Abstract
Although intraocular tumours reside in an immune-privileged site, they can circumvent immune privilege and undergo rejection. Ocular tumour rejection typically follows one of two pathways. One pathway involves CD4+ T cells, delayed-type hypersensitivity (DTH), and culmination in ischemic necrosis of the tumour and phthisis (atrophy) of the eye. The second pathway is DTH-independent and does not inflict collateral injury to ocular tissues, and the eye is preserved. In this study, we used a well-characterized tumour, Ad5E1, to investigate the role of CD4+ T cells in the non-phthisical form of intraocular tumour rejection. It has been previously documented that CD4+ T cells and interferon (IFN)-γ are necessary for rejection of these tumours in the eye. In this study, we demonstrate that CD4+ T cells can circumvent immune privilege and infiltrate intraocular Ad5E1 tumours. Following tumour rejection, CD4+ T cells from tumour rejector mice could be adoptively transferred to severe combined immunodeficiency (SCID) mice and protect them from intraocular Ad5E1 tumour growth. Tumour-specific CD4+ T cells produced IFN-γ in response to Ad5E1 tumour antigens. Macrophages also contributed to rejection, as they were present in intraocular Ad5E1 tumours, and local depletion of macrophages resulted in progressive tumour growth. Ocular macrophages contributed to Ad5E1 tumour rejection, as Ad5E1 tumour rejection did not occur in macrophage-depleted SCID mice reconstituted with rejector CD4+ T cells. This demonstrates that macrophage and CD4+ T-cell co-operation is needed for non-phthisical rejection of intraocular tumours.
Keywords: T cells, interferon-γ, tumours, immune privilege, macrophages
Introduction
Ocular immune privilege is necessary to maintain the eye's physiological function of sight. Immune-mediated inflammation has devastating consequences for normal ocular cells and can lead to blindness. To reduce the risk of immune-mediated injury, the eye invokes several immunosuppressive mechanisms to maintain immune privilege. The anterior chamber (AC) of the eye is particularly endowed with such mechanisms. The reduced expression or absence of major histocompatibility complex (MHC) class I antigens on the corneal endothelium lessens the likelihood of injury caused by infiltrating CD8+ cytotoxic T lymphocytes (CTLs).1 The aqueous humour contains a number of anti-inflammatory and immunosuppressive factors that serve to quench inflammation.2–4 Also, antigens introduced into the AC induce an antigen-specific down-regulation of T helper type 1 (Th1) immune responses, a phenomenon known as anterior chamber-associated immune deviation (ACAID).4 Immune privilege allows the prolonged and sometimes permanent survival of foreign tissues and tumours.5,6 In spite of all these mechanisms, ocular immune privilege can be circumvented. Ocular tumours can induce a robust systemic immune response that culminates in tumour rejection.7 Immune rejection of intraocular tumours follows two mutually divergent patterns. One pattern of rejection occurs by a CD4+ T-cell-mediated process that coincides with the development of delayed type hypersensitivity (DTH) responses leading to ischemic necrosis and damage to both the tumour and innocent bystander ocular cells, and culminates in phthisis of the eye.7 The second pattern of T-cell-mediated intraocular tumour rejection is characterized by an intratumour T-cell infiltrate, piecemeal necrosis of intraocular tumour cells, preservation of normal ocular cells, and the absence of phthisis.7,8 The immunoregulatory process that determines which of these two pathways is invoked has an enormous impact on the fate of the eye and the preservation of vision.
We have employed the adenovirus type 5 early region 1 (Ad5E1) tumour to investigate how ocular immune privilege is circumvented. This tumour expresses human Ad5E1 oncogenes and has been used extensively in studies examining the immunopathology and immune mechanisms of intraocular tumour rejection. Although there is no current evidence for an adenoviral aetiology for human intraocular tumours, adenoviral tumours have been successfully used to explore fundamental immune responses to tumour-associated antigens and the MHC restriction of T-cell epitopes on virally induced tumours.9 The Ad5E1 tumour undergoes spontaneous immune rejection following transplantation into the AC of syngeneic C57BL/6 mice. Tumour rejection requires CD4+ T cells and IFN-γ, but does not require tumour necrosis factor (TNF)-α, Fas ligand (FasL), tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), perforin, B cells, natural killer (NK) cells, or CD8+ T cells.10,11 The process of rejection is non-phthisical, as the tumour is eradicated without damage to neighbouring ocular tissue.10 This pristine rejection is attributed to IFN-γ and its effect on the Ad5E1 tumour. We have recently reported that IFN-γ induces apoptosis, reduces proliferation, and inhibits angiogenesis of Ad5E1 tumours.12 As both IFN-γ and CD4+ T cells are required for the non-phthisical rejection of Ad5E1 tumours, we hypothesized that IFN-γ derived from CD4+ T cells was the primary mediator of Ad5E1 tumour rejection. In this study, we discovered that CD4+ T cells migrated to the intraocular tumour and produced IFN-γin vitro when confronted with Ad5E1 tumour antigens.
Macrophages are also involved in Ad5E1 tumour rejection. Recently, it was discovered that depletion of ocular macrophages through subconjunctival (SCJ) injections of liposomes containing the macrophagicidal drug clodronate resulted in progressive Ad5E1 tumour growth in the eye.13 It is well known that macrophages play a central role in immune responses and antitumour immunity.14 Macrophages participate by acting as mediators of tumour cytotoxicity, as activated macrophages are directly cytolytic to tumour cells and are potent producers of IFN-γ,15 and they serve as antigen-presenting cells (APCs) for stimulating CD4+ T cells.16 The latter function may be especially important, as the Ad5E1 tumour is MHC class II negative, and it is unlikely that tumour-specific CD4+ T cells directly recognize and kill Ad5E1 tumour cells. In this study, we found that ocular macrophages function as APCs in the generation of primed CD4+ T cells, but do not appear to be directly cytotoxic to Ad5E1 tumour cells. Depletion of ocular macrophages prevented the generation of tumour-specific CD4+ T cells that were able to produce IFN-γ in response to Ad5E1 tumour antigens, supporting the proposition that macrophages act primarily as APCs. Also, adoptive transfer of primed CD4+ T cells to severe combined immunodeficiency (SCID) mice resulted in progressive tumour growth when macrophages were absent, indicating that macrophages were necessary for the efferent arm of tumour rejection. Together, the results demonstrate that CD4+ T-cell and macrophage co-operation is necessary for the rejection of intraocular tumours without the generation of ocular tissue damage.
Materials and methods
Animals
C57BL/6 (H-2b) mice were obtained from either The Jackson Laboratory (Bar Harbor, ME) or the National Cancer Institute (Frederick, MD). CD4 knockout (KO) mice (B6·129S2-Cd4tm1Mak/J), IFN-γ KO mice [C.129S7(B6)-Ifngtm1Ts/J] and SCID mice (B6.CB17-Prkdcscid/SzJ) were obtained from The Jackson Laboratory. For CD4-depleted mice, C57BL/6 mice were injected intraperitoneally (IP) with 500 µg of goat anti-mouse CD4 (GK1·5) on days −2 and 0 post-tumour injection, and then twice weekly throughout the course of tumour growth. All animals were housed and cared for in accordance with the guidelines of the University Committee for the Humane Care of Laboratory Animals, National Institutes of Health Guidelines on Laboratory Animal Welfare, and the Association for Research in Vision and Ophthalmology statement about the Use of Animals in Ophthalmic and Vision Research.
