamm Res. 1995;44:125–30. doi: 10.1007/BF01782023. [DOI] [PubMed] [Google Scholar]
- 49.Schwyzer R. Membrane-assisted molecular mechanism of neurokinin receptor subtype selection. EMBO J. 1987;6:2255–9. doi: 10.1002/j.1460-2075.1987.tb02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Jonge F, De Laet A, Van Nassauw L, Brown JK, Miller HR, van Bogaert PP, Timmermans JP, Kroese AB. In vitro activation of murine DRG neurons by CGRP-mediated mucosal mast cell degranulation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G178–G191. doi: 10.1152/ajpgi.00528.2003. [DOI] [PubMed] [Google Scholar]
- 51.Kitabatake Y, Kawamura S, Yamashita M, Okuyama K, Takayanagi M, Ohno I. The expression of mRNA for calcitonin gene-related peptide receptors in a mucosal type mast cell line, RBL-2H3. Biol Pharm Bull. 2004;27:896–8. doi: 10.1248/bpb.27.896. [DOI] [PubMed] [Google Scholar]
- 52.Kawamoto K, Aoki J, Tanaka A, Itakura A, Hosono H, Arai H, Kiso Y, Matsuda H. Nerve growth factor activates mast cells through the collaborative interaction with lysophosphatidylserine expressed on the membrane surface of activated platelets. J Immunol. 2002;168:6412–9. doi: 10.4049/jimmunol.168.12.6412. [DOI] [PubMed] [Google Scholar]
- 53.Mousli M, Bronner C, Landry Y, Bockaert J, Rouot B. Direct activation of GTP-binding regulatory proteins (G-proteins) by substance P and compound 48/80. FEBS Lett. 1990;259:260–2. doi: 10.1016/0014-5793(90)80023-c. [DOI] [PubMed] [Google Scholar]
- 54.Chahdi A, Mousli M, Landry Y. Substance P-related inhibitors of mast cell exocytosis act on G-proteins or on the cell surface. Eur J Pharmacol. 1998;341:329–35. doi: 10.1016/s0014-2999(97)01480-5. [DOI] [PubMed] [Google Scholar]
- 55.Lorenz D, Wiesner B, Zipper J, Winkler A, Krause E, Beyermann M, Lindau M, Bienert M. Mechanism of peptide-induced mast cell degranulation. Translocation and patch-clamp studies. J Gen Physiol. 1998;112:577–91. doi: 10.1085/jgp.112.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wershil BK, Turck CW, Sreedharan SP, Yang J, An S, Galli SJ, Goetzl EJ. Variants of vasoactive intestinal peptide in mouse mast cells and rat basophilic leukemia cells. Cell Immunol. 1993;151:369–78. doi: 10.1006/cimm.1993.1246. [DOI] [PubMed] [Google Scholar]
- 57.Goetzl EJ, Sreedharan SP, Turck CW. Structurally distinctive vasoactive intestinal peptides from rat basophilic leukemia cells. J Biol Chem. 1988;263:9083–6. [PubMed] [Google Scholar]
- 58.Lygren I, Revhaug A, Burhol PG, Giercksky KE, Jenssen TG. Vasoactive intestinal polypeptide and somatostatin in leucocytes. Scand J Clin Lab Invest. 1984;44:347–51. doi: 10.3109/00365518409083818. [DOI] [PubMed] [Google Scholar]
- 59.Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249–90. doi: 10.1124/pr.56.2.7. [DOI] [PubMed] [Google Scholar]
- 60.Groneberg DA, Welker P, Fischer TC, et al. Down-regulation of vasoactive intestinal polypeptide receptor expression in atopic dermatitis. J Allergy Clin Immunol. 2003;111:1099–105. doi: 10.1067/mai.2003.1477. [DOI] [PubMed] [Google Scholar]
- 61.Waschek JA, Bravo DT, Richards ML. High levels of vasoactive intestinal peptide/pituitary adenylate cyclase-activating peptide receptor mRNA expression in primary and tumor lymphoid cells. Regul Pept. 1995;60:149–57. doi: 10.1016/0167-0115(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 62.Lewis MJ, Short AL, Lewis KE. Autonomic nervous system control of the cardiovascular and respiratory systems in asthma. Respir Med. 2006;100:1688–705. doi: 10.1016/j.rmed.2006.01.019. Epub ahead of print Mar 9. [DOI] [PubMed] [Google Scholar]
- 63.Beck LA, Dalke S, Leiferman KM, Bickel CA, Hamilton R, Rosen H, Bochner BS, Schleimer RP. Cutaneous injection of RANTES causes eosinophil recruitment: comparison of nonallergic and allergic human subjects. J Immunol. 2006;159:2962–72. 1997. [PubMed] [Google Scholar]
- 64.Conti P, DiGioacchino M. MCP-1 and RANTES are mediators of acute and chronic inflammation. Allergy Asthma Proc. 2001;22:133–7. doi: 10.2500/108854101778148737. [DOI] [PubMed] [Google Scholar]
- 65.Munitz A, Piliponsky AM, Levi-Schaffer F. IgE-independent activation of human mast cells indicates their role in the late phase reaction of allergic inflammation. Cell Tissue Bank. 2003;4:25–8. doi: 10.1023/A:1026307812980. [DOI] [PubMed] [Google Scholar]