Tumour cells
Ad5E1 tumour cells were kindly provided by Dr Rene E. M. Toes (Leiden University Medical Center, Leiden, the Netherlands). The tumour cells were generated by the transformation of C57BL/6 mouse embryo cells with the human Ad5E1 adenovirus and propagated as previously described.17 The Ad5E1 tumour elicits both MHC class I-restricted and MHC class II-restricted immune responses.18,19 Single-cell suspensions of Ad5E1 tumour cells were washed in Hanks' balanced salt solution (HBSS; Cambrex, East Rutherford, NJ) and suspended in HBSS for AC injections. Tumour cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL, Grand Island, NY) containing 10% heat-inactivated fetal calf serum, 1%l-glutamine, 1% sodium pyruvate, 1% non-essential amino acids, 1% HEPES buffer, and 1% antibiotic–antimycotic solution.
AC injections
Tumour cell suspensions were injected into the AC as previously described.11 Mice were anaesthetized with 0·66 mg/kg of ketamine hydrochloride (Vetalar; Parke-Davis and Co., Detroit, MI) given intraperitoneally (i.p.). The eye was viewed at low power (×8) under a dissecting microscope, and a sterile 30-gauge needle was used to puncture the cornea at the corneoscleral junction, parallel and anterior to the iris. A glass micropipette (diameter ∼80 μm) was fitted onto a sterile infant feeding tube (#5 French; Tyco Healthcare Group, Mansfield, MA) and mounted onto a 0·1-ml Hamilton syringe (Hamilton, Whittier, CA). A Hamilton automatic dispensing apparatus was used to inject 5 µl of a monocellular suspension of Ad5E1 tumour cells (3 × 105 cells/5 µl). Eyes were examined three times per week, and the tumour volume was recorded as the percentage of AC occupied with tumour.11
Flow cytometry of tumour-bearing eyes
C57BL/6 mice bearing intraocular Ad5E1 tumours were killed between days 11 and 13 post-tumour injection, and tumour-bearing eyes, naïve eyes, and spleens were collected. Tissues were homogenized, and cell suspensions were passed through a 70-µm nylon cell strainer. Erythrocytes were lysed, and tissues were resuspended in HBSS containing 0·3% bovine serum albumin (BSA). Cells were then double-stained with fluorochrome-conjugated antibodies (1 µg/ml): either immunoglobulin G (IgG)-fluorescein isothiocyanate (FITC) and IgG-phycoerythrin (PE), or anti-CD4-FITC and anti-CD3-PE (BD Pharmingen, San Diego, CA). The presence of macrophages was also examined by staining the cells with anti-F4/80-PE (eBioscience, San Diego, CA). Cells were incubated with antibodies for 30 min at 4°, washed three times with HBSS, and resuspended in phosphate-buffered saline (PBS) containing 0·3% BSA. Cells were then assessed for fluorescence in a FACScan flow cytometer (BD Biosciences, Palo Alto, CA), and the results were analysed using CellQuest version 3·1f software (BD Biosciences).
Adoptive transfer experiments
C57BL/6 mice were injected with Ad5E1 tumours as described above. The tumours were spontaneously rejected in these animals within 2–3 weeks post-tumour injection. Following rejection, these animals were killed, splenocytes were collected, and erythrocytes were lysed. Splenocytes were then incubated with CD4-specific microbeads (10 µl of beads per 107 cells; Miltenyi Biotec, Auburn, CA) in 0·5% BSA in PBS for 15 min at 4°. The cells were washed with 0·5% BSA in PBS followed by magnetic separation using LS+ columns as described by the manufacturer (Miltenyi Biotec). The retained cells were eluted from the column. To ensure that no contaminating CD8+ T cells were present, the cells were subsequently treated with 1 µg/ml anti-mouse CD8 (BD Pharmingen), followed by incubation with Low-Tox rabbit complement (Cedarlane Laboratories, Burlington, NC). Cells were washed three times and resuspended in HBSS, and injected intravenously (i.v.) into SCID mice using a 1 : 1 donor to recipient ratio. Following adoptive transfer, recipient mice were injected with Ad5E1 tumour cells into the AC as described above.
In vitro stimulation of CD4+T cells and IFN-γ enzyme-linked immunosorbent assay (ELISA)
Intraocular tumour-bearing animals (day 17 post-tumour injection) were killed and spleens obtained. Spleens were homogenized and spleen cells dissociated through nylon mesh. CD4+ T cells were isolated from spleens using mouse CD4 microbeads and magnetic cell sorting as described above.
To obtain APCs, naïve mouse spleens were homogenized and dissociated through nylon mesh. Spleen cells were incubated at 37° for 30 min with 1 mg/ml collagenase D (Roche, Indianapolis, IN), then plated on Primaria™ tissue culture dishes (BD Biosciences) and incubated for 4 hr at 37°. Non-adherent cells were aspirated, leaving adherent macrophages and dendritic cells to serve as APCs.
CD4+ T cells (1 × 106) were incubated alone (negative control), with 5 µg of anti-CD3 (positive control), with Ad5E1 stimulator cells (1 × 106) treated with mitomycin C (Sigma-Aldrich, St Louis, MO) (40 µg/ml; 45 min; 37°), or with APCs and mitomycin C-treated Ad5E1 stimulator cells (1 × 106) in 35 mm × 10 mm tissue culture dishes (BD Biosciences) in triplicate. Cells were incubated for 72 hr at 37°. Supernatants were harvested, and levels of IFN-γ in cell supernatants were determined using a mouse IFN-γ Quantikine ELISA kit (R & D Systems, Minneapolis, MN).
NK cell cytotoxicity assay
NK-cell-mediated cytolysis of tumour targets was assessed using a conventional 4-hr 51Cr release assay as previously described.20 Lymphokine-activated killer (LAK) cells were used as effectors and were obtained from C57BL/6 mice. LAK cells have been previously used in lieu of resting NK cells for a variety of in vitro and in vivo experiments, as they give higher and more consistent levels of cytolysis than resting NK cells. Moreover, LAK cells demonstrate the same in vivo effector function as NK cells.21 Briefly, spleens were collected, erythrocytes were lysed, and splenocytes were washed with HBSS before 4 days of culture at 37°, 10% CO2, in complete DMEM medium containing recombinant human interleukin (IL)-2 (100 U/ml; Hoffman-La Roche, Nutley, NJ). This procedure has previously been shown to enrich for NK cells.22 Cells were also incubated with recombinant mouse IFN-γ (2, 20, or 200 U/ml; R & D Systems). After 4 days of culture, LAK cells were collected and used as a source of effector cells in cytotoxicity assays where 51Cr-labelled Ad5E1 tumour cells were used as targets.
Liposome-encapsulated dichloromethylene diphosphonate
Multilamellar liposomes were prepared as described previously.23 Briefly, 8 mg of cholesterol and 86 mg of phosphatidylcholine (Sigma) were dissolved in 10 ml of chloroform in a round-bottomed flask. After low-vacuum rotary evaporation at 37°, a thin film was formed on the inner surface of the flask. This film was then dispersed by gentle rotation for 10 min in PBS for the preparation of PBS-containing liposomes (PBS-LIP). For clodronate liposomes (C12MDP-LIP), 2·5 g of C12MDP (kindly provided by Roche, Mannheim, Germany) was dissolved in 10 ml of PBS. The suspension was kept for 2 hr at room temperature and sonicated for 3 min at 20°. To remove free C12MDP, the liposomes were washed twice by centrifugation in PBS at 100 000 g for 30 min and resuspended in 4 ml of PBS that contained approximately 20 mg of C12MDP. Each 100 µl of C12MDP-LIP suspension contained 1 mg of clodronate. The cytotoxicities of C12MDP-LIP and PBS-LIP were tested for in vitro toxicity against RAW 264·7 macrophages before use. Liposomes were stored at 4° for up to 1 month.
Macrophage depletion
Depletion of ocular macrophages was established by subconjunctival injection of C12MDP-LIP. Under an operating microscope, the conjunctiva was lifted and the C12MDP-LIP suspension was injected into the bulbar conjunctiva using a 30-gauge needle mounted on a 1-ml tuberculin syringe. Injection of the C12MDP-LIP suspension resulted in a bleb around the injection site. To obtain a more equal distribution of the suspension around the limbus, the dose was divided by injecting at four different sites around the limbus until we obtained a circular conjunctival bleb. PBS-LIP was used as a negative control for macrophage depletion. Injections occurred at day 0 post-tumour injection, and were repeated every 3–4 days throughout tumour observation.
Statistics
Student's t-test was used to assess the statistical significance of the differences between experimental and control groups. A P-value of < 0·05 was considered significant.
Results
Progressive growth of intraocular tumours in CD4+ T-cell-deficient mice
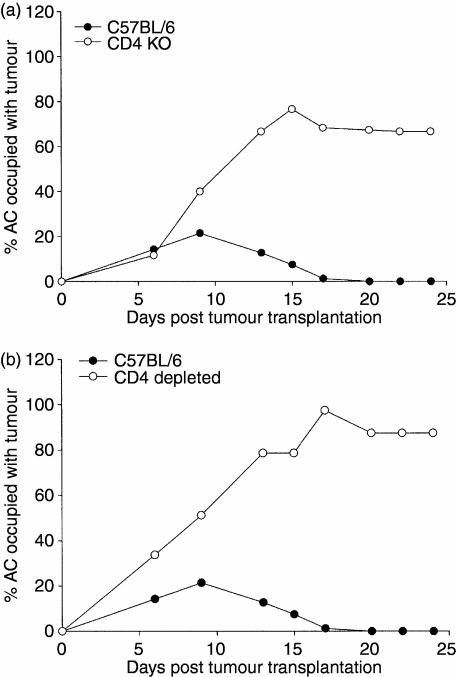
We confirmed that CD4+ T cells were required for the rejection of intraocular Ad5E1 tumours. Ad5E1 tumours were injected into the AC of wild-type C57BL/6 and CD4 KO mice. Whereas Ad5E1 tumours were rejected in C57BL/6 mice, tumours grew progressively in CD4 KO mice (Fig. 1a). Also, C57BL/6 mice that were depleted of CD4+ T cells by periodic injections of anti-CD4 (GK1·5) antibody had progressively growing intraocular Ad5E1 tumours (Fig. 1b), confirming previous work carried out by other researchers.10
Figure 1.
Intraocular tumour growth in C57BL/6, CD4-depleted, and CD4 knockout (KO) mice. Adenovirus type 5 early region 1 (Ad5E1) tumour cells (3 × 105 cells/5 µl) were injected into the anterior chamber (AC) on day 0. Tumour growth was scored as the percentage of AC occupied by tumour. (a) CD4 KO mice (n = 5). This experiment was repeated an additional time with similar results. (b) CD4-depleted mice (n = 5). To obtain CD4-depleted mice, C57BL/6 mice were injected intraperitoneally with 500 µg of goat anti-mouse CD4 (GK1·5) on days −2 and 0 post-tumour injection, and then twice weekly throughout the course of tumour growth. This experiment was repeated an additional time with similar results.
CD4+ T-cell-independent tumour rejection occurs at extraocular sites, but is excluded from the eye
The requirement of CD4+ T cells for rejection of intraocular Ad5E1 tumours raised the question as to whether CD4+ T cells were necessary for rejection of Ad5E1 tumours in general or if this was a unique property of the intraocular tumours. Accordingly, Ad5E1 tumour cells (3 × 105 cells/5 µl) were injected subcutaneously (s.c.) into panels of CD4 KO mice, IFN-γ KO mice, and wild-type C57BL/6 mice and s.c. tumour growth was assessed three times per week. Although previous results indicated that Ad5E1 tumour cells grew progressively when transplanted into the eyes of CD4 KO mice and IFN-γ KO mice, none of the previously mentioned naïve mice injected s.c. with Ad5E1 tumour cells developed tumours, indicating that a CD4+ T-cell- and IFN-γ-independent form of tumour rejection was induced at subcutaneous sites, but was excluded from the eye (Table 1). Interestingly, CD4 KO and IFN-γ KO mice that rejected a primary s.c. challenge of Ad5E1 tumour cells also rejected a secondary AC challenge of Ad5E1 tumour cells (Table 1). This demonstrates a striking discordance in the nature of the immune responses induced by subcutaneous and intraocular tumours. CD4- and IFN-γ-independent immune effector mechanisms are induced by subcutaneous tumours, but not by intraocular tumours. However, once induced, these mechanisms are expressed within the eye, and culminate in tumour rejection.
Table 1.
Interferon (IFN)-γ-independent and CD4+ T-cell-independent tumour rejection can be induced by subcutaneous tumours, but not by intraocular tumours
| First tumour challenge1 | Second tumour challenge | ||
|---|---|---|---|
| Host | Anterior chamber | Subcutaneous | AC tumour growth of s.c. challenged mice2 |
| C57BL/6 | Rejected (30/30) | Rejected (10/10) | Rejected (5/5) |
| IFN-γ KO | Not rejected (30/30) | Rejected (10/10) | Rejected (5/5) |
| CD4 KO | Not rejected (10/10) | Rejected (5/5) | Rejected (5/5) |
Adenovirus type 5 early region 1 (Ad5E1) tumour cells (3 × 105 cells) were injected either into the anterior chamber (AC) or subcutaneously (s.c.). Tumour growth was observed three times per week either by biomicroscopy (anterior chamber) or by palpation (subcutaneous).
Ad5E1 tumour cells were injected s.c. 15–17 days prior to AC challenge.
KO, knockout.
CD4+ T cells infiltrate intraocular Ad5E1 tumours
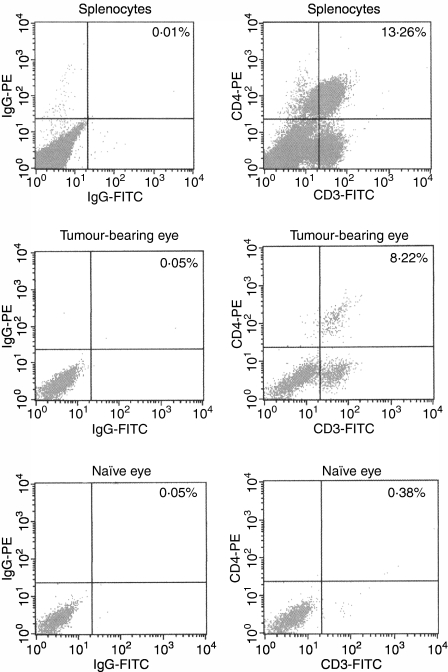
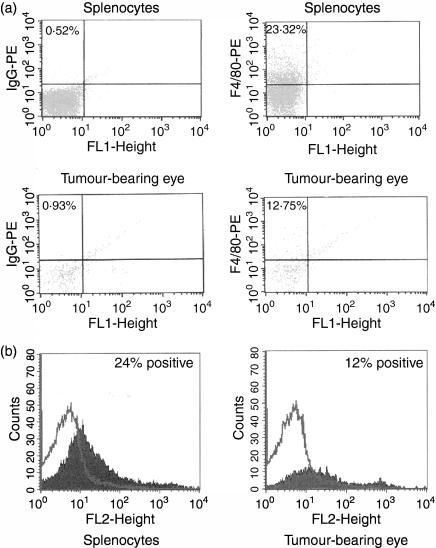
Previous studies briefly mentioned that CD4+ T cells were present in intraocular Ad5E1 tumours on the basis of histological observation.10 In order to verify and quantify the relative numbers of CD4+ T cells infiltrating these tumours, we harvested tumour-bearing eyes (experimental group), naïve eyes (negative control), and splenocytes (positive control) at the early onset of intraocular tumour resolution (days 11–13) and performed fluorescence-activated cell sorter (FACS) analysis to detect the presence of CD4+ CD3+ T cells. A significant number of CD4+ CD3+ cells were found to be present in Ad5E1 tumour-bearing eyes (Fig. 2). By contrast, no CD4+ CD3+ cells were found in naïve eyes (Fig. 2).
Figure 2.
CD4+ T cells are present in adenovirus type 5 early region 1 (Ad5E1) tumour-bearing eyes. C57BL/6 mice bearing Ad5E1 tumours were killed between days 11 and 13 post-tumour injection, and tumour-bearing eyes, naïve eyes, and spleens were harvested. All tissues were stained for the presence of CD4+ CD3+ cells, using anti-CD4-phycoerythrin (PE) and anti-CD3-fluorescein isothiocyanate (FITC) antibodies. Staining was then assessed by flow cytometric analysis. CD4+ CD3+ cells were present in splenocytes (positive control) and Ad5E1 tumour-bearing eyes. CD4+ CD3+ cells were absent in naïve eyes, indicating that these cells only migrate to tumour-bearing eyes following injection of tumour. IgG, immunoglobulin G.
Adoptively transferred CD4+ T cells from tumour rejector mice confer tumour protection to SCID mice
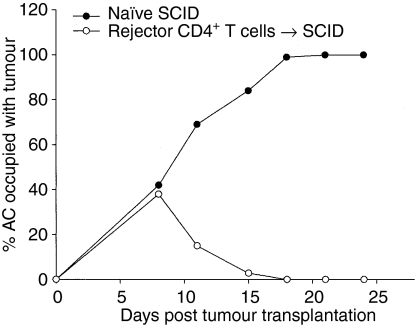
Many studies have demonstrated that CD4+ T cells are needed as helper cells for the generation of CD8+ CTLs, and do not directly play a role in tumour cell cytotoxicity. To determine whether CD4+ T cells act as effector cells that directly mediate intraocular Ad5E1 tumour rejection, we adoptively transferred CD4+ T cells from C57BL/6 mice that previously rejected an intraocular challenge of Ad5E1 tumour cells into SCID mice. Briefly, splenocytes from rejector mice were harvested on day 21 post-tumour injection, and CD4+ T cells were isolated by magnetic bead sorting. Contaminating CD8+ T cells were removed by antibody plus complement treatment. Following the adoptive transfer of CD4+ T cells (∼1 × 107 cells/mouse), SCID mice were challenged in the AC with Ad5E1 tumour cells. Figure 3 shows that SCID mice that received CD4+ T cells from rejector mice eliminated their intraocular Ad5E1 tumours, whereas tumours grew progressively in naïve SCID mice. Also, SCID mice that received CD4– CD8– cells from rejector mice did not reject Ad5E1 tumours, indicating that neither NK cells nor macrophages were sufficient to reject intraocular Ad5E1 tumours (data not shown).
Figure 3.
Adoptive transfer of CD4+ T cells from C57BL/6 mice that have previously rejected adenovirus type 5 early region 1 (Ad5E1) tumours protects severe combined immunodeficiency (SCID) recipients from Ad5E1 tumour outgrowth. Following rejection of an anterior chamber (AC) challenge of Ad5E1, C57BL/6 mice were killed, and their splenocytes harvested. CD4+ T cells were isolated from splenocytes by magnetic bead sorting, and further purified by treatment with anti-CD8 antibody plus complement. One donor-equivalent of CD4+ T cells (∼1 × 107 cells) was injected intravenously into each SCID mouse. SCID mice were challenged in the AC with Ad5E1 tumour cells on the same day as adoptive transfer, and tumour growth was monitored 2–3 times per week. Tumour growth was scored as the percentage of AC occupied by tumour. Tumours grew progressively in naïve SCID mice (n = 5), but were rejected in SCID mice that received rejector CD4+ T cells (n = 5). This experiment was repeated two additional times with similar results.
CD4+ T cells produce IFN-γ in response to Ad5E1 tumour antigens
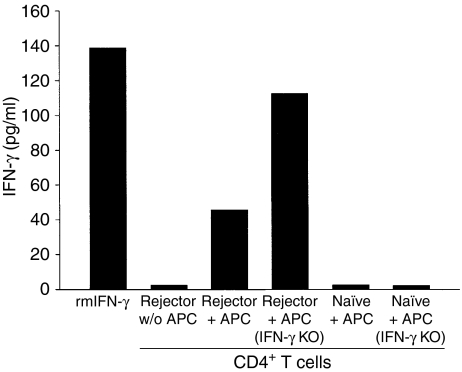
IFN-γ secretion by CD4+ T cells is a primary mediator for the in vivo antitumour effects of CD4+ T cells.24,25 An IFN-γ ELISA was used to confirm that CD4+ T cells from mice that had rejected intraocular Ad5E1 tumours produced IFN-γ when they encountered Ad5E1 tumour antigens. Splenocytes were harvested from rejector mice, and CD4+ T cells were isolated by magnetic bead separation. Contaminating CD8+ T cells were removed by antibody plus complement treatment. CD4+ T cells were then subjected to various treatments: medium alone (negative control), anti-CD3 stimulation (positive control), Ad5E1 tumour cells, or APCs pulsed with Ad5E1 tumour cells (experimental controls). Although Ad5E1 tumour cells are MHC class II negative, CD4+ T cells were still able to recognize Ad5E1 tumour antigens and produce IFN-γ(Fig. 4). Importantly, the IFN-γ was produced by CD4+ T cells and not the APCs in the coculture, as the supernatants from CD4+ T cells incubated with APCs from IFN-γ KO donors contained amounts of IFN-γ that actually exceeded those found in supernatants from CD4+ T cells pulsed with APCs from wild-type donors.
Figure 4.
CD4+ T cells produce interferon (IFN)-γ in response to adenovirus type 5 early region 1 (Ad5E1) tumour antigens. CD4+ cells from tumour-bearing (day 25–30 post-tumour inoculation) C57BL/6 mice were assessed for their ability to secrete IFN-γ. Cells were incubated for 72 hr in the presence of Ad5E1 tumour antigen-pulsed antigen-presenting cells (APCs) from either wild-type C57BL/6 mice or IFN-γ knockout (KO) mice. Supernatants were harvested and IFN-γ secretion was determined by enzyme-linked immunosorbent assay (ELISA). CD4+ T cells produced significant levels of IFN-γ in response to Ad5E1 tumour antigens, through the recognition of tumour antigens presented by APCs. This experiment was repeated two additional times with similar results. w/o, without. rmIFN-γ, recombinant mouse IFN-γ.
IFN-γ activated NK cells are not cytotoxic to Ad5E1 tumours
IFN-γ produced by CD4+ T cells might affect tumour rejection by several mechanisms. One effect is that IFN-γ directly binds to Ad5E1 tumours, leading to increased apoptosis, decreased proliferation, and the production of antiangiogenic chemokines, as we have previously shown.2 In addition, CD4+ T-cell-derived IFN-γ activates tumour-infiltrating cells, which could be cytotoxic to Ad5E1 cells and result in tumour rejection. IFN-γ-activated NK cells have been shown to be cytotoxic towards tumour cell targets.26 We observed the presence of NK1·1+ cells in tumour-bearing eyes by FACS analysis (data not shown). To determine whether NK cells play a role in Ad5E1 tumour rejection, we activated NK cells with IL-2 (positive control) or various concentrations of IFN-γ, and tested cytotoxic activity against Ad5E1 tumours in a 4-hr 51Cr release assay. IL-2-activated NK cells (LAKs) were cytotoxic to Ad5E1 tumours at various effector:target (E:T) ratios. However, IFN-γ-activated NK cells were unable to lyse Ad5E1 tumour targets at any dose or any E:T ratio (data not shown). These data, along with previous results demonstrating that mice depleted of NK cells with anti-NK1·1 antibody rejected Ad5E1 tumours,10 suggest that it is unlikely that the immune rejection of Ad5E1 tumours involves NK cells.
Macrophages are necessary for rejection of intraocular Ad5E1 tumours
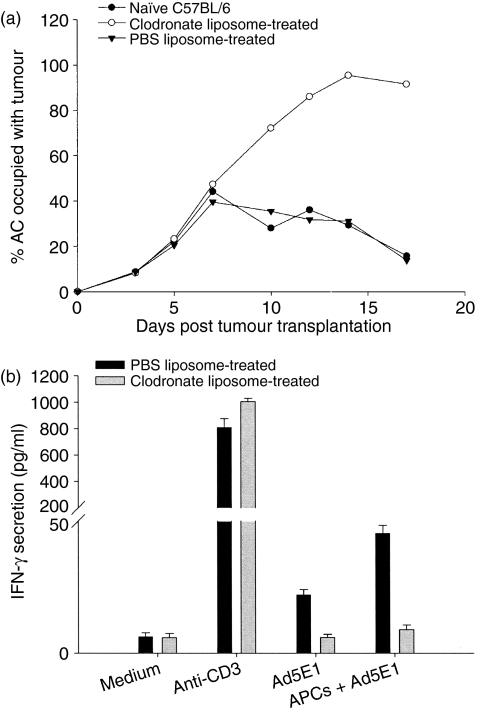
Macrophages are another accessory cell that may be activated by CD4+ T-cell-derived IFN-γ and become cytotoxic to Ad5E1 tumour cells. Macrophages are crucial in the spontaneous rejection of Ad5E1 tumors.13 Boonman et al. have shown that depletion of ocular macrophages through subconjunctival injection of clodronate liposomes leads to progressive Ad5E1 tumour growth.13 However, the role of macrophages as either APCs for the activation of CD4+ T cells or as cytotoxic effector cells has not been determined. Accordingly, we examined tumour-bearing eyes for the presence of F4/80+ macrophages. FACS analysis revealed the presence of macrophages infiltrating Ad5E1 tumours (Fig. 5), confirming the histological results of Boonman et al.13 We also confirmed that local depletion of ocular macrophages through subconjunctival injection of clodronate liposomes leads to progressive Ad5E1 tumour growth (Fig. 6a), indicating that macrophages are necessary for tumour rejection.
Figure 5.
Macrophages are present in adenovirus type 5 early region 1 (Ad5E1) tumour-bearing eyes. C57BL/6 mice bearing Ad5E1 tumours were killed between days 11 and 13 post-tumour injection, and tumour-bearing eyes, naïve eyes, and spleens were harvested. All tissues were stained for the presence of F4/80+ cells, using an anti-F4/80-phycoerythrin (PE) antibody. (a) Staining was then assessed by flow cytometric analysis. F4/80+ cells were present in splenocytes (positive control) and Ad5E1 tumour-bearing eyes, with percentages of PE-positive cells indicated in the upper left-hand corner of fluorescence-activated cell sorter (FACS) profiles. F4/80+ cells were absent in naïve eyes (data not shown), indicating that these cells only migrate to tumour-bearing eyes following injection of tumour. (b) FACS results of an experiment similar to that shown in (a). IgG, immunoglobulin G.
Figure 6.
Macrophages are necessary for rejection of intraocular adenovirus type 5 early region 1 (Ad5E1) tumours. C57BL/6 mice were injected in the anterior chamber (AC) with 3 × 105 Ad5E1 tumour cells. Following tumour injection, two groups of mice were injected subconjunctivally with clodronate-containing or phosphate-buffered saline (PBS)-containing liposomes, respectively. Liposome injection was repeated every 3–4 days. (a) Ad5E1 tumours were rejected in naïve and PBS liposome-treated mice, but grew progressively in clodronate liposome-treated mice. (b) CD4+ T cells were harvested from the spleens of PBS-treated or clodronate liposome-treated mice, and incubated for 72 hr in medium alone (negative control), with anti-CD3 antibody (positive control), or with antigen-presenting cells (APCs) pulsed with Ad5E1 tumour antigens. Following incubation, supernatants were harvested and interferon (IFN)-γ production examined by enzyme-linked immunosorbent assay (ELISA). CD4+ T cells from clodronate liposome-treated mice were unable to produce IFN-γ in response to Ad5E1 tumour antigens.
To determine whether macrophages function as APCs or cytotoxic effector cells, we first examined whether CD4+ T cells from mice depleted of macrophages produced IFN-γ in response to Ad5E1 tumour antigens. If macrophages function solely as APCs to activate CD4+ T cells, then mice depleted of macrophages should not generate primed T cells that can respond to Ad5E1 tumour antigens. We found that mice depleted of macrophages developed CD4+ T cells that were unable to produce IFN-γ in response to Ad5E1 tumour cells (Fig. 6b), indicating that macrophages serve as APCs that prime and activate Ad5E1-specific CD4+ T cells.
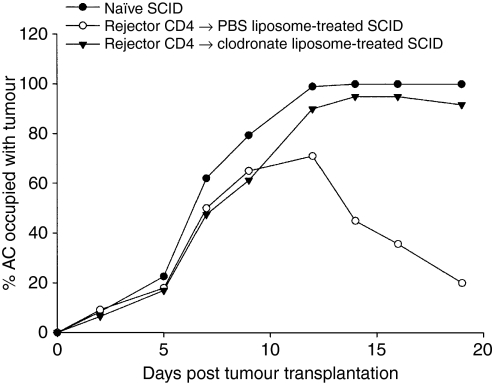
To determine whether macrophages also function in the effector phase in Ad5E1 tumour rejection, we adoptively transferred CD4+ T cells from tumour rejector donors into SCID recipients, as described earlier. However, SCID mice were depleted of ocular macrophages by subconjunctival injection of clodronate liposomes prior to receiving the adoptively transferred CD4+ T cells. Therefore, these mice received primed effector CD4+ T cells, but did not have ocular macrophages present to serve as effector cells. SCID mice that received immune CD4+ T cells, and were depleted of ocular macrophages, failed to reject intraocular Ad5E1 tumours (Fig. 7). By contrast, SCID mice that had both immune CD4+ T cells and macrophages (PBS liposome control) were able to reject Ad5E1 tumours. These results indicate that macrophages are involved in the effector phase of the Ad5E1-specific antitumour immune response. However, macrophages alone are not sufficient to mediate tumour rejection, as naïve SCID mice had progressively growing Ad5E1 tumours.
Figure 7.
Macrophages are necessary for CD4+ T cell-dependent rejection of adenovirus type 5 early region 1 (Ad5E1) tumours. Severe combined immunodeficiency (SCID) mice received adoptively transferred CD4+ T cells from C57BL/6 mice that rejected an earlier anterior chamber (AC) challenge of Ad5E1 tumours, and were then injected with 3 × 105 Ad5E1 tumours into the eye. Following tumour injection, mice were treated with subconjunctival injections of either clodronate- or phosphate-buffered saline (PBS)-containing liposomes. Liposome injections were repeated every 3–4 days. Primed CD4+ T cells alone were insufficient to mediate tumour rejection, as mice that received clodronate-containing liposomes developed progressively growing Ad5E1 tumours. Primed CD4+ T cells and macrophages together mediated tumour rejection, as mice that received PBS-containing liposomes rejected Ad5E1 tumours. Macrophages alone did not mediate Ad5E1 tumour rejection, as naïve SCID mice developed progressively growing tumours.
Discussion
The eye expresses immune privilege, which silences the immune response to various antigens, including many tumour antigens. However, some highly immunogenic tumours circumvent ocular immune privilege and undergo immune rejection when transplanted into the anterior chamber.6,27,28 The nature of the tumour antigens that circumvent immune privilege and the lymphoid tissues in which the ocular tumour antigens are presented to the immune system are poorly understood. Syngeneic tumours induced by ultraviolet light, chemical carcinogens, or viruses can abrogate ocular immune privilege and undergo spontaneous immune rejection in the AC of the eye.6,27,28 One study reported that intraocular tumour antigens localize only in the draining submandibular lymph nodes and that CTL activation occurs only in this draining lymph node and CTL do not disseminate systemically. In other intraocular tumour models, tumour-specific CTL can be detected in peripheral lymph nodes and the spleen, and evidence of systemic DTH responses to tumour antigens is readily obtained in footpad and ear swelling assays.7,8,29,30
Regardless of which lymphoid tissue tumour antigens are processed, they are recognized by both CD4+ and CD8+ T cells, which contribute to tumour rejection. Many tumour models describe the role of CD8+ T cells as being the primary mediators of tumour rejection,31–33 which occurs through the direct recognition of tumour antigens expressed on MHC class I determinants and subsequent cytolysis by CD8+ CTLs. However, the role of CD4+ T cells in the rejection of tumours is not entirely clear. Many studies have focused on the role of CD4+ T cells as accessory cells that are needed only for the induction of CD8+ CTLs.34–37 However, there is evidence that CD4+ T cells orchestrate several effector mechanisms in an antitumour immune response, including (1) activation of APCs to present antigen to CD8+ CTLs, (2) activation of tumoricidal macrophages and eosinophils, (3) secretion of IFN-γ, (4) enhancement of chemotherapy efficacy, and (5) involvement in direct tumoricidal activity.38–42 This study characterizes the direct and indirect roles of CD4+ T cells in the rejection of intraocular Ad5E1 tumours in a non-phthisical pattern that circumvents immune privilege, yet preserves ocular integrity.
As most tumour cells are MHC class II-negative, direct cytolysis of tumours by CD4+ T cells seems unlikely. This suggests that CD4+ T cells may use indirect effector mechanisms for tumour rejection, including cytokine secretion. Several studies suggest that IFN-γ plays a critical role in CD4+ T-cell-mediated tumour rejection.24,25,39,40,43 This study supports the hypothesis that CD4+ T-cell-derived IFN-γ is needed for rejection of Ad5E1 tumours. Ad5E1 tumour-infiltrating CD4+ T cells are observed in the eyes of IFN-γ KO mice (data not shown), yet tumours grow progressively. This suggests that IFN-γ is not needed for CD4+ T cells to recognize and infiltrate the intraocular tumour, but is needed for the rejection of intraocular Ad5E1 tumours. However, IFN-γ is necessary for tumour rejection at the immune-privileged ocular site, but is not necessary for tumour rejection at non-immune-privileged sites, as subcutaneous Ad5E1 tumours are rejected in IFN-γ KO mice.12
One mechanism that IFN-γ may utilize to facilitate rejection of intraocular Ad5E1 tumours is direct binding of the IFN-γ receptor expressed by Ad5E1 tumour cells. Signalling through the IFN-γ receptor can induce multiple effects that promote tumour eradication, including the up-regulation of MHC class I and II expression,25 the inhibition of tumour cell proliferation,44 the induction of tumour cell apoptosis,45 and the induction of tumour cell secretion of antiangiogenic chemokines.46–49 In many cases, the antiangiogenic effect of IFN-γ requires tumour cell responsiveness to IFN-γ, rather than host cell responsiveness.46 IFN-γ binds to IFN-γ receptor on tumour cells, causing the local release of antiangiogenic chemokines such as interferon-inducible protein-10 (IP-10), monokine-induced by IFN-γ (Mig) and interferon-inducible T-cell alpha chemoattractant (I-TAC).48–50 These chemokines bind to CXCR3, which is expressed on both vascular endothelial cells and activated T cells.51,52 These chemokines not only inhibit tumour angiogenesis, but also serve as chemoattractants for activated tumour-specific T cells, further augmenting antitumour immunity. The effect of IFN-γ on Ad5E1 tumour cells is pleiotropic, as IFN-γ inhibited proliferation, induced apoptosis, and inhibited angiogenesis of Ad5E1 tumour cells.12
Another mechanism IFN-γ may utilize to mediate rejection of intraocular Ad5E1 tumours is macrophage activation.43,45,53 CD4+ T cells co-ordinate several antitumour effector pathways independent of CTLs, including the activation of macrophages, which migrate to the tumour site, leading to tumour eradication.39,43 Others have shown, and we have confirmed, that macrophages are necessary for rejection of intraocular Ad5E1 tumours, as depletion of ocular macrophages through subconjunctival injection of clodronate-containing liposomes leads to progressive Ad5E1 tumour growth.13 We also found that macrophages infiltrate intraocular Ad5E1 tumours. The importance of macrophages may be twofold, as they may serve both as APCs for the activation of CD4+ T cells and as effector cells contributing to tumour cytotoxicity. In the Ad5E1 tumour model, it appears that macrophages serve as APCs, as the lack of IFN-γ production by CD4+ T cells isolated from clodronate-treated mice strongly suggests a role for macrophages as APCs. As stated above, macrophages can mediate tumour rejection in antitumour immunity.39,43 IFN-γ, which is necessary for rejection of intraocular Ad5E1 tumours,11 is produced by both tumour-specific CD4+ T cells and macrophages.15,54 We investigated the role of macrophages in the rejection of Ad5E1 tumours with macrophage-depleting clodronate-containing liposome injections prior to the adoptive transfer of rejector CD4+ T cells. Adoptive transfer of rejector CD4+ T cells was sufficient to mediate Ad5E1 tumour rejection. However, we found that clodronate-containing liposome-treated SCID mice that received the adoptive transfer of rejector CD4+ T cells were unable to reject intraocular Ad5E1 tumours, suggesting that macrophages might contribute to rejection of Ad5E1 tumours. Macrophages alone were unable to mediate Ad5E1 tumour rejection, as naïve SCID mice with no rejector CD4+ T cells present were unable to reject intraocular Ad5E1 tumours. Macrophage-mediated tumour cell killing is exceptionally slow compared with CTL-mediated cytolysis of tumour cells. We were unable to detect macrophage-mediated killing of Ad5E1 tumour cells in 24-, 48- or 72-hr in vitro cytotoxicity assays (data not shown). Collectively, the data support the conclusion that macrophages contribute to tumour rejection at both the inductive and the effector stages of the CD4+ T-cell response. Although we have not ruled out a role for cytotoxic macrophages, the data thus far argue against it.
Therefore, macrophages and CD4+ T cells appear to co-operate at the efferent arm of the immune rejection of intraocular Ad5E1 tumours.
We suggest the following model for CD4+ T-cell-mediated rejection of Ad5E1 tumour cells. Naïve CD4+ T cells are primed by ocular macrophages. Following activation, activated CD4+ T cells produce multiple cytokines, including IFN-γ, that activate macrophages. Activated macrophages then produce IFN-γ themselves, which supplements the IFN-γ produced by CD4+ T cells. Also, ocular macrophages continuously present antigen to CD4+ T cells in the eye, keeping the CD4+ T cells in an activated state and increasing their IFN-γ production in response to Ad5E1 tumour antigens. This combination of CD4+ T-cell- and macrophage-derived IFN-γ leads to the rejection of Ad5E1 tumours in the eye through the induction of tumour cell apoptosis, the inhibition of tumour cell proliferation, and a prevention of tumour cell angiogenesis.
Acknowledgments
We would like to thank Christina Stevens for her technical assistance with liposome preparation and subconjunctival injections. This work was supported by National Institutes of Health grants EY05631 and EY016664, and an unrestricted grant from Research to Prevent Blindness, New York, NY.
Abbreviations
- AC
anterior chamber
- Ad5E1
adenovirus type 5 early region 1
- CTL
cytotoxic T lymphocyte
- IFN-γ
interferon-γ
References
- 1.Whitsett CF, Stulting RD. The distribution of HLA antigens on human corneal tissue. Invest Ophthalmol Visual Sci. 1984;25:519–24. [PubMed] [Google Scholar]
- 2.Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–89. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 3.Niederkorn JY. See no evil, hear no evil, do no evil: the lessons of immune privilege. Nat Immunol. 2006;7:354–9. doi: 10.1038/ni1328. [DOI] [PubMed] [Google Scholar]
- 4.Niederkorn JY. Immune privilege in the anterior chamber of the eye. Crit Rev Immunol. 2002;22:13–46. [PubMed] [Google Scholar]
- 5.Medawar PB. Immunity to homologous grafted skin. III. The fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br J Exp Pathol. 1948;29:58–69. [PMC free article] [PubMed] [Google Scholar]
- 6.Niederkorn JY, Wang S. Immunology of intraocular tumors. Ocular Immunol Inflammation. 2005;13:105–10. doi: 10.1080/09273940490518586. [DOI] [PubMed] [Google Scholar]
- 7.Knisely TL, Luckenbach MW, Fischer BJ, Niederkorn JY. Destructive and nondestructive patterns of immune rejection of syngeneic intraocular tumors. J Immunol. 1987;138:4515–23. [PubMed] [Google Scholar]
- 8.Knisely TL, Niederkorn JY. Emergence of a dominant cytotoxic T lymphocyte antitumor effector from tumor-infiltrating cells in the anterior chamber of the eye. Cancer Immunol Immunother. 1990;30:323–30. doi: 10.1007/BF01786881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toes RE, Hoeben RC, van der Voort EI, Ressing ME, van der Eb AJ, Melief CJ, Offringa R. Protective anti-tumor immunity induced by vaccination with recombinant adenoviruses encoding multiple tumor-associated cytotoxic T lymphocyte epitopes in a string-of-beads fashion. Proc Natl Acad Sci USA. 1997;94:14660–5. doi: 10.1073/pnas.94.26.14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schurmans LR, Diehl L, den Boer AT, et al. Rejection of intraocular tumors by CD4(+) T cells without induction of phthisis. J Immunol. 2001;167:5832–7. doi: 10.4049/jimmunol.167.10.5832. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Boonman ZF, Li HC, He Y, Jager MJ, Toes RE, Niederkorn JY. Role of TRAIL and IFN-gamma in CD4+ T cell-dependent tumor rejection in the anterior chamber of the eye. J Immunol. 2003;171:2789–96. doi: 10.4049/jimmunol.171.6.2789. [DOI] [PubMed] [Google Scholar]
- 12.Dace DS, Chen PW, Alizadeh H, Niederkorn JY. Ocular immune privilege is circumvented by CD4+ T cells, leading to the rejection of intraocular tumors in an IFN-γ-dependent manner. J Leukoc Biol. 2007;81:421–9. doi: 10.1189/jlb.0806489. [DOI] [PubMed] [Google Scholar]
- 13.Boonman ZF, Schurmans LR, van Rooijen N, Melief CJ, Toes RE, Jager MJ. Macrophages are vital in spontaneous intraocular tumor eradication. Invest Ophthalmol Visual Sci. 2006;47:2959–65. doi: 10.1167/iovs.05-1427. [DOI] [PubMed] [Google Scholar]
- 14.Whitworth PW, Pak CC, Esgro J, Kleinerman ES, Fidler IJ. Macrophages and cancer. Cancer Metastasis Rev. 1990;8:319–51. doi: 10.1007/BF00052607. [DOI] [PubMed] [Google Scholar]
- 15.Munder M, Mallo M, Eichmann K, Modolell M. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J Exp Med. 1998;187:2103–8. doi: 10.1084/jem.187.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homma S, Komita H, Sagawa Y, Ohno T, Toda G. Antitumour activity mediated by CD4+ cytotoxic T lymphocytes against MHC class II-negative mouse hepatocellular carcinoma induced by dendritic cell vaccine and interleukin-12. Immunology. 2005;115:451–61. doi: 10.1111/j.1365-2567.2005.02179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toes RE, Offringa R, Blom RJ, Brandt RM, van der Eb AJ, Melief CJ, Kast WM. An adenovirus type 5 early region 1B-encoded CTL epitope-mediating tumor eradication by CTL clones is down-modulated by an activated ras oncogene. J Immunol. 1995;154:3396–405. [PubMed] [Google Scholar]
- 18.Dace DS, Chen PW, Niederkorn JY. CD8+ T cells circumvent immune privilege in the eye and mediate intraocular tumor rejection by a TNF-α-dependent mechanism. J Immunol. 2007;178:6115–22. doi: 10.4049/jimmunol.178.10.6115. [DOI] [PubMed] [Google Scholar]
- 19.Schurmans LR, den Boer AT, Diehl L, van der Voort EI, Kast WM, Melief CJ, Toes RE, Jager MJ. Successful immunotherapy of an intraocular tumor in mice. Cancer Res. 1999;59:5250–4. [PubMed] [Google Scholar]
- 20.Koo GC, Dumont FJ, Tutt M, Hackett J, Jr, Kumar V. The NK-1.1(–) mouse: a model to study differentiation of murine NK cells. J Immunol. 1986;137:3742–7. [PubMed] [Google Scholar]
- 21.Murphy WJ, Kumar V, Bennett M. Natural killer cells activated with interleukin 2 in vitro can be adoptively transferred and mediate hematopoietic histocompatibility-1 antigen-specific bone marrow rejection in vivo. Eur J Immunol. 1990;20:1729–34. doi: 10.1002/eji.1830200816. [DOI] [PubMed] [Google Scholar]
- 22.Charley MR, Mikhael A, Bennett M, Gilliam JN, Sontheimer RD. Prevention of lethal, minor-determinate graft-host disease in mice by the in vivo administration of anti-asialo GM1. J Immunol. 1983;131:2101–3. [PubMed] [Google Scholar]
- 23.Van Rooijen N. The liposome-mediated macrophage ‘suicide’ technique. J Immunol Meth. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg PD, Kern DE, Cheever MA. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2– T cells. Tumor eradication does not require participation of cytotoxic T cells. J Exp Med. 1985;161:1122–34. doi: 10.1084/jem.161.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber J, Rosenberg S. Modulation of murine tumor major histocompatibility antigens by cytokines in vivo and in vitro. Cancer Res. 1988;48:5818–24. [PubMed] [Google Scholar]
- 26.Ellis TM, McKenzie RS, Simms PE, Helfrich BA, Fisher RI. Induction of human lymphokine-activated killer cells by IFN-alpha and IFN-gamma. J Immunol. 1989;143:4282–6. [PubMed] [Google Scholar]
- 27.Niederkorn JY. The immunopathology of intraocular tumour rejection. Eye (London) 1991;5:186–92. doi: 10.1038/eye.1991.33. [DOI] [PubMed] [Google Scholar]
- 28.Niederkorn JY. Immunoregulation of intraocular tumours. Eye (London) 1997;11:249–54. doi: 10.1038/eye.1997.60. [DOI] [PubMed] [Google Scholar]
- 29.Ma D, Alizadeh H, Comerford SA, Gething MJ, Sambrook JF, Anand R, Niederkorn JY. Rejection of intraocular tumors from transgenic mice by tumor-infiltrating lymphocytes. Current Eye Res. 1994;13:361–9. doi: 10.3109/02713689409167300. [DOI] [PubMed] [Google Scholar]
- 30.Niederkorn JY, Meunier PC. Spontaneous immune rejection of intraocular tumors in mice. Invest Ophthalmol Visual Sci. 1985;26:877–84. [PubMed] [Google Scholar]
- 31.Dailey MO, Pillemer E, Weissman IL. Protection against syngeneic lymphoma by a long-lived cytotoxic T-cell clone. Proc Natl Acad Sci USA. 1982;79:5384–7. doi: 10.1073/pnas.79.17.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kast WM, Offringa R, Peters PJ, Voordouw AC, Meloen RH, van der Eb AJ, Melief CJ. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989;59:603–14. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- 33.Greenberg PD. Therapy of murine leukemia with cyclophosphamide and immune Lyt-2+ cells: cytolytic T cells can mediate eradication of disseminated leukemia. J Immunol. 1986;136:1917–22. [PubMed] [Google Scholar]
- 34.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–30. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 35.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–3. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 36.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–80. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 37.Ridge JP, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–8. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 38.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–94. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 39.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mumberg D, Monach PA, Wanderling S, Philip M, Toledano AY, Schreiber RD, Schreiber H. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci USA. 1999;96:8633–8. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenberg PD, Cheever MA, Fefer A. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2– lymphocytes. J Exp Med. 1981;154:952–63. doi: 10.1084/jem.154.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujiwara H, Fukuzawa M, Yoshioka T, Nakajima H, Hamaoka T. The role of tumor-specific Lyt-1+2– T cells in eradicating tumor cells in vivo. I. Lyt-1+2– T cells do not necessarily require recruitment of host's cytotoxic T cell precursors for implementation of in vivo immunity. J Immunol. 1984;133:1671–6. [PubMed] [Google Scholar]
- 43.Corthay A, Skovseth DK, Lundin KU, Rosjo E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–83. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 44.Coughlin CM, Salhany KE, Gee MS, et al. Tumor cell responses to IFNgamma affect tumorigenicity and response to IL-12 therapy and antiangiogenesis. Immunity. 1998;9:25–34. doi: 10.1016/s1074-7613(00)80585-3. [DOI] [PubMed] [Google Scholar]
- 45.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–95. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 46.Beatty G, Paterson Y. IFN-gamma-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-gamma. J Immunol. 2001;166:2276–82. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 47.Sgadari C, Angiolillo AL, Tosato G. Inhibition of angiogenesis by interleukin-12 is mediated by the interferon-inducible protein 10. Blood. 1996;87:3877–82. [PubMed] [Google Scholar]
- 48.Arenberg DA, Kunkel SL, Polverini PJ, et al. Interferon-gamma-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastases. J Exp Med. 1996;184:981–92. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sgadari C, Farber JM, Angiolillo AL, et al. Mig, the monokine induced by interferon-gamma, promotes tumor necrosis in vivo. Blood. 1997;89:2635–43. [PubMed] [Google Scholar]
- 50.Belperio JA, Keane MP, Arenberg DA, Addison CL, Ehlert JE, Burdick MD, Strieter RM. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 51.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–60. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Loetscher M, Gerber B, Loetscher P, Jones S, Piali L, Clark-Lewis I, Baggiolini M, Moser B. Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes. J Exp Med. 1996;184:963–9. doi: 10.1084/jem.184.3.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xie QW, Whisnant R, Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993;177:1779–84. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blankenstein T, Qin Z. The role of IFN-gamma in tumor transplantation immunity and inhibition of chemical carcinogenesis. Curr Opin Immunol. 2003;15:148–54. doi: 10.1016/s0952-7915(03)00007-4. [DOI] [PubMed] [Google Scholar